Development of a solid phase microextraction (SPME) method for the sampling of VOC traces in indoor air†
Virginie
Larroque
a,
Valérie
Desauziers
*a and
Pierre
Mocho
b
aLaboratoire Génie de l’Environnement Industriel, Ecole des Mines d’Alès, Hélioparc – 2 avenue Pierre Angot, 64053, Pau Cedex 9, France. E-mail: valerie.desauziers@ema.fr; Fax: 33 5 5930 6368; Tel: 33 5 5930 5425
bLaboratoire Thermique Energétique et Procédés, Université de Pau et des Pays de l’Adour, B.P. 1155, 64000, Pau, France. E-mail: pierre.mocho@univ-pau.fr; Fax: 33 5 5940 7725; Tel: 33 5 6256 3510
First published on 30th November 2005
Abstract
Solid-phase microextraction (SPME) was studied for the measurement of volatile organic compounds (VOCs) in indoor air. An adsorptive PDMS/Carboxen fibre was used and an analytical methodology was developed in order to overcome competitive adsorption. Kinetics and adsorption isotherms were investigated for different sample volumes and model compounds. In order to evaluate competitive adsorption on the fibre, these compounds were studied alone and in mixture. From the results obtained, the operating conditions allowing co-adsorption of the target compounds were determined: the air sample is enclosed in a 250 mL glass bulb where the SPME fibre is exposed until adsorption equilibrium. This procedure was combined with GC/MS analysis for the identification and quantification of VOCs in indoor air. The performances were determined by using a standard gas containing 10 VOCs representative of indoor environments (acetaldehyde, acetone, BTX, α-pinene, trichloroethylene, alkanes). The detection limits were determined in single ion monitoring mode and for a signal to noise ratio of 3. Except acetaldehyde (6 μg m−3), they are all below 0.5 μg m−3. Calibration curves are linear up to 10 μmol m−3 for all the compounds with good correlation coefficients (above 0.99). The reproducibility ranges from 6 to 12% according to the compound. The methodology was then applied to the comparison of the VOCs content in classrooms of two different schools.
 Valérie Desauziers Valérie Desauziers | Valérie Desauziers was born in France in 1965. She received her PhD in 1991 on "Analysis of tributyltin in marine sediments—Preliminary study for the development of a reference material" from the University of Pau, France. In 1992, she joined the Laboratory of Industrial Environmental Engineering of the Mining School of Alès as assistant-professor. Since 2002, she develops a research group in Pau on VOCs and odour analysis. Her research interest is the development of pre-concentration methods for the sampling of micropollutants in the environment, particularly focused on volatile organic compounds in air and odour. |
Introduction
Recently, indoor air quality has been associated with adverse health effects (headaches, nausea,…) observed in French schools. The few French studies undertaken on the subject have highlighted considerable levels of indoor pollutant concentrations in the schools investigated. It can be also pointed out that contrary to the biological agents, chemical pollution, including volatile organic compounds (VOCs), is studied. The most identified molecules are aldehydes, especially formaldehyde, which mainly comes out of agglomerated wooden furniture,1 BTEX (benzene, toluene, ethyl benzene and xylenes) which predominantly have outdoor sources2,3 and halogenated compounds and terpenes which are emitted by household products.1 The concentrations measured are in the μg m−3 order and were shown to be similar to those observed in residential buildings.2–5For the individual analysis of VOCs in indoor atmospheres, two different sampling strategies can be envisaged with regards to the analytical objectives: one consists of active sampling which gives information about the evolution of pollutant concentrations; another approach involves the time weighted average (TWA) sampling which allows the determination of average concentrations for the evaluation of long term exposure. Generally, adsorbent devices are used for active6,7 or diffusive8 sampling followed by thermal desorption on line with gas chromatography (GC). Air samples can also be collected in adapted containers such as canisters previously evacuated. VOCs are then pre-concentrated in the laboratory by using adsorbent traps or cryogenic concentrators.9 As these techniques require the use of specific and expensive analytical equipment (i.e. automated thermal desorber), solid-phase microextraction (SPME)10–13 was studied as an alternative method. The interest of this sampling tool consists of its ease of use and its direct thermal desorption in a classic split/splitless GC injection port. In a previous work,14 it was shown that the adsorptive PDMS (polydimethylsiloxane)/Carboxen fibre was the most effective for extraction of VOCs at trace levels. However, competitive adsorptions, leading to inaccurate quantification, were observed.15,16 To overcome this drawback, analytical methodologies were developed12,15 to determine co-adsorption conditions of VOCs on adsorptive coatings. They are based on non-equilibrium short-time extraction to reduce the quantity of molecules to be adsorbed on the fibre.15,17,18 Generally, extraction times of several minutes are applied. This paper intends to describe another experimental approach for the SPME sampling of VOCs in air. The methodology involves extraction under adsorption equilibrium conditions to increase sensitivity and to minimize the importance of extraction time. The co-adsorption conditions were determined from the study of adsorption kinetics and isotherms for isolated model compounds and compounds in mixture. Even if air velocity has less influence in indoor environments than in ambient air, we chose to perform extraction in static mode by enclosing the air sample in glass bulbs; different volumes were tested. All the development experiments were carried out using standard atmospheres and GC/FID analysis. The performances (limits of detection, reproducibility and calibration curves) of the final method were determined by using GC/MS.
