Laboratory and field comparison of measurements obtained using the available diffusive samplers for ozone and nitrogen dioxide in ambient air†
Michel
Gerboles
*,
Daniela
Buzica
,
Luciano
Amantini
and
Friedrich
Lagler
Emissions and Health Unit, Institute for Environment and Sustainability, Joint Research Centre, I – 21020, Ispra, Varese, Italy. E-mail: michel.gerboles@jrc.it; Fax: +39-0332-785652; Tel: +39-0332-789364
First published on 10th November 2005
Abstract
This study presents an evaluation of the extent of differences between measurements performed by O3 and NO2 diffusive samplers and by the reference methods for diffusive samplers commercially available. The tests were performed in an exposure chamber under extreme conditions of controlling factors and under field conditions. For NO2, the results of the laboratory experiments showed that most of the diffusive samplers were affected by extreme exposure conditions. The agreement between the samplers and the reference method was better for the field tests than for the laboratory ones. The estimate of the uptake rate for the exposure conditions using a model equation improved the agreement between the diffusive samplers and the reference methods. The agreement between O3 measured by the diffusive samplers and by the reference method was satisfactory for 1-week exposure. For 8-hour exposures, the diffusive samplers with high uptake rates quantifiyed better the O3 concentration than the samplers with low uptake rates. As for NO2, the results of the O3 field tests were in better agreement with the reference method than the ones of the laboratory tests. The field tests showed that the majority of diffusive samplers fulfils the 25% uncertainty requirement of the NO2 European Directive and the 30% uncertainty requirement of the O3 European Directive for 1-week exposure.
 Michel Gerboles Michel Gerboles | Michel Gerboles was born in France, in 1964. He received his Master of Science in Analytical Chemistry from the University of Bordeaux (France). In 1990, he joined the Joint Research Centre of the European Commission. Currently he has been the quality officer of the Emissions and Health unit at the Institute for Environment and Sustainability of the JRC. His current research interests are: the development of primary methods of calibration and indicative methods like diffusive samplers for the measurement of ambient air pollution, the organisation of proficiency testing and intercomparison exercises for indicative methods in support to the implementation of European Directives on air quality. |
Introduction
The diffusive sampling technique is largely used as an indicative method for monitoring concentrations of pollutants in ambient air. According to the European Daughter Directives 1999/30/EC1 and 2002/3/EC2 indicative methods can be used to assess the level of nitrogen dioxide (NO2) and ozone (O3) in ambient air provided that they meet the data quality objective for uncertainty of 25% for NO2 and 30% for O3. In recent years, several diffusive sampler designs have been implemented for the determination of NO2 and O3 in ambient air.3–7 However, the quality of the results from these samplers has been often criticized. In fact, several inter-comparisons8–10 have been performed, none of which included experiments under controlled conditions in an exposure chamber, under field conditions and including all commercially available diffusive samplers.In this study, the extent of differences between measurements performed by diffusive samplers and the reference methods defined in the European Directives are evaluated under extreme conditions of controlling factors and according to the CEN protocol11 for the validation of diffusive samplers. The inter-comparison consisted of laboratory and field experiments. First, experiments were carried out in an exposure chamber so that the parameters affecting the performance of the diffusive samplers could be controlled. Second, field tests were performed in order to evaluate the consistency of the results of the laboratory tests.
The objective of this paper is to present, to potential users of diffusive samplers, useful results about the whole range of diffusive samplers commercially available for measuring NO2 and O3 in ambient air.
