Biological monitoring of ozone: the twenty-year Italian experience†‡
C.
Nali
*,
A.
Francini
and
G.
Lorenzini
Dipartimento di Coltivazione e Difesa delle Specie Legnose “Giovanni Scaramuzzi”, Università di Pisa, Via del Borghetto, 80, 56124, Pisa, Italy. E-mail: cnali@agr.unipi.it; Tel: +39 050 960092
First published on 24th November 2005
Abstract
Tropospheric ozone is a growing environmental menace in Italy and in the whole Mediterranean basin. The importance of active biomonitoring of this pollutant with hypersensitive Bel-W3 tobacco plants is stressed, and several examples of field studies carried out in Italy with this technique are presented. Current limitations are discussed, with special emphasis on data quality assessment and the opportunity of adopting easy-to-use kits based on tobacco germlings instead of adult plants. A standardization of methodologies (from cultivation to scoring and data elaboration), also at an international level, is strongly felt to be needed, in order to get official acknowledgement of biomonitoring procedures. Potential educational implications, with the active involvement of students and environmentalists, are shown. Other biological indicators are used, namely sensitive and resistant white clover (Trifolium repens) clones (as descriptors of biomass reduction in crops species) and Centaurea jacea (brown knapweed) as a model species to evaluate the relationship between ozone exposure and effects on the performance and injury symptoms of native plants which are largely used in the framework of European programmes.
 C. Nali C. Nali | Cristina Nali was born in Italy, in 1965. She received her PhD in Phytotoxicology from University of Pisa, Italy in 1997. During this time and thereafter she worked as a researcher on plant pathology, with special concern about the interactions between fungal diseases and gaseous air pollutants. Throughout this time, she also engaged in biological education projects, with particular regard to biological monitoring of ground-level ozone; another major field of interest has been the study of the effects of environmental pollutants upon qualitative performances of agricultural and medicinal plant species. Currently, she is Assistant Professor at the Faculty of Agricultural Sciences of the University of Pisa. Her research interests are interactions between air pollutants and plants, especially biological monitoring and the study of physiological and molecular bases for differential response of plants to the oxidative stress. |
Introduction
In all Europe and especially in the Mediterranean basin there is growing evidence of an increase in the concentration in the troposphere of photochemically-produced ozone;1 it is expected that this will continue to rise in the coming years.2 This means that ‘clean air’ today (and tomorrow) differs from that present a few decades ago, and this could have serious consequences for human welfare.3 The increasing interest in the distribution of ozone is properly due to its complex environmental impact on organisms and on non-living targets. Apart from direct effects, ozone plays important roles in controlling the chemical composition and climate of the troposphere. Urban areas are the main sources of precursor pollutants (i.e. nitrogen oxides and non-methanic hydrocarbons), but the presence of significant ozone levels is also verified in rural and remote areas, albeit with large temporal and spatial variations. Detailed mapping of ozone levels in vulnerable areas is strongly needed.The on-ground ozone distribution in Italy is a relatively young field of research: in this country, measurements of ambient ozone are mainly made using instruments operating on the principle of UV absorption. Some 50 analyzers were regularly operating about ten years ago:4 unfortunately, their spatial distribution was irregular and large geographical areas—especially in the south—were not covered. For instance, in Tuscany (central Italy, 22 997 km2, about 3.5 × 106 inhabitants, 287 municipalities), ozone analyzers operate routinely in only 13 municipalities, covering merely 6% of the total surface area, 33% of the inhabitants and 4% of the municipalities. Moreover, only a handful of these sites have long-term records. Information is scattered and no nationwide database has been set up so far.