A feasibility study was then performed to check the potential of the developed method for the identification and quantification of VOCs in indoor air, and particularly in school environments. Two schools were investigated: one was built according to the regulations and traditional rules of design, the other was built according to the “HQE” (“High Environmental Quality”) approach which is the transposition of sustainable development concepts to the building trade.
Experimental
Reagents and materials
Studied VOCs were acetaldehyde, acetone, benzene, cyclohexane, trichloroethylene, toluene, butyl acetate, p-xylene, α-pinene and n-decane purchased from Acros Organics (New Jersey, USA). They were all at least 99% purity.Standard solutions for GC/FID external calibration were prepared by diluting different amounts of the studied VOCs in n-butanol of analytical grade (Acros Organics, New Jersey, USA). The concentrations ranged from 0.005 to 0.1 mol L−1 for each compound. This calibration was performed to determine the extracted amount of analyte by the SPME fibre.19 So, to be representative of fibre thermal desorption, n-butanol was chosen for its small vapor volume in order to be compatible with the SPME injection liner.19 A set volume of 0.1 μL of standard solutions was injected manually, using a 1 μL SGE syringe (without dead volume) (Fisher Scientific, Elancourt, France).
A manual SPME holder was used with 75 μm PDMS/Carboxen fibres purchased by Supelco (Bellefonte, PA, USA). New SPME fibres were conditioned into the GC injection port at 280 °C for at least 5 h. Before each sampling, fibres were conditioned for 10 min in the injection port at 320 °C to avoid contamination from the laboratory atmosphere.
Standard gas generating device
The device used was especially designed and purchased from Calibrage (Saint Chamas, France). It consists of the continuous injection of liquid VOCs, using a mass-flow meter, into a controlled airflow where the compounds were vaporized. Successive air dilutions of concentrated standard gas were then applied to reach the desired concentrations. The detailed principle of gas generation is described in a previous paper.20 The sampling chambers were 250 mL, 375 mL and 1000 mL glass bulbs equipped with Teflon stopcocks and septum for SPME fibre introduction. Sampling was realised in static mode (stagnant air).Instrumentation
Analytical performances and analysis of indoor air samples were performed by using a Varian 3800 gas chromatograph coupled to a 1200Q quadrupole mass spectrometer (MS) (Varian, Les Ulis, France). The PTV 1079 injection port was equipped with a 0.75 mm id liner and operated at 320 °C in splitless mode. The carrier gas was helium with a flow rate of 1 mL min−1. The chromatographic column was a Varian VF5ms (Varian, Les Ulis, France), 60 m × 0.25 mm × 1 μm. The oven was programmed as follows: 40 °C for 4 min, ramped to 190 °C at 7 °C min−1 then ramped to 250 °C at 10 °C min−1. The transfer line to MS was maintained at 280 °C and the ion source at 250 °C. Acquisition was performed in electronic impact (EI) mode and started 4.3 min after sample introduction. The mass range used was 30–250 a.m.u. and the acquisition rate was 0.5 scan s−1.