Experimental procedure
Inter-comparison protocol
Several laboratories were involved in the inter-comparison exercise. They were asked to send their own diffusive samplers to the JRC Ispra. The JRC Ispra was responsible for exposing the samplers and sending them back to the participants for analysis. Seven diffusive samplers (six exposure samplers and one travel blank) were supplied by each laboratory. Travel blanks accompanied the exposure samplers to and from the sampling site. They were isolated in sealed bags and refrigerated throughout the exposure period. Small protective boxes gathering six samplers to limit the effect of wind velocity on the response of the samplers were installed in the exposure chamber. Larger rain shields and shelters could not fit in the chamber. For field tests, the samplers were installed inside a shelter as requested by each participant (Table 1). Upon completion of exposure, the samplers were closed and returned to the participants for analysis.| NO2 | O3 | ||||||
|---|---|---|---|---|---|---|---|
| Shelter | Shelter | ||||||
| Samplers | Lab | Preparationa | Lab | Field | Preparationa | Lab | Field |
| a Preparation refers to the cleaning and assembly of the samplers and the coating of the absorbent. | |||||||
| Gradko | 1 | Lab 1 (membrane) | No | No | — | — | — |
| 2 | Lab 2 | Yes | Yes | — | — | — | |
| 3 | Lab 3 | Yes | Yes | — | — | — | |
| 4 | Manufacturer | No | No | Manufacturer (membrane) | Yes | Yes | |
| 5 | Lab 5 | No | No | — | — | — | |
| 6 | — | — | — | Lab 6 (membrane) | No | No | |
| Passam | 7 | Manufacturer | Yes | Yes | Manufacturer | Yes | Yes |
| 8 | Manufacturer | Yes | Yes | — | — | — | |
| 9 | — | — | — | Lab 9 | Yes | Yes | |
| Passam badge | 10 | — | — | — | Manufacturer | — | Yes |
| Radiello | 11 | Manufacturer | No | Yes | Manufacturer | No | Yes |
| 12 | Manufacturer | No | Yes | Manufacturer | No | Yes | |
| 13 | — | — | — | Lab 13 | No | Yes | |
| Ogawa | 14 | Manufacturer | No | Yes | Manufacturer | Yes | Yes |
| 15 | Lab 15/manufacturer | No | Yes | Lab 15 | Yes | Yes | |
| IVL badge | 16 | Manufacturer | No | Yes | Manufacturer | No | Yes |
| Analyst® | 17 | Manufacturer | No | Yes | Manufacturer | — | Yes |
Material and methods
There are basically 3 types of diffusive samplers: longitudinal tube (Palmes tube), radial and badge type. A summary of the diffusive samplers and protective boxes or shelters used by the laboratories are shown in Table 1. They are not associated with individual laboratories to ensure confidentiality. The materials used for the preparation of the samplers (Fig. 1) were manufactured by Gradko International Ltd (UK), Passam ag (CH), the National Research Council (CNR-I), Fondazione Salvatore Maugeri (I), Ogawa & Co Inc. (USA) and the IVL Swedish Environmental Research Institute (S). It is important to notice that several participants did not use samplers prepared by the manufacturer but did their own preparation (Table 1). Some did not implement the shelter specified by the manufacturer (Table 1) or did not use the advised analytical method. The type of samplers is presented in the following paragraphs. Their uptake rate value11 used for the calculation of concentrations is discussed as this is the major characteristic of each sampler.11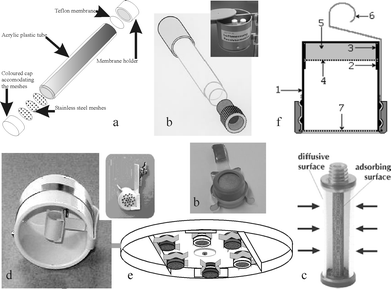 | ||
| Fig. 1 Diffusive samplers: (a) Gradko samplers (with or without membrane), (b) Passam samplers (Palmes tube and badge), (c) Radiello sampler, (d) Ogawa sampler, (e) IVL badge, (f) Analyst. | ||
More details on the implementation of these samplers are available.23,24
Laboratory experiments
The laboratory experiments were performed in an exposure chamber25 that allowed controlling of concentration level, temperature, relative humidity and wind velocity (Fig. 2). The exposure chamber was an “O”-shaped ring-tube system, covered with dark insulation material. A permeation system26 was used to generate NO2. The exposure chamber could hold up to 72 samplers installed on the wall of the chamber and some samplers with their protective box inside the chamber. NO2 was monitored by a nitrogen oxides analyser (Thermo Environmental Instruments TEI 42 C) based on the chemiluminescence method.27 The analyser was checked before and after each experiment with a NO-in-nitrogen cylinder certified using the permeation method and crosschecked with the static volumetric method.28 The NO2 concentrations measured by the continuous analyser were corrected for the humidity interference and for the drift, assuming linear drift between calibration adjustments.29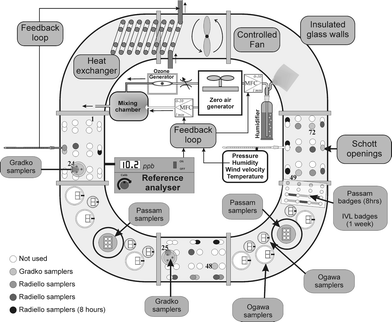 | ||
| Fig. 2 Example of implementation of the exposure chamber for the O3 tests. | ||
For generating O3, a MicroCal 5000 Umwelttechnik MCZ Gmbh O3 generator was used instead of the NO2 permeation system (Fig. 2). O3 was monitored using a TEI 49C. The analyser was calibrated before and after the inter-comparison with an O3 primary standard (TEI Model 49 C Primary Standard, Thermo Environmental Instruments) cross-checked against a long-path UV photometer (UMEG, GmbH). During the inter-comparison, the analyser was submitted to several multi-point calibration checks using a portable O3 generator SYCOS KTO 3 (Ansyco, GmbH) certified against the primary standard.