Evidence of ozone-induced visible phytotoxic markings was first reported in Tuscany in the early 1980s,5 but the proof of detrimental effects of regional air pollution on plant productivity under field conditions was only obtained several years later. The establishment of air filtration experiments in northern Italy, based on the open-top chamber exposure technique,6,7 showed the relevant beneficial effect of filtering ambient air on productive performances of several crops. In bean plants8 the appearance of typical interveinal brown stipple associated with mesophyll histopathological alterations—which were reproduced by fumigating healthy plants with realistic doses of ozone—was positively attributed to ozone toxicity. Specific symptoms were observed on the leaves of peach trees grown in open-top chambers at a rural site in central Italy.9,10 reported ozone-induced visible injury in Italy on several species, such as bean, courgette, grapevine, peach, pepper, radish, soybean, spinach and tomato. Symptoms attributed to ozone have also been reported for natural vegetation: black locust, heaven tree and poplar,11 as well as for conifers growing in urban areas.12
Comparison of the observed ozone concentrations with United States’ crop damage criteria suggests that in summers the quantitative yield of many crops will be reduced in a significant way. Potential ozone damage in connection with crop susceptibility has been evaluated for the Province of Milan via a Geographical Information System (GIS) approach.13
Ozone exposure at rural and forest sites in Italy exceeds by far concentration-based critical levels.14 The computation of the AOT40 (accumulated exposure over a threshold of 40 ppb) values for the area of Pisa (Tuscany)15 (Fig. 1) has systematically indicated values up to 20 ppm h (for three months, June–August, daylight hours): these figures are well above the long-term critical level for agricultural crops (3 ppm h for three months).16 Similar results have been reported from other Italian regions, such as Campania.17 A common trait is that daily patterns of ozone concentrations in hilly areas are different from those of sites located in plains, the differences between daily maximum and minimum being quite low at the hilly sites.
 | ||
| Fig. 1 Cumulate AOT40s of ozone during the photochemical season 2004 in the Tuscan sites of Lari (rural, 130 m above sea level), Montecerboli (remote, 370 m a.s.l.) and Pisa (urban, 5 m a.s.l.); the bold lines indicate the thresholds for critical levels of 3 ppm h (for three consecutive months, the target to be protected being quantitative yield of agricultural crops) and 10 ppm h (for six consecutive months, the target to be protected being biomass reduction of forest trees).16 | ||
The estimated yield losses for soybean due to ozone pollution in some Tuscan districts ranged from 17 to 27% of the potential in ozone-free air.18
An assessment of the biological implications of air pollution should improve global knowledge of environmental contamination also in economic terms and contribute to urge authorities and decision-makers to put into action proper measures to improve the quality of our atmosphere. It is interesting to note that the first perception of an air pollution problem in the Los Angeles area emerged from the observation of ‘unusual’ plant injury in the late 1940s.19 Indicator plants can be successfully used to detect the presence of phytotoxic ambient levels of ozone over large territories, integrating the data from analyzers.
The rationale of biological monitoring
Biological monitoring may be defined as the measurement of the response of living organisms to changes in their environment. As a rule, plants are more sensitive (in terms of physiological reaction) to the most prevalent air pollutants (such as ozone) than humans and animals. This methodology may be performed through observations and analyses of the native and cultivated vegetation present in a given study area (so-called ‘passive monitoring’) or carried out with selected test plants of standard genetic origin and developmental state, which are exposed to ambient air under standardized conditions (‘active monitoring’). Biomonitoring may allow a detailed and reliable coverage of the territory with relatively low costs, it does not require an electrical supply and its educational and didactical values are incomparable. These techniques provide information on the response of living organisms to the integrated effects of environment and contamination which cannot be determined by direct analytical measurements. An ideal indicator plant must satisfy some key requirements, such as: (i) to be very sensitive to a specific pollutant and then to have a low threshold for visible injury (the reaction must occur quickly when exposed to realistic pollutant concentrations); (ii) the visible response should be precise, characteristic, possibly specific and definitively reproducible; (iii) the intensity of visible response must be easily quantified and an appropriated calibration procedure against the actual pollutant doses must be possible; this allows the extrapolation of pollution levels from biological data; (iv) the indicator should have an adequate period of vegetation and be easily cultivable, well adapted to the environment and tolerant to the major stress factors, both of biotic (pests, parasites) and abiotic (thermal extremes, water deficit, other chemical pollutants) nature.Suitable bioindicators may be useful in involving ordinary people in environmental diagnosis. One of the key aspects of sustainable development is the active participation of citizens in environmental issues, such as air quality monitoring.20 However, the final goal of biomonitoring is not to replace the conventional physico-chemical approach: an integration of the two systems is the most appropriate solution for air quality assessment.