Measurement of temperature, relative humidity and air velocity
At the sampling sites, relative humidity and temperature of air samples were measured with a Kimo HD200 electronic thermo-hygrometer (Kimo, Montpon, France). The ranges measured were 3 to 98% RH with a 0.1% RH resolution, and −20 to +80 °C with a 0.1 °C resolution. The air velocity was measured with a Kimo VT100 anemometer (Kimo, Montpon, France). The range measured was 0–3 m s−1 with a 0.01 m s−1 resolution.Theoretical models
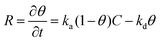 | (1) |
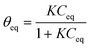 | (2) |
If Ceq → 0 then θeq = KCeq where K is the origin slope of the isotherm. This simplified Langmuir equation, similar to the Henry law, can be assumed in this application because of the low concentration of VOCs in indoor air.
Results and discussion
Development of the analytical methodology
To obtain good sensitivity and to be independent of the extraction time, equilibrium extraction was preferred to the short-time method previously developed.15,18 Equilibrium conditions were determined through adsorption kinetics of toluene. This compound was selected because it was shown to have a high affinity with the adsorptive Carboxen coating of the SPME fibre.14,16 Consequently, it can be regarded as representative of the VOCs for which adsorption equilibrium takes a long time to reach (equilibrium times for p-xylene, α-pinene and n-decane were checked in the 250 mL sampling bulb and were found to be established around 240 min, as toluene).Three sample volumes were tested. The 1000 mL glass bulb was the same as that used in the short-time extraction method.15,18 Smaller volumes, 375 mL and 250 mL, were studied to limit the quantity of extractable VOCs and hence to overcome competitive adsorption. Another reason to select small volumes was to shorten the equilibrium time. Toluene atmospheres were generated as described in the experimental section. Concentration in air was 200 μmol m−3 (from 8800 μg m−3 to 27 248 μg m−3 according to the compound) for 250 mL and 1000 mL, and 135 μmol m−3 for 375 mL.
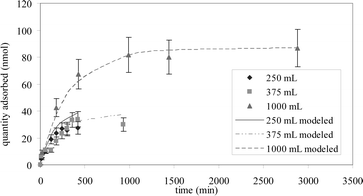
Adsorption kinetics are presented in Fig. 1. The amount of toluene adsorbed onto the fibre was determined using the GC/FID external calibration described in the Reagents and Materials section. The results of the adsorption kinetics of toluene on SPME fibres are reported in Table 1.
 | ||
| Fig. 1 | ||
| k a/m3 s−1 mol−1 | k d/s−1 | K/m3 mol−1 | |
|---|---|---|---|
| Toluene | 1.28 × 10−1 | 1.54 × 10−5 | 8.312 × 103 |
The high value of K provides evidence that PDMS/Carboxen SPME fibres are particularly suitable for adsorbing toluene at low concentrations. Hence, the adsorbate/adsorbent interactions are strong and toluene is not affected by competitive adsorption.
Equilibrium is reached after 240 min (4 h), 360 min (6 h) and 990 min (16.5 h) for 250 mL, 375 mL and 1000 mL, respectively. For the latter (375 mL and 1000 mL), extraction times would be too long to be applied in a realistic analytical procedure, even if the sensitivity were higher than for smaller air volumes. A sample volume of 250 mL was hence selected.
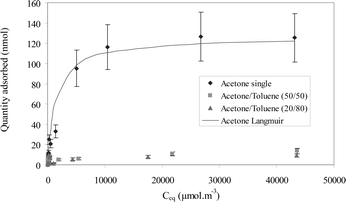
Due to the small quantity of adsorbent available on the SPME fibre, saturation occurs rapidly, leading to competitive adsorption and then to inaccurate quantification. Therefore, co-adsorption conditions were determined from the adsorption isotherms of acetone, which has a low affinity with Carboxen and hence, can be considered as a limitant. Indeed, this compound was shown to be significantly desorbed by other molecules when it was studied in VOC complex mixtures.15,16 Thus, only the adsorption isotherms of acetone were studied. A binary isotherm was obtained from an equimolar mixture and an acetone/toluene (20/80) mixture (Fig. 2). The shape of the adsorption isotherm of single acetone in Carboxen is a type I isotherm for a concentration lower than 42 500 μmol m−3. This type represents a monolayer adsorption in small pores (micropores) not significantly greater than the molecular diameter of the adsorbate.22 The curve fitting of this isotherm with the Langmuir model gives a value of 700 m3 mol−1 for the equilibrium constant, K and 126 nmol for the monolayer adsorption capacity, qo. Compared to the K value of toluene, acetone is less strongly linked to the adsorbent and so it is more sensitive to competitive adsorption.
 | ||
| Fig. 2 | ||
The binary adsorption isotherm shows clearly the competitive adsorption between acetone and toluene. However, the adsorption of acetone is not modified in the linear part of the isotherms, even when regarding binary mixtures. This co-adsorption zone is detailed in Fig. 3.