Previous experiments in the exposure chamber13,30 suggested the combination of environmental parameters that would lead to one low and high uptake rate. This is in accordance with the requirements of the protocol for diffusive samplers.11 The conditions were also chosen to match the yearly limit value of the European Directives of 40 μg m−3 for NO2 and the 8-hour limit value of 120 μg m−3 for O3. The conditions of the experiments in the exposure chamber are given in Table 2.
| Pollutant | Test no | Uptake rate | Concentration/μg m−3 | Wind speed/m s−1 | Temperature/°C | Relative humidity (%) | Exposure time |
|---|---|---|---|---|---|---|---|
| NO2 | 1 | High level | 80 | 2.5 | 25 | 75 | 1 week |
| 2 | Low level | 40 | 1.0 | 5 | 30 | 2 weeks | |
| O3 | 5 | High level | 80 | 2.0 | 15 | 80 | 1 week |
| 6 | Low level | 40 | 0.5 | 25 | 40 | 1 week | |
| 9 | High level | 180 | 2.0 | 15 | 80 | 8 hours | |
| 10 | Low level | 120 | 0.5 | 32 | 40 | 8 hours |
Field tests
For NO2, two monitoring stations of the automatic network of AIRPARIF in Paris (F), one urban at Genevilliers and one rural at Fointainebleau were used. AIRPARIF is accredited under ISO 1702531 for the measurement of several pollutants including NOx and O3 at the automatic stations of its monitoring network. NO2 was measured continuously using a NOx analyser (an Environment s.a model AC 32 M at the urban site and a TEI model 42C at the rural site). The measurements were corrected to the exact value of the NO2-to-NO converter efficiency, for humidity and for the calibration drifts.29For O3, the urban site was also situated at Genevilliers. A TEI 49 analyser was used for the continuous monitoring of O3. The zero and span of the instrument were checked every 14 days. The measurements are traceable to the UV primary standard (long-path UV NIST photometer) of the Laboratoire National d’Essais (F). The rural test was performed at one of the NABEL monitoring stations (Swiss Air Pollution Monitoring Network), situated in Cadenazzo (CH). A TEI 49C instrument was used for the ozone continuous measurements. The zero and span of the instrument were automatically checked every 25 hours; every 14 days a manual calibration (zero and one span point) was performed. The analyser was compared to the Swiss Federal Laboratories for Materials Testing and Research (EMPA) transfer standard (TEI 49C-PS) every three months. EMPA has been designated by the World Meteorological Organisation to operate the World Calibration Centre for Surface Ozone, Carbon Monoxide and Methane (WCC–EMPA). EMPA is also the Quality Assurance/Scientific Activity Centre in Switzerland. For both monitoring sites, the measurements were not corrected for calibration drifts, the drifts being up to 1.5%. The conditions of exposure during the field experiments are shown in Table 3. All samplers, protective boxes and rain shields were installed on the roof of the monitoring stations at about 2 metres far from the inlet of the sampling heads.
| Pollutants | Test no | Site type | Concentration/μg m−3 | Wind speed/m s−1 | Temperature/°C | Relative humidity (%) | Exposure time |
|---|---|---|---|---|---|---|---|
| NO2 | 3 | Urban | 41.6 | 2.8 | 10 | 74 | 2 weeks |
| 4 | Rural | 14.1 | 4.2 | 9 | 79 | 2 weeks | |
| O3 | 11 | Urban | 62 | 21.4 | 68.5 | 2 | 8 hours |
| 7 | 98 | 26.2 | 57.1 | 2.3 | 1 week | ||
| 12 | Rural | 122 | 26.9 | 40.9 | 3.2 | 8 hours | |
| 8 | 92 | 23.2 | 63.1 | 2.8 | 1 week |
Results and discussion
Table 4 shows the average concentration of the 6 samplers analysed by each laboratory for each test together with their standard deviation. The blank values were substracted. In the last column of the table, the reference value of each test is given. It corresponds to the measurement by the reference method. The expanded uncertainty of the O3 and NO2 reference values for the laboratory tests is given in Table 4 under each reference value. The NO2 expanded uncertainty of the laboratory tests was evaluated using the GUM method:29 conversion efficiency, repeatability and linearity of the analyser, uncertainty of standard and zero air for calibration and of zero/span drift were the factors taken into account. For the laboratory O3 tests, the