Ozone bioindication
Ozone biomonitoring is a detection and monitoring technique that involves documenting ozone-induced visible injury to known ozone-sensitive species under conditions of ambient exposure. Since 1962, ozone-hypersensitive tobacco (Nicotiana tabacum L.) Bel-W3 has been used worldwide as a reliable and sensitive bioindicator of ozone.21 It shows a characteristic and specific foliar response. The typical foliar lesions induced by ozone under realistic exposure to ambient air are bi-facial greyish necrotic spots, scattered over the lamina. The tobacco system has been extensively investigated, also from the quantitative point of view. Biomonitoring campaigns have been successfully performed all over the world. Ozone-resistant tobacco plants (cv. Bel-B) are routinely inserted in the plots; their sensitivity threshold, in terms of visible injury, for 2 h exposures is 220 ppb vs. 100 ppb of Bel-W3.21 So the appearance of injury on Bel-W3 but not on Bel-B provides further confirmation that such injury is due to ozone.‘Conventional’ biomonitoring is performed with adult tobacco plants (about 2 months old, 60 cm in height). Important operational constraints are to be stressed: (i) a large space (and a long time) in filtered-air, controlled-environment growing facilities is required; (ii) due to leaf fragility, long-distance transport of plants is difficult; (iii) visual assessment of leaf injury should be performed by remote operators, all of whom must be adequately selected and skilled; (iv) indicator plants stay in a site for many (usually 4) weeks, and their sensitivity may change over time (as a rule, during the first days of exposure in the open-air the plants are overresponsive).22 A preliminary attempt to standardise the procedure of tobacco exposure has been carried out in Germany.23
The Italian experience
The pioneer biomonitoring experiences started in Tuscany in 1983.24 Since then many studies have been carried out in Tuscany, Liguria, Umbria, several areas of the Po Valley and Rome, and also in the framework of EU pilot projects (see ref. 25 for a preliminary review). All of these experiences have adopted the methodologies developed by Dutch scientists who first established a network of indicator plant sites throughout their country (see ref. 26) and which were formalized.27In all these surveys, hypersensitive tobacco Bel-W3 plants always showed visible injury, demonstrating that the threshold concentration of ozone required for the appearance of visible symptoms (40–50 ppb for exposures of about 5 h, or 30 ppb for 8 h21) had been largely exceeded. Weekly variation in the amount of leaf injury was constantly reported and generally was associated with meteoclimatic parameters. A linear multiple correlation model has shown that leaf damage on indicator plants in Milan was influenced positively by temperature and negatively by vapour pressure deficit.28 A campaign involving many stations29 showed, through a chronological analysis, a high correlation between all the sites in the Po Valley, even for those hundreds of kilometres distant from each other. This can be explained by the existence of synchronous patterns of ozone pollution on a wide geographical scale.
Integrated ambient air studies have been conducted in the city of Florence.30,31 The results obtained with biomonitoring were in agreement with those offered by instrumental monitoring; as expected, the city centre has the lowest leaf injury values. This pattern is consistent with what it is expected for ozone in large urban areas, as ozone depletion by nitrogen oxide is higher in the most polluted environments.32 In the same way, the amount of visible injury on the indicator plants was negatively correlated with the presence of lead in/on the leaf tissues of ryegrass (accumulator plant for heavy metals). The reason for this is that most ambient lead came at that time from vehicle exhausts, which also contain a mass of other pollutants, which easily react with ozone molecules and deplete them.