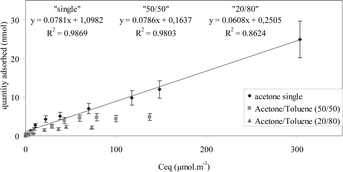 | ||
| Fig. 3 | ||
From this figure, it has been deduced that co-adsorption conditions are obtained for acetone concentrations up to 20 μmol m−3. Within this range, it can be assumed that reliable quantification can be achieved.
These operating conditions (250 mL air sample, static extraction at equilibrium and concentration range below 20 μmol m−3) were applied to determine the performance of the method by using GC/MS analysis (Table 2). A mixture of 10 different VOCs, representative of indoor environments, was used (see Experimental section). Limits of detection (LOD) were determined in single ion monitoring (SIM) and for a signal to noise ratio of 3. They are all below the LOD, acetaldehyde excepted (Table 2). The reproducibility was evaluated for an individual concentration of 2 μmol m−3 by using 5 different SPME fibres and 5 different 250 mL glass bulbs. Under these conditions, the relative standard deviation (RSD) varies from 6 to 12% (Table 2).
The calibration curves were plotted for the ten compounds in the mixture. The ions used for quantification are described in Table 2. Calibration curves were plotted from 0.05 μmol m−3 to 10 μmol m−3 and were found linear within this range. Equation parameters and correlation coefficients are given in Table 3.
| Compounds | y axis intercept/counts | Slope/counts μg−1 m3 | Correlation coefficient, R2 |
|---|---|---|---|
| Acetaldehyde | 2.3×106 | 3.8×107 | 0.9917 |
| Acetone | −2.2×108 | 1.3×109 | 0.9915 |
| Benzene | −4.7×108 | 3.3×109 | 0.9956 |
| Cyclohexane | −3.3×108 | 2.5×109 | 0.9918 |
| Trichloroethylene | −7.9×108 | 6.3×109 | 0.9924 |
| Toluene | −3.2×108 | 3.6×109 | 0.9958 |
| Butyl acetate | −2.3×108 | 2.9×109 | 0.9909 |
| p-Xylene | 1.4×107 | 3.1×109 | 0.9906 |
| α-Pinene | 3.7×108 | 1.4×109 | 0.9992 |
| n-Decane | 9.7×107 | 8.2×108 | 0.9945 |
With regards to these results, a feasibility study was envisaged to check the ability of SPME to sample indoor air.
Sampling sites
The indoor atmospheres of two different schools were studied. They are located in the Aquitaine region (France), in a small town near Bordeaux. The school named A was built in 1956, following traditional rules of design, and school B was built in 2003 according to the “HQE” (“High Environmental Quality”) approach.23 This involved numerous objectives, including improvement of indoor air quality, therefore “HQE” buildings are equipped with mechanical ventilation and building materials are chosen for their low pollutant emission rates.For each school, two classrooms were studied. They are described in Table 4. Outdoor air was also sampled in each playground.
| A (traditional building) | B (“HQE” building) | |||
|---|---|---|---|---|
| Classroom A1 | Classroom A2 | Classroom B1 | Classroom B2 | |
| Area/m2 | 38.4 | 36.5 | 60.3 | 60.3 |
| Volume/m3 | 110 | 95 | 150 | 150 |
| Children age | 5 | 3 | 5 | 3 |
| Children number | 28 | 28 | 28 | 28 |
| Adult number | 2 | 2 | 2 | 2 |
The sampling campaign was carried out on 15th March 2005. Sampling conditions are summarized in Table 5.