method by Zucco et al.32was implemented with a small modification24 to add the calibration drift and the linearity of the analyser. Gas chromatographic analysis showed that the zero air generator was free of organic compounds which could create an interference to the UV-photometry O3 measurement. It was also checked that water vapour did not interfere with the O3 measurements under the exposure conditions of the inter-comparison by connecting a nafion dryer at the inlet of the analyser. For all field tests, the data quality objective of the European Directive, 25% for NO2 and 30% for O3 of uncertainty, are used instead of an uncertainty estimation. Fig. 4 shows the ratio of NO2 and O3 measured by the diffusive samplers versus the reference methods for all the laboratories. The closer this ratio is to 1.0 the better the agreement between diffusive samplers and the reference methods.| Gradko samplers | Passam samplers | Passam badge | Radiello | Ogawa | IVL | Analyst | Reference values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |||
| a The conditions of the tests are defined in Tables 2 and 3. | |||||||||||||||||||
| NO2 | Lab high level (1) | 76 | 97 | 97 | 126 | 139 | 92 | 87 | 54 | 58 | 132 | 188 | 77 | 111 | 77 | ||||
| ±1.9 | 8.9 | ±11 | ±17 | ±11 | ±12 | ±13 | ±3.7 | ±7.2 | ±19 | ±44 | ±1.3 | 13 | ±5.1 | ||||||
| Lab Low level (2) | 43 | 33 | 36 | 46 | 37 | 37 | 37 | 30 | 27 | 47 | 44 | 31 | 43 | 44 | |||||
| ±1.7 | ±1.8 | ±2.3 | ±4.2 | ±2.1 | ±1.6 | ±1.3 | ±2.4 | ±1.5 | ±2.5 | ±2.0 | ±0.9 | 2.4 | ±4.8 | ||||||
| Urban (3) | 42 | 47 | 41 | 45 | 42 | 50 | 47 | 45 | 41 | 41 | 68 | 65 | 56 | 42 | |||||
| ±1.1 | ±2.3 | ±1.3 | ±2.6 | ±1.1 | ±0.6 | ±1.3 | ±3.2 | ±2.8 | ±0.9 | ±2.4 | ±8.0 | 3.5 | ±10 | ||||||
| Rural (4) | 13 | 12 | 12 | 13 | 12 | 15 | 15 | 12 | 13 | 13 | 32 | 15 | 15.0 | 14 | |||||
| ±0.4 | ±0.9 | ±0.3 | ±0.6 | ±0.4 | ±0.8 | ±1.0 | ±2.2 | ±1.4 | ±0.3 | ±4.9 | ±3.1 | 1.2 | ±3.5 | ||||||
| O3-1 week | Lab high level (5) | 94 | 60 | 132 | 137 | 60 | 59 | 84 | 93 | 88 | 85 | ||||||||
| ±11 | ±4.5 | ±7.2 | ±23 | ±2.5 | ±4.1 | ±2.8 | ±5.7 | ±2.6 | ±5.9 | ||||||||||
| Lab Low level (6) | 24 | 18 | 35 | 30 | 56 | 53 | 28 | 38 | 37 | 40 | |||||||||
| ±1.7 | ±3.1 | ±1.7 | ±1.7 | ±4.0 | ±3.1 | ±3.7 | ±1.7 | ±1.3 | ±5.2 | ||||||||||
| Urban (7) | 108 | 175 | 51 | 87 | 114 | 115 | 100 | 73 | 91 | 83 | 98 | ||||||||
| ±11 | ±49 | ±4.7 | ±4.6 | ±2.8 | ±3.4 | ±2.9 | ±4.2 | ±1.5 | ±3.6 | ±29 | |||||||||
| Rural (8) | 98 | <6.9 | 100 | 119 | 98 | 98 | 104 | 78 | 87 | 86 | 92 | ||||||||
| ±11 | ±8.2 | ±11 | ±4.5 | ±5.3 | ±5.5 | ±5.5 | ±1.6 | ±4.7 | ±28 | ||||||||||
| O3-8 hours | Lab high level (9) | 166 | <80 | 253 | 250 | 215 | 197 | <40 | ND | 180 | |||||||||
| ±37 | ±8.5 | ±16 | ±8.5 | ±11 | ±5.4 | ||||||||||||||
| Lab Low level (10) | 149 | <80 | 185 | 190 | 131 | 146 | <40 | ND | 123 | ||||||||||
| ±52 | ±20 | ±16 | ±4.3 | ±8.3 | ±4.9 | ||||||||||||||
| Urban (11) | 266 | 678 | 67 | 57 | 54 | 64 | 38 | 63 | |||||||||||
| ±172 | ±6.3 | ±13 | ±4.9 | ±9.3 | ±12 | ±19 | |||||||||||||
| Rural (12) | 265 | 2553 | 100 | 99 | 170 | 144 | 84 | 122 | |||||||||||
| ±172 | ±506 | ±4.9 | ±18 | ±13 | ±38 | ±14 | ±37 | ||||||||||||
Nitrogen dioxide
The majority of diffusive samplers overestimated the reference value of test no 1 when NO2, temperature, humidity and wind speed are set to the highest level (Table 4). On the contrary when NO2, temperature, humidity and wind speed were set to the lowest level, the majority of diffusive samplers underestimated the reference value of the laboratory experiment (test no 2).The Fondazione S. Maugeri changed the model equation used to estimate the NO2 uptake rate of the sampler in between the laboratory and field tests. At the same time, the manufacturing process and method of analysis of the sampler did not change. By applying the equation of the operational manual vs. 1/200318 to the laboratory tests, the bias changed from −30 and −31% for Lab 11 and −25 and −39% for Lab 12 to −5.6 and −1.6% for Lab 11 and +1.0 and −13.3% for Lab 12 (values shown in Table 4 and Fig. 3). The corrected concentrations were in closer agreement with the reference values than with those calculated with the previous model of uptake rate.