Some years ago, an innovative procedure for ozone biomonitoring was developed and patented at the University of Pisa.5 Tobacco seeds are germinated in an ozone-free environment and when 15 days old (the first leaf 1 cm long), the germlings are singly transplanted in commercial polystyrene tissue culture plates (13 × 9 cm) with 24 round wells (16 mm diameter, 20 mm depth), filled with an organic compost (Fig. 2). The plates are modified to allow the watering of the wells. After a further day in ozone-free air for acclimatization, the plates are exposed to ambient air. A typical configuration is with 16 seedlings of the hypersensitive Bel-W3 tobacco and 7 of the ozone-resistant Bel-B. The 24th well is connected to the mouth of an inverted feeding bottle containing deionized water, to provide continuous sub-irrigation of the seedlings. At least 3 plates are routinely positioned in each monitoring site under a metal cage covered with a fine shading net. Shading is a normal practice in biomonitoring with tobacco, as the cultivars utilized are shade-grown. These plates are exposed to ambient air for 7 days. The intensity of the injury of cotyledons and the first leaf is visually assessed and related to the dose of ozone to which the seedlings have been exposed. The necrotic area percentage was estimated by comparison with standards and reported in terms of Cotyledonar (CII) and Leaf Injury Index (LII), based on a 1 to 5 scale (1: no lesion; 5: more than 50% of surface covered by necrosis). Concerning the pathometric scale, every plant pathologist is aware of the crucial role of the Horsfall–Barratt (H–B) rating scale for measuring rust and other fungal diseases, but in our opinion this is not the right tool to measure the extent of foliar injury induced by ozone. This is due to the fact that the H–B scale punctually covers all the span from 0 to 100% damaged surface; since long we know that ozone induced lesions may spontaneously coalesce causing the collapse of large regions of mesophyll, even several hours after the end of the exposure. This implies the inopportunity of rating large necrotic surfaces, so it is common in the field of ozone monitoring with tobacco to ignore injury above about 40% of the whole surface (see ref. 27 for a detailed analysis of the matter).
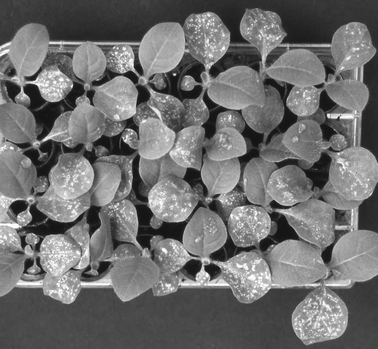 | ||
| Fig. 2 A kit of tobacco Bel-W3 germlings after seven days of exposure to ambient air. | ||
The advantages of ozone biomonitoring with this miniaturized kit may be summarized in the following way: (i) only a very limited space is required in the growing facility; (ii) a large number of individuals may be exposed; (iii) the biomonitoring units may be easily dispatched to long distances and returned to the main laboratory for visual assessment of injury; this ensures a standardization of the reading; (iv) the permanence of germlings for a standard time (e.g. 24 h) in an ozone-free environment allows the full maturation of symptoms due to the last-day exposure.
This innovative methodology is reliable and allows an inexpensive survey of the geographical distribution of tropospheric ozone over large areas, including remote sites, where conventional monitoring would be difficult. A large scale campaign with the use of the miniaturized kit has been performed in seven districts of Tuscany.33 Another trial demonstrated the presence of phytotoxic ozone in the small remote islands of Capraia and Gorgona (Tyrrhenian Sea), where local sources of precursors are negligible.34 Assessments with this kit were also carried out in France and the UK.35,36
Epiphytic lichen biodiversity distribution has been widely used as a bioindicator of air pollution throughout the world, mainly due to their high sensitivity to sulfur dioxide.37 Comparative evaluations of ozone hypersensitive tobacco responses and lichen biodiversity in several locations indicated that the two variables investigated did not show any degree of association.38
As visual estimates of foliar injury involve subjectivity, there are limitations to the tobacco systems. A methodology based on non-subjective measures, rather than estimated responses to ozone, and not significantly influenced by other factors would clearly be an addition to, and an improvement over, present systems. Ozone-sensitive (NC-S) and ozone-resistant (NC-R) clones of white clover (Trifolium repens L.) cv. Regal have been tested in the USA to determine this feasibility to indicate concentrations of ozone and its potential effects on plants.39 The clones display measurable differences in growth response to ozone while responding similarly to the other factors that influence plant growth. The growth of NC-S and NC-R is similar when ozone concentration is low, whereas the NC-S grows much less than NC-R when ozone concentration is high (Fig. 3). A comparison of