SPME static sampling was performed in 250 mL glass bulbs, under the operating procedure previously described (single extraction in each room and playground). Sampling was done at a height of one meter from the floor to be representative of the air breathed by the children, and far from the walls. Bulbs were flushed for several minutes by the air samples, then they were closed for extraction. To avoid cross contamination, one bulb per sample was used. Losses by adsorption were overcome by treating the walls of the 250 mL glass bulbs with deactivated material (Silcosteel) (Restek, Evry, France). After use, SPME fibres were stored at ambient temperature in hermetically closed stainless steel tubes. Blank fibres were stored under the same conditions as the samples. All the analyses were performed within one day, at a maximum of 24 h after sampling. For this storage time, at ambient temperature, it was shown that the average recovery for the ten model compounds was 93% (from 79% for acetaldehyde to 120% for acetone). The test was carried out by using standard gas at 1 μmol m−3 (individual concentration) and a relative humidity of 50%.
Relative humidity may influence the VOCs adsorption, despite Carboxen hydrophobicity. Low molecular weight compounds are of particular concern, whereas adsorption of “heavier” compounds seems not to be influenced.24 This parameter will be further studied to determine its effects.
VOCs identified in the two schools studied
Results dealing with the traditional and the HQE schools, (respectively “A” and “B”) are given in Table 6.| A (Traditional building) | B (“HQE” building) | |||||
|---|---|---|---|---|---|---|
| VOCs | Playground | Classroom A1 | Classroom A2 | Playground | Classroom B1 | Classroom B2 |
| a Not detected. b Detected but not quantified (see text). c Methyl ethyl ketone. d Methyl isobutyl ketone. | ||||||
| Butane | Nd a | Nd | Nd | Nd | 12.0 | Nd |
| Isobutene | 5.0 | Nd | 5.0 | Nd | Nd | Nd |
| 2-methyl butane | Nd | Nd | Nd | Nd | 13.0 | 13.0 |
| Cyclohexane | Nd | Nd | Nd | Nd | 14.0 | 9.0 |
| Sum of alkanes | 5.0 | — | 5.0 | — | 39.0 | 22.0 |
| Benzene | 0.5 | 1.0 | Nd | 0.3 | 0.5 | 1.0 |
| Toluene | 3.0 | 2.0 | Nd | Nd | 0.5 | 3.0 |
| Xylenes | Nd | 3.0 | 6.0 | Nd | Nd | 3.0 |
| Trimethylbenzenes | Nd | 2.0 | 2.0 | Nd | Nd | 2.0 |
| Sum of aromatics | 3.5 | 8.0 | 8.0 | 0.3 | 1.0 | 9.0 |
| Acetonitrile | Nd | Nd | 8.0 | Nd | Nd | Nd |
| Acetic acid | Nd | 36.0 | 18.0 | Nd | Nd | 1.0 |
| Acetone | d b | d | d | d | d | d |
| Ethanol | 2.0 | 8.0 | 47.0 | Nd | 10.0 | 14.0 |
| 1-Methoxy-2-propanone | 7.0 | 10.0 | 7.0 | Nd | 37.0 | 16.0 |
| MEK c | 7.0 | Nd | 2.0 | Nd | Nd | 3.0 |
| MIBK d | Nd | 37.0 | 11.0 | Nd | Nd | Nd |
| Cyclohexanone | 10.0 | 13.0 | Nd | Nd | Nd | Nd |
| 4-Pentan-2-ol | Nd | Nd | Nd | Nd | Nd | 46.0 |
| Butyl acetate | Nd | 48.0 | 7.0 | Nd | 5.0 | Nd |
| Nonanal | 4.0 | 16.0 | 1.0 | 42.0 | Nd | 3.0 |
| Decanal | Nd | 3.0 | Nd | 0.7 | Nd | Nd |
| Sum of oxygenated | 30.0 | 171.0 | 101.0 | 42.7 | 52.0 | 80.0 |
| α-Pinene | 3.0 | 6.0 | 8.0 | Nd | 20.0 | 10.0 |
| β-Phellandrene | Nd | Nd | Nd | Nd | 3.0 | Nd |
| D-Limonene | 2.0 | 17.0 | 15.0 | Nd | 7.0 | Nd |
| Sum of terpenes | 5.0 | 23.0 | 23.0 | — | 30.0 | 10.0 |
| Sum of VOCs | 43.5 | 202.0 | 137.0 | 43.0 | 122.0 | 121 |
The model compounds (in bold lettering in Table 6) were quantified using their own calibration curves (SIM mode). For the other VOCs, only an estimation of the concentrations is given: they were determined through the calibration curves, obtained in full scan mode, of model compounds belonging to the same chemical family. For example, alkanes were quantified by using the cyclohexane calibration curve, trimethylbenzene, by using the toluene curve, oxygenated compounds by using the acetone or the butyl acetate curves, and terpenes by using the α-pinene curve. It can be also noted that acetone was identified in all the samples but a contamination, highlighted by the blank fibres, did not allow its quantification.