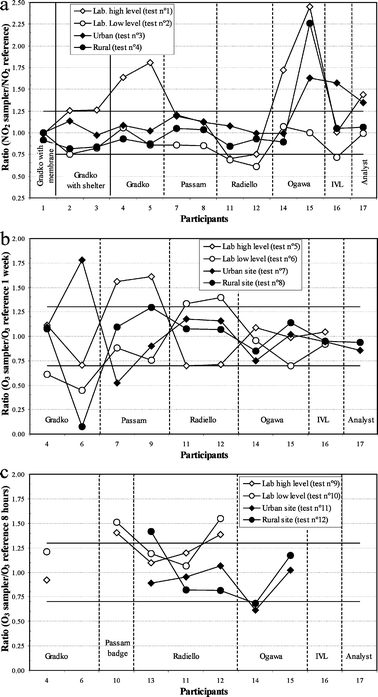 | ||
| Fig. 3 Ratio of concentrations measured by diffusive samplers to the measured by reference methods for laboratory and field tests. (a) NO2, (b) O3, one-week exposure and (c) O3, 8-hour exposure. The horizontal solid lines show the data quality objectives: 25% of uncertainty for NO2 and 30% for O3. | ||
For the Gradko sampler with a protective box, Lab 2 initially calculated NO2 for the laboratory experiments using the theoretical uptake rate of 72.8 cm3 h−1. By using the model equation elaborated by the laboratory14 to estimate the uptake rate, the bias changed from +26%, −25%, +13% and −18% to +14%, −17%, +18%, and −13%. The corrected concentrations agreed better with the reference values. The correction using the model is especially necessary for the exposure under extreme conditions, as show tests no 1 and 2.
Initially, Lab 7 intended to use an uptake rate of 0.9047 cm3 min−1 estimated at 20 °C. However, Lab 7 has been monitoring NO2 since 1986 using an uptake rate of 0.8536 cm3 h−1. In order to remain consistent with previous measurements and to retain the possibility to evaluate long-term trend of air pollution level without a sudden correction of the uptake rate, Lab 7 decided to use the uptake rate estimated at 9 °C. The use of an uptake rate estimated at 9 °C instead of 20 °C worsened the agreement of test no 1 (at 25 °C). It has also improved the agreement of test no 2 (at 5 °C). It is therefore anticipated that with an uptake rate determined for the experimental conditions, the response of the sampler would agree better with the reference values.
For some samplers, the uptake rate can be corrected (for example using a model equation) according to exposure conditions, mainly temperature and humidity. For example, subsequent calculation of the uptake rate was applied by Lab 7 for the Passam sampler, Lab 2 for the Gradko diffusion tube, Labs 11 and 12 for the Radiello sampler, Labs 14 and 15 on a Ogawa sampler. The changes of Lab 7 for the Passam sampler showed a relative deviation between reference values and sampler measurements of about 10%. For the Passam sampler the agreement would be better if the uptake rate was estimated at the actual temperature conditions of exposure. The correction of the Radiello sampler by Labs 11 and 12 using a model developed by the Fondazione S. Maugeri gave an agreement within about 10%. Although showing an improvement, the correction of Lab 15 on Ogawa results still gave a relative deviation of about 35%. However, Lab 14 obtained much better agreement with the model equation developed by Ogawa. The correction using a model developed by Lab 1 gave bias lower than 10%. The correction of Lab 2 on the Gradko diffusion tube with open end placed in a protective box allowed to limit the deviation between reference values and Gradko measurements to about 15%. This was a lower bias than the one of Lab 3 using the same sampler without correction. Therefore it is possible to improve the agreement between the diffusive sampler response and the reference method by calculating an uptake rate for each condition of exposition. The Gradko sampler were implemented by several laboratories (1, 2, 3, 4 and 5) with and without protective box and membrane. Like the majority of samplers, all the implementations of Gradko diffusion tubes gave satisfactory results in the field experiments. Test no 1 showed that under high wind velocities a protective box improves the agreement between sampler response and the reference value.
Lab 16 using the IVL badge obtained good results apart for test no 3 for which the laboratory reported an error in the analytical instrument, which could not be detected at once.
Lab 17, using the Analyst sampler, overestimated test no 1 with high wind speed. The sampler was exposed without using any shelter while the manufacturer requires to use a simple rain shield. The lack of agreement with the reference value for test no 1 may be explained by the lack of rain shield.