this method with the classic exposure in open-top chambers (filtered vs. unfiltered air) conducted by an Italian group confirmed its suitability.40 The utility of this method has been acknowledged by workers who carry out research on effects of air pollution on crops and non-woody plants within the framework of a UN/ECE International Cooperative Programme (ICP-Vegetation, formerly ICP-Crops41) for the Convention on Long-Range Transboundary Air Pollution. Within this programme,42 clover experiments are performed in several Italian locations. Data so far available in Italy show no clear relationship between actual AOT40 and yield losses of white clover: in experiments in Milan, Naples and Rome (1996–2001), these were not significantly correlated with their respective AOT40 (n = 22; R2 = 0.08), whereas they did correlate in a survey carried out in Pisa in 1999–2000.15 The same survey, using white clover NC-S/NC-R epigeous biomass ratio as response indicator showed that AOT60 and AOT80 yield higher R2 than AOT10, AOT20, AOT30 and AOT40 (Table 1).15 Our determination coefficients were much higher than those reported for the European and Mediterranean scenarios,43–45 and slightly lower than those reported in the USA.46Table 2 reports the statistical analysis performed with the combined O3 (AOT40 accumulated over three months) and biomass data, omitting the data of the last two harvests, to remove any late season climatic effects on plant biology. The extent of biomass reduction was closely related to the O3 dose received over the previous three months (June, July and August). This is in accordance with ref. 47 whose authors hypothesized a residual carry-over effect in the biomass responses of NC-S/NC-R between subsequent harvests in a given growth season. Fig. 4 describes the typical response of the NC-S/NC-R biomass ratio. It should be stressed that this experimental approach can help to assess the economic losses caused by the effects of ozone on crops. A modified approach, based on the use of clover ‘mini-stations’ has been successfully developed by Roman scientists.48 AOT40 descriptor were also positively correlated with the visible injury of hypersensitive tobacco cv. Bel-W3 (n = 8; R2 = 0.75).38
 | ||
| Fig. 3 Biomass production of white clover (Trifolium repens) cv. Regal NC-S clone (left) compared to that of NC-R clone (right) in a monitoring campaign concerning tropospheric ozone conducted in Pisa. | ||
 | ||
| Fig. 4 Response of the NC-S/NC-R biomass ratio of white clover to cumulated AOT40 over 28 monthly harvests between 1997 and 2004 in Pisa; broken lines represent the confidence interval (P = 0.05) (y = 0.928–2.830 e−5x; n = 28; R2 = 0.66). | ||
| Year | Regressors | Equation | R 2 |
|---|---|---|---|
| 1999 | AOT10 vs. NC-S/NC-R | Y = −9.08 X + 1.06 | 0.41 |
| AOT20 vs. NC-S/NC-R | Y = −0.02 X + 1.10 | 0.45 | |
| AOT30 vs. NC-S/NC-R | Y = −0.03 X + 1.14 | 0.50 | |
| AOT40 vs. NC-S/NC-R | Y = −0.06 X + 1.20 | 0.55 | |
| AOT60 vs. NC-S/NC-R | Y = −20.64 X + 61.86 | 0.64 | |
| AOT80 vs. NC-S/NC-R | Y = −448.68 X + 63.11 | 0.49 | |
| 2000 | AOT0 vs. NC-S/NC-R | Y = −1.69 X + 0.71 | 0.55 |
| AOT10 vs. NC-S/NC-R | Y = −2.30 X + 0.72 | 0.57 | |
| AOT20 vs. NC-S/NC-R | Y = −3.33 X + 0.72 | 0.59 | |
| AOT30 vs. NC-S/NC-R | Y = −5.06 X + 0.72 | 0.62 | |
| AOT40 vs. NC-S/NC-R | Y = −8.33 X + 0.72 | 0.65 | |
| AOT60 vs. NC-S/NC-R | Y = −0.08 X + 0.73 | 0.68 | |
| AOT80 vs. NC-S/NC-R | Y = −1.26 X + 0.71 | 0.65 |
| Year | Regressors | Equation | R 2 |
|---|---|---|---|
| 1999 | AOT40 vs. NC-S/NC-R (F.W.) | Y = −9.78 * 10−5X + 1.35 | 0.95 |
| AOT40 vs. NC-S/NC-R (D.W.) | Y = – 9.78 * 10−5X + 1.32 | 0.95 | |
| 2000 | AOT40 vs. NC-S/NC-R (F.W.) | Y = −3.74 * 10−5X + 1.01 | 0.88 |
| AOT40 vs. NC-S/NC-R (D.W.) | Y = −2.69 * 10−5X + 0.85 | 0.84 |
Extrapolation of responses of these sentinel plants to natural and semi-natural vegetation is difficult because of differences in genetic structure, community composition, and especially environmental adaptations.49 Native (detector) plants, growing in situ, are preferred for vegetation surveys. These established detector bioindicators visibly respond to O3 only when sufficient soil moisture and climatic conditions allow uptake of enough O3 for a sufficient time to allow inactivation of antioxidant defence mechanisms.50 As an alternative, brown knapweed (Centaurea jacea L.) has been suggested as a potential native bioindicator agent because this species shows characteristic symptoms of O3 injury in the form of bronze stipples or leaf discoloration after only a few days of field exposure to elevated O3.51 Symptoms were found to be identical to those observed in open-top chamber experiments.72 Similarly, Manning et al.50 proposed the closely related Centaurea nigra L. as a bioindicator species in central European forests. Due to its broad climatic adaptation, C. jacea is indigenous in many European countries. Recently, the above mentioned European project introduced a common protocol involving C. jacea plants as a model species to evaluate the relationship between ozone exposure and effects on the performance and injury symptoms of native plant species.42 As expected, a strong intraspecific variation in ozone response has been detected in this sensitive wild species:52 the extent of visible injury under identical environmental conditions varies among populations from different regions with different climates and pollution histories, and differences in plant ontogeny.