About 20 compounds, belonging to four different chemical families (alkanes, aromatic compounds, oxygenated compounds and terpenes) were identified. As it is commonly stated in the literature, VOCs are more numerous and more concentrated in buildings than in ambient air.
The concentration of benzene is the same indoor and outdoor due to the external source (exhaust fumes) of this compound. However, the sum of aromatic compound concentrations is slightly higher in the classrooms, assuming indoor sources of toluene, xylenes and trimethylbenzenes.25
A lot of carbonyl compounds were identified such as acetone, MEK and MIBK, which can come from adhesives and solvents.25
The concentration of terpenes is also higher in the classrooms than in playgrounds: this can be explained by their use in household products.
In classrooms, the sum of VOCs ranged from 121 μg m−3 to 202 μg m−3. These data are in good agreement with those obtained from an English study performed on 8 classrooms and where the annual average concentration of TVOC (total VOC) ranged from 87 to 378 μg m−3.26 In our study, the higher level is observed in the classroom for 5 year old children, in the traditional school. It is essentially due to the high concentrations of acetic acid, MIBK and butyl acetate. These two latter compounds may come from pen felts27 and so, this high VOCs level could be attributed to the childrens’ activities rather than to the difference in building design (traditional/HQE).
The links to health effects are difficult to establish as VOCs differ in their toxicity by more than four orders of magnitude.28 The studies investigating causal relationships between health symptoms and exposures to pollutants suggest that asthma and “Sick Building Syndrome” (SBS) in schools are related to VOCs together with moulds, microbial VOCs and allergens.29 At this time, research should be continued to improve the scientific basis on which to establish limit values and guidelines for VOCs in non-industrial buildings.30
Conclusion
This study shows that SPME can be used for the screening of VOCs in indoor air. The limits of detection obtained for 10 representative model compounds range from 0.05 μg m−3 (p-xylene) to 5.9 μg m−3 (acetaldehyde), and the reproducibility varies from 6 to 12% according to the compound. Linearity of the calibration curves is achieved for concentrations up to 10 μmol m−3 (440 μg m−3 to 1360 μg m−3 according to the compound), which is sufficient to quantify most VOCs in indoor air.The feasibility study confirms the complexity of contamination of indoor environments by VOCs, which is strongly influenced by the multiplicity of sources, rules of construction, household and occupant activities. Even if these first results are in good agreement with recent works on the subject, they do not allow us to establish precisely the relationship between VOCs and their potential sources or to highlight a significant difference between the two building concepts studied (traditional and HQE). Therefore, further studies will involve more numerous data and average measurements for a better understanding of VOCs indoor pollution and their links to health effects.
Acknowledgements
Authors acknowledge TREF LE laboratory (ENSAM, Bordeaux) coordinator of the project “Performance monitoring of HQE buildings”, and FEDER for financial support.References
- D. G. Shendell, A. M. Winer, T. H. Stock, L. Zhang, J. F. Zhang, S. Maberti and S. D. Colombe, J. Exposure Anal. Environ. Epidemiol., 2004, 14, 44–59 Search PubMed.
- H. Guo, W. M. Li and J. J. Cao, Atmos. Environ., 2003, 37, 73–82 CrossRef CAS.
- E. Vega, V. Mugica, R. Carmona and R. Valencia, Atmos. Environ., 2000, 34, 4121–4129 CrossRef CAS.
- J. L. Adgate, T. R. Church, A. D. Ryan, G. Ramachandran, A. L. Fredrickson, T. H. Stock, M. T. Morandi and K. Sexton, Environ. Health Perspect., 2004, 112, 1386–1392 CAS.
- H. Guo, S. C. lee, L. Y. Chan and W. M. li, Environ. Res., 2004, 94, 57–66 CrossRef CAS.