Ozone
Overall, for the laboratory tests, the diffusive samplers gave a better quantification of O3 concentration for the 1-week experiments than for the 8-hour experiments. In fact, for the 1-week experiments all samplers quantifyed the O3 concentration, while for the 8-hour laboratory experiments 3 out of 8 samplers were not able to quantify the O3 concentration (Table 4).The 1-week field experiments showed that the majority of measurements by diffusive samplers are in good agreement with the reference method and fulfil the 30% accuracy requirement of the European Directive2 for monitoring O3 (about 9 out of 10 times, the results lie within 30% of the reference value in Fig. 3b). On the other hand, for the 8-hours field experiments the agreement with the reference method was worse (only about 4 out of 7 results lie within 30% of the reference value).
The concentrations measured with the IVL sampler were in good agreement with the reference value (within 8%) and lie within 30% of the UV photometric values for all inter-comparison experiments. Good results were also obtained with the Analyst sampler, which was involved only in the field tests. These samplers however were used only for the 1-week exposures.
The Radiello sampler was implemented by several laboratories (11, 12, 13). Similar results were obtained for 1-week exposure by Labs 11 and 12. No obvious improvement of the agreement with the reference value of the results of Lab 13 was observed by applying its model equation.14 For test no 12, Lab 13 noticed an increase in the blank value and therefore did not substract any blank value for this test. This resulted in a high positive bias (Fig. 3c). Labs 11 and 12 also reported anomalous blank values in test nos 11 and 12 (Table 3 and Fig. 3c). Lab 11 used the actual blank value and obtained better results than Lab 12 which used a statistical blank based on previous values. It can be noticed that the use of a constant uptake rate by Labs 11 and 12 on tests nos 5 and 6 gave once a positive bias and once a negative bias (Fig. 3b). This suggests that the uptake rate might not be constant and that a correction according to exposure conditions could be appropriate.
The results obtained by the laboratories using Gradko samplers (4 and 6) are quite different. The relative biases obtained by Lab 4 were less than 30% for 1-week exposure apart from test no 6, while for the 8-hour exposures Lab 4 obtained much better agreement for the laboratory tests than for the field ones (Fig. 3). Lab 4 indicated that the standard deviation values for some sets of exposed tubes were more variable than it would have been expected from previous tests. The results obtained by Lab 6 for the 1-week laboratory exposure underestimated the O3 concentration. The samplers of Lab 6 did not work properly during the field tests and the 8-hour laboratory experiments. The preparation and analysis of the samplers were performed according to the procedure of each laboratory, which could explain the difference in the results. In Table 4, it can also be noticed that for the 8-hour exposure for which the Gradko sampler could quantify O3, their standard deviations are at least 3 times bigger than the standard deviations obtained for the Radiello, Passam badge and Ogawa samplers.
The Passam diffusive sampler was implemented by Labs 7 and 9 for the 1-week experiments. The temperature correction applied by Lab 9 did not improve significantly the results comparing with Lab 7 which did not correct its results. Lab 7 suggested that high temperatures during sampling could have been the source of the underestimation of O3 for test no 7.33 The Passam badge was used for the laboratory tests with exposure of 8 hours and gave overestimation of O3 with respect to the reference method.
O3 measured by Ogawa sampler were in good agreement with the reference method for the field tests and the 1-week laboratory experiments. The sampler did not measure properly O3 during the 8-hour tests in the exposure chamber independently of the methods of preparation and analysis.
Conclusions
Some participants used their own preparation of samplers instead of using the samplers prepared by the manufacturers, implemented their own analytical method or did not use the shelter as specified by the manufacturer (see Table 1). Therefore, the results of this study can be either characteristic of the participants’ own implementations of the diffusive samplers or typical of the implementation requested by the manufacturer.Using the NO2 results of tests 1 and 2, it would be possible to evaluate the contribution of the controlling factors on the uncertainty for diffusive samplers according to the CEN protocol.11 However, the lack of shelters for Ogawa and Analyst samplers, may have been responsible for the bias between the sampler responses and the reference values rather than the performance of these samplers. In fact, during tests 1 and 2, high wind speed combined with the presence of protective boxes may have created turbulence, hence creating bias for the Ogawa and Analyst samplers. However, the absence of rain shield did not give an increase of the IVL badge, the Gradko diffusion tube with membrane and Radiello responses for the laboratory exposure at high exposure condition (see Table 4). It is believed that by using a thick membrane, samplers could remain unaffected by the conditions in the exposition chamber, in particular by wind velocity.
For O3, the experiments of the inter-comparison showed that no general statement could be given about the performance of each diffusive sampler. The agreement between diffusive sampler measurements and the reference methods was satisfactory for the 1-week exposure. For the 8-hour experiments, the diffusive samplers with high uptake rates quantified better the O3 concentration than the samplers with low uptake rates.