Searching for an alternative O3 bioindicator, another Solanacea has been tested, Lycopersicon pimpinellifolium, also known as the currant tomato. This is a wild plant (0.5–1 m height) native to western South America, that can be easily cultivated in any kind of drained soil, and only requires a sunny position.53 Though its sensitivity to O3 was described by Gentile et al.,54 no other reports have been published since then on the level and traits of this sensitivity.74 Besides confirming that L. pimpinellifolium is a highly O3-sensitive species, it is suggest that it can be positively evaluated as an effective bioindicator of the pollutant.
How realistic is the response of indicator plants?
In southern Europe, O3 uptake can be limited because several environmental factors can reduce stomatal conductance (gsto75). They include summer water deficit, salinification of water tables and soils, high air temperature, vapour pressure deficit (VPD) and high O3 level itself. Summer water deficit is probably the most widespread. Recent data (1999–2004) from rural and forest sites located throughout Italy show summer water deficits (rainfalls minus ETP) ranging from −243 mm (63% of ETP, mountain site in southern Italy) to −411 mm (87% of ETP, Rome). Only alpine sites show water surplus or little deficit during the summer period. Irrigation can only mitigate the effects of water limitations on gsto, as gsto can be reduced by 50% in the period between two consecutive irrigations. Water stress reducing the gsto at the time O3 levels are high can reduce O3 uptake and yield losses. OTC experiments with white clover demonstrated no significant relationship between O3 uptake and yield loss under water stress (n = 4; R2 = 0.079), while a significant reduction (n = 4; R2 = 0.99) occurred when water limitation was removed.It is known that the Mediterranean vegetation may tolerate potentially harmful O3 levels.73 The prevailing weather conditions in the Mediterranean area (excess light, elevated temperature, reduced precipitation) reduce gsto at the time of the highest O3 levels, and promote sclerophylly, VOC emission and antioxidant activity. These avoidance and detoxification mechanisms may be of greater importance for Mediterranean vegetation than for that from central and northern Europe.
Data quality
Uncertainty is inherent in most biological data—and particularly in the field of environmental biology—much more than in the physical and chemical disciplines. Most biological phenomena are characterized by a high degree of variability depending on several factors, such as variability of the subject under study, range of the data spread, measurement errors and sampling intensity.55 In spite of this, there are relatively few studies devoted to the analysis of factors influencing the variability of data related to biological indicators.Quality assurance (QA) is still a relatively new topic in biomonitoring. QA is ‘an organized group of activities defining the way in which tasks are to be performed to ensure an expressed level of quality’. This means that all steps of the programme should be correctly addressed, from the design of the survey to data collection, processing and reporting. Four main activities are considered in a QA program: Quality Management (QM), whose major task is to ensure that activities are performed in a proper way; Quality Assurance (QA), whose task is to provide consistent methods with verified data quality; Quality Control (QC), whose major task is to ensure that data are appropriately collected and quality assurance is carried out; Quality Evaluation, which allows precision and accuracy of determinations to be evaluated, providing a basis to evaluate the comparability of data. A brilliant example of management of data quality in the environmental field is given by American and European forest health monitoring programs.56,57
QA procedures were adopted to ensure proper design and implementation of the study conducted in Florence.30 Results obtained before the beginning of the campaign were reassuring and, in terms of data quality limits, 90% of the scores of injured tobacco Bel-W3 leaves examined fell into the exact class or in the adjacent ones.