- T. Seko, K. Usukura and N. Onda, Bunseki Kagaku, 2003, 52, 1215–1220 CrossRef CAS.
- M. Hippelein, J. Environ. Monit., 2004, 6, 745–752 RSC.
- A. Pennequin-Cardinal, H. Plaisance, N. locoge, O. Ramalho, S. Kirchner and J. C. Galloo, Atmos. Environ., 2005, 39, 2535–2544 CrossRef CAS.
- S. C. Lee, W. M. Li and C. H. Ao, Atmos. Environ., 2002, 36, 225–237 CrossRef CAS.
- J. Pawliszyn, Solid-Phase MicroExtraction: theory and practice, Wiley VCH, New York, 1st edn, 1997, pp. 1–247 Search PubMed.
- E. Razote, I. Jeon, R. Maghirang and W. Chobpattana, J. Environ. Sci. Health, 2002, 37, 365–378 Search PubMed.
- M. Y. Jia, J. Koziel and J. Pawliszyn, Field Anal. Chem. Technol., 2000, 4, 73–84 Search PubMed.
- Y. Chen and J. Pawliszyn, Anal. Chem., 2003, 75, 2004–2010 CrossRef CAS.
- L. Tuduri, V. Desauziers and J. L. Fanlo, J. Chromatogr. Sci., 2001, 39, 521–529 CAS.
- L. Tuduri, V. Desauziers and J. L. Fanlo, Analyst, 2003, 128, 1028–1032 RSC.
- L. Tuduri, PhD thesis, University of Pau, 2002.
- J. Koziel, M. Y. Jia and J. Pawliszyn, Anal. Chem., 2000, 72, 5178–5186 CrossRef CAS.
- L. Tuduri, V. Desauziers and J. L. Fanlo, J. Chromatogr., A, 2002, 963, 49–56 Search PubMed.
- L. Tuduri, V. Desauziers and J. L. Fanlo, J. Microcolumn Sep., 2000, 12, 550–557 CrossRef CAS.
- L. Tuduri, V. Desauziers and J. L. Fanlo, J. Chromatogr. Sci., 2001, 39, 521–529 CAS.
- C. L. Chuang, P. C. Chiang and E. E. Chang, Chemosphere, 2003, 53, 17–27 CrossRef CAS.
- S. Brunauer, L. S. Deming, N. S. Deming and E. Teller, J. Am. Chem. Soc., 1940, 62, 1723–1728 CrossRef CAS.
- Association pour la Haute Qualité Environmentale des Bâtiments, http://www.assohqe.org/.
- F. Lestremau, F. A. T. Anderson, V. Desauziers and J. L. Fanlo, Anal. Chem., 2003, 75, 2626–2632 CrossRef CAS.
- International standard ISO 16000-1: 2004 Indoor air - Part 1: General aspects of sampling strategy, AFNOR, Saint Denis La Plaine, July 2004 Search PubMed.
- D. Crump, Indoor air quality and ventilation in some schools in England, in Proceedings of the Scientific day on Qualité de l’air intérieur dans les écoles, Paris, 2005, Ineris, Verneuil en Halatte, 2005, http://rsein.ineris.fr/actualite/actualite.html#Manifestations%20passees Search PubMed.
- A. M. Laurent, Bilan de la qualité de l’air dans les écoles, in Proceedings of the Scientific day on Qualité de l’air intérieur dans les écoles, Paris, 2005, Ineris, Verneuil en Halatte, 2005, http://rsein.ineris.fr/actualite/actualite.html#Manifestations%20passees Search PubMed.
- H. Rothweiler and C. Schlatter, Toxicol. Environ. Chem., 1993, 40, 1–4.
- J. M. Daisey, W. J. Angell and M. G. Apte, Indoor Air, 2003, 13, 53–64 CrossRef CAS.
- K. Andersson, J. V. Bakke, O. Bjorseth, C. G. Bornehag, G. Clausen, J. K. Hongslo, M. Kjellman, S. Kjaergaard, F. Levy, L. Molhave, S. Skerfving and J. Sundell, Indoor Air, 1997, 7, 78–91 CrossRef CAS.
Footnote |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| This journal is © The Royal Society of Chemistry 2006 |