The NO2 and 1-week O3 field tests, carried out under conditions of exposure which were a mixture of the laboratory experiments, show better agreement between the reference values and the response of samplers. This is probably due to the lower scattering of the exposure parameters under field conditions as compared to laboratory conditions. The difference between field and laboratory agreements also indicates the presence of unknown factors that affected the exposure environment during the laboratory tests. Even though it is not possible to compare field and laboratory experiments for the obvious lack of correspondence in temperature and humidity conditions, the overall results suggest the usefulness of some further investigation in order to identify all the factors that could affect the exposure environment of the chamber. The results of the field tests showed that the majority of diffusive samplers fulfils the 25% uncertainty requirement of the European Directive1 and the 30% accuracy requirement of the European Directive2 for the averaging time of 1 week.
In the future, a solution must be found in order to accommodate several diffusive samplers using large shelters and rain shields in exposition chamber to run inter-comparison. The number of inter-comparison proposed to participants should be extended in order to draw conclusions based on a higher number of exposure conditions, reflecting more accurately the range of values expected for the controlling factor all the year long.
Acknowledgements
The authors wish to acknowledge the participation of the following laboratories in the inter-comparison: Consiglio Nazionale della Ricerca (I)—Dr Franco De Santis; Ecole des Mines de Douai (F)—Dr Hervé Plaisance; Fondazione Salvatore Maugeri (I) Dr Paolo Sacco and Vincenzo Cocheo; Gradko Ltd (UK)—Dr Gerry Stuchburry; Harwell Scientifics (UK)—Dr Andy Parish; Instituto Carlos III (S)—Dr Rosalia Fernandez Patier; IVL Swedish Environmental Research Institute (S)—Dr Martin Ferm; Laboratoire d’Hygiène de la ville de Paris (F)—Dr Yvon Le Moullec; Passam A. G. (CH) Dr Markus Hangartner; Surveillance de la Qualité de l’air en Ile-de-France, (F)—Dr Esthel Le Bronnec.The authors would like to thank Dr Hélène Marfaing, Dr Patrick Garnoussi, Laurent Gauvin and Christophe Ampe, of the laboratory Surveillance de la Qualité de l’air en Ile-de-France (AIRPARIF) and Dr Cristoph Hueglin, Mr. Mario Bertozza of the Swiss Federal Laboratories for Material Testing and Research (EMPA) for the installation of diffusive samplers and for providing reference measurements of the monitoring stations of the automatic network of AIRPARIF and Dr Ioannis Drossinos of the Institute of Environment and Sustainability (EC, DG—Joint Research Centre) for the language revision of the manuscript.
References
- EEC Directive, L 163/41, 1999, n° 30.
- EEC Directive, L 67/14, 2002, n° 3.
- K. Stevenson, T. Bush and D. Mooney, Atmos. Environ., 2001, 35, 281–287 CrossRef CAS.
- M. Ferm and H. Rodhe, J. Atmos. Chem., 1997, 27, 17–29 CrossRef CAS.
- G. R. Carmichael, M. Ferm, N. Thongboonchoo, J.-H. Woo and L. Y. Chan, Atmos. Environ., 2003, 37, 1293–1308 CrossRef CAS.
- F. De Santis, C. Vazzana, S. Menichelli, I. Allegrini and S. Morimoto, Phys. Chem. Earth, 2003, 28, 1213–1216 Search PubMed.
- F. Detimmerman, M. Gerboles, S. Boudaa, C. Duchemann and E. De Saeger, The Diffusive Monitor issued by Health and Safety Executive/CAR Working Group 5, 2000, vol. 11, pp. 9–14, Health and Safety Executive, Buxton, UK, 2000 Search PubMed.
- M. Hangartner, M. Kirchner and H. Werner, Analyst, 1996, 121, 1269–1272 RSC.
- M. Kirchner, S. Braeutigam, M. Ferm, M. Haas, M. Hangartner, P. Hofschreuder, A. Kasper-Giebl, H. Rommelt, J. Striedner, W. Terzer, L. Thoni, H. Werner and R. Zimmerling, J. Environ. Monit., 1999, 1, 259–265 RSC.
- M. Gerboles, D. Buzica, L. Amantini, H. Plaisance and H. Marfaing, Pollut. Atmos., 2004, 183, 345–359 Search PubMed.
- European Committee for Standardization, Ambient air quality-Diffusive samplers for the determination of concentrations of gases and vapours-Requirements and test methods-Part 2: Specific requirements and test methods, EN 13528-2, Brussels, 2002 Search PubMed.
- E. D. Palmes, A. F. Gunnison and C. Tomczik, Am. Ind. Hyg. Assoc. J., 1976, 37, 570–577 CAS.
- M. Gerboles, D. Buzica and L. Amantini, Atmos. Environ., 2005, 39, 2595–2608.
- H. Plaisance, A. Piechocki-Minguy, S. Garcia-Fouqué and J. C. Galloo, Atmos. Environ., 2004, 38, 573–5801 CrossRef CAS.
- M. Hangartner, P. Burri and C. Monn, Passive sampling of nitrogen dioxide sulfur dioxide, and ozone in ambient air, Proceedings of the 4th World Clean Air Congress, The Hague, The Netherlands, 11th–15th September, 1989, vol. 3, pp. 661–666 Search PubMed.