One of the advantages in biomonitoring is in measuring rapidly the magnitude of the injury. The impracticability of counting and measuring individual lesions on indicator plants motivated research workers to rely on rapid visual methods with the help of iconographic material and pathometric scales. The observer is a significant source of measurement error. Large scale campaigns, which involve several observers, are exposed to significant risks of estimation errors. Because disease scoring apparently follows the Weber–Fechner law, which states that the response of an organism to a stimulus is a linear function of the logarithm of the stimulus, graded readings are often converted to percentages on a logarithmic curve.58 Psychological studies provide evidence that the eye often grades inaccurately.59 Shape, orientation, shading, surrounding figures and personal traits enter into perception.
Visual assessments must be made quickly and do not require expensive equipment, chemical analysis or highly trained personnel, but their subjective nature creates concern. For ozone-induced injury, various attempts at improving the reading procedures have been reported.60 Mortensen described an integrated unit which illuminates the leaves from behind and allows the taking of standardized photos which are analyzed by a computer.60 Della Mea et al.61 presented a method of evaluation by computerized image analysis. However, the applicability of these techniques for large-scale surveys is doubtful due to operational limitations.
A specific study has been performed in Pisa.62 Fifty volunteers were selected to evaluate the accuracy of the visual method for assessing lesions induced by ozone on Bel-W3. Results indicate an easy estimation of the highest and lowest classes and a general difficulty in evaluating intermediate ones.
Biomonitoring and biological education
Biomonitoring of ozone is a powerful tool for understanding the link between the causes and effects of environmental pollutants; moreover, pupils act as multipliers for information about conservation and environment-friendly behaviour. Pilot studies involving several schools in mapping ozone distribution by means of indicator plants have been carried out in Italy,63 and represented true complete exercises in problem-solving. So, under the guidance of their teachers, thousands of students (aged from 6 to 18) had several opportunities to practise basic and applied study techniques in a multidisciplinary way, such as biology, chemistry, ecology, geography, computer graphics, statistics and so on. Unfortunately, in Italy the ordinary school calendar ends at the beginning of June, when the photochemical season is not at its peak, so the data set collected does not represent the worst possible environmental scenario.Concluding remarks
Tropospheric ozone is recognized to be a priority issue in Europe64 and its concentration occurring in Italy during the warm season systematically exceeds the long- and short-term critical levels defined for trees, crops and natural vegetation.65 Consequences for organisms must be considered a priority and biomonitoring of air pollutants is a crucial tool for understanding critical environmental issues. A widespread coverage of the territory is required and is only possible with the help of bioindicators, even if the logistical involvement in establishing a large number of biological monitoring stations is considerable. However, there is no question of replacing present ambient-air monitoring by physico-chemical methods with monitoring of effects on plants: both should be used jointly with an integrated approach.The visible foliar response of tobacco Bel-W3 can be used at least as a qualitative indicator of ambient ozone pollution.66,67 One of the reasons for the difficulty in relating ozone injury on adult plants to concentrations may be that prolonged exposures to ambient oxidants modify the response to subsequent exposures, when the same plants are used for several weeks; in the same way, the behaviour of plants immediately after being transported to the sites may be influenced by the radical change in environmental conditions. The adoption of methodologies based on short exposure times (i.e. 7 days, as in the case of the miniaturized kit) proved to be very useful. The effectiveness of the traditional biomonitoring method, based on the use of adult plants, was compared with the innovative miniaturized kit of seedlings: correlation studies among cotyledonar and foliar injury index and ozone levels monitored through physico-chemical measurements and results of analysis of variance emphasized the high degree of sensitivity of the innovative methodology.22
The mass media are very interested in spreading information on biomonitoring. Knowledge provided by indicator plants has incomparable value from the didactic and educational points of view: the vision of the severe macroscopic injury which ambient air causes to sensitive organisms may stimulate in the citizen a deeper involvement in environmental issues. Several pilot study projects have been launched with selected groups of teachers.