- M. Hangartner, The diffusive Monitor HSE/CAR Working Group 5, 2000, vol. 11, pp. 23–24, Health and Safety Executive, Buxton, UK, 2000 Search PubMed.
- S. Garcia-Fouque, H. Plaisance, F. Mathé, J. L. Houdret, J. C. Galloo and R. Guillermo, Improvements of passive sampling techniques for the measurements of ozone nitrogen dioxide and sulfur dioxide in ambient air, presented at the International Conference Air Quality in Europe: Challenges for the 2000s, Venice, Italy, 1999, 19th–21st May, Fondazione Salvatorre Maugeri, Padova, Italy, 1999 Search PubMed.
- Fondazione Salvatore Maugeri, Instruction manual for Radiello sampler, version 1/2003, http://www.radiello.com.
- A. Piechocki, H. Plaisance, A. Kasprowiak, B. Herbin and E. Tison, Etude de la préparation et de l’analyse des tubes Radiello O3 et intercomparaison des methods sur site, no 5–2003, Ecole d’Ingénieurs Centre de Recherche Mines de Douai, Douai, 2003, http://www.lcsqa.org Search PubMed.
- Ogawa Company, NO, NO2, NOx and SO2, Sampling Protocol using the Ogawa sampler, Pompano Beach, Florida, USA; Protocol for ozone measurements using the ozone passive sampler badge, revision 3, February 2001. http://www.ogawausa.com Search PubMed.
- M. Ferm, The theories behind diffusive sampling, EUR 20242 EN, Office for Official Publications of the European Communities, European Commission, Luxembourg, 2002, pp. 31–40 Search PubMed.
- (a) F. De Santis, C. Vazzana, B. D’Angelo, T. Dogeroglu, S. Menichelli and I. Allegrini, in Air Pollution X, ed. C. A. Brebbia, J. F. Martin-Duque, WIT Press, Ashurst Lodge, Southampton, UK, 2002, pp. 371–380 Search PubMed; (b) F. De Santis, T. Dogeroglu, S. Menichelli, C. Vazzana and I. Allegrini, Sci. World, 2001, 1, 475–482 Search PubMed; (c) F. De Santis, T. Dogeroglu, A. Fino, S. Menichelli, C. Vazzana and I. Allegrini, Anal. Bioanal. Chem., 2002, 373, 901–907 CrossRef CAS; (d) F. De Santis, A. Fino, S. Menichelli, C. Vazzana and I. Allegrini, Anal. Bioanal. Chem., 2004, 378, 782–788 CrossRef CAS.
- D. Buzica, M. Gerboles and L. Amantini, Inter-comparison of NO2 diffusive samplers under laboratory and field conditions, EUR 20860 EN, Office for Official Publications of the European Communities, Luxembourg, 2003 Search PubMed.
- D. Buzica, M. Gerboles, L. Amantini and F. Lagler, Laboratory and field inter-comparison of O3 diffusive samplers, EUR 21754 EN, Office for Official Publications of the European Communities, Luxembourg, 2005 Search PubMed.
- M. Gerboles, D. Buzica, L. Amantini, F. Lagler and T. Hafkenscheid, J. Environ. Monit. 10.1039/b509559j.
- International Standards Organisation, Gas analysis—Preparation of calibration gas mixtures using dynamic volumetric methods—Part 10 Permeation method, ISO 6145-10:2002, Geneva, CH, 2002 Search PubMed.
- European Committee for Standardization, Ambient air quality-Measurement method for the determination of the concentration of nitrogen dioxide and nitrogen monoxide by chemiluminescence, EN 14211, Brussels, 2005 Search PubMed.
- International Standards Organisation, Gas analysis—Preparation of calibration gas mixtures—Static volumetric method, ISO 6144:2002, Geneva, 2002 Search PubMed.
- M. Gerboles, F. Lagler, D. Rembges and C. Brun, J. Environ. Monit., 2003, 5, 529–540 RSC.
- F. Detimmerman, M. Gerboles, L. Amantini and E. De Saeger, Validation of Radiello diffusive sampler for monitoring ozone in ambient air, EUR 19594 EN, Office for Official Publications of the European Communities, Luxembourg, 2000 Search PubMed.
- International Standards Organisation, General requirements for the competence of testing and calibration laboratories, ISO 17025:2005, Geneva, 2005 Search PubMed.
- M. Zucco, S. Curci, G. Castrofino and M. P. Sassi, Meas. Sci. Technol., 2003, 14, 1683–1689 CrossRef CAS.
- S. Garcia-Fouque, PhD thesis, Study of ozone measurement using diffusion tubes—Field implementation, Technological University of Compiègne, 1998 Search PubMed.
Footnote |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| This journal is © The Royal Society of Chemistry 2006 |