A standardization of methodologies, even at an international level (e.g. EU) is strongly felt to be needed, in order to get official acknowledgement of biomonitoring procedures. A first attempt of the Italian Environmental Agency (ANPA, Rome) to describe fundamental standard procedures of bioindication of ozone (from seed acquisition to plant raising, to personnel training and data analysis) has been successfully performed.68 International co-operation could allow the setting up of an extensive and inexpensive network for monitoring biological effects of air pollutants over large areas. As an ozone climatology for Europe and the Mediterranean basin is needed, a coordinated effort is desirable in order to integrate physico-chemical and biological data concerning the spatio-temporal distribution of this pollutant.
In Italy a fundamental reorganization of the national and regional health and environmental services has been launched: this could be a good opportunity for organizing large scale integrated surveys, where conventional monitoring facilities are coupled to biomonitoring units. A nation-wide monitoring network which gives complete coverage of the country should be organized, by implementing the present availability of analyzers and integrating it with biomonitors. A task-force should be put in charge of supervising all the aspects of the problem, including meteorological and biological ones, following the example first offered by the United Kingdom Photochemical Oxidants Review Group69 and then by the National Expert Group on Transboundary Air Pollution.70 It is important to continue discussion concerning the future use of tobacco, both as an ‘ozone monitor’ and as an indicator of ozone stress on other types of vegetation. The use of a biomonitor less sensitive than Bel-W3 tobacco could be envisaged, due to the very extreme response that this indicator systematically gives under the Italian pollution situation.
A glance to the future: selected perennial plants could be individualized to form permanent plots. The differential response to ozone is characterized in two poplar clones, indicating that the differences in phenomenological response are the effect of biochemical and physiological differences.71 In addition, biosensors and genetic engineering open up new application fields.
Data quality assessment should be a rule in biomonitoring activities. The quality of biological measurements, as with physico-chemical methods, depends on the reproducibility of the results. The degree of error intrinsic in the variability of all biological data is compensated for by the density of sampling points; this permits the construction of reliable pollution maps of large areas that could not have been obtained by instrumental recording due the scarcity of sampling points. This is particularly true in the case of the miniaturized kits, which allow an adequate sampling within the study site.
Biomonitoring, however, must overcome the present limitations, which include: a poor understanding of its validity, importance and versatility by the decision-makers, which may also be related to the poor ‘commercial’ inclination of the biomonitoring advocates; enthusiasm from the local environmental protection agencies, which—as a rule—are managed by chemists, who approach bioindication with scepticism; the lack of true standardization and formalization of the bioindication methodologies, as well as of a sound procedure for selection, instruction and evaluation of the field operators. The use of simple techniques, such as the miniaturized kit developed in Italy, should be encouraged. Basic research should be intensified, field courses and seminars organized: biomonitoring should be more ‘visible’!
Acknowledgements
This work was performed as part of the PRIN/MIUR activities.References
- A. Ribas and J. Peñuelas, Atmos. Environ., 2004, 38, 985 CrossRef CAS.
- J. E. Jonson, J. K. Sundet and L. Tarrasòn, Atmos. Environ., 2001, 35, 525 CrossRef CAS.
- J. L. Levy, S. M. Chemerynski and J. A. Sarnat, Epidemiology, 2005, 16, 458 CrossRef.
- G. Lorenzini, Medit, 1993, 4(2), 53 Search PubMed.
- G. Lorenzini, Appl. Biochem. Biotechnol., 1994, 48, 1 Search PubMed.
- G. Schenone and G. Lorenzini, Agric., Ecosyst. Environ., 1992, 38, 51 CrossRef CAS.
- G. Schenone, G. Botteschi, I. Fumagalli and F. Montinaro, New Phytol., 1992, 122, 689 CAS.
- A. Panattoni, G. Lorenzini and G. Schenone, Inf. Fitopatol., 1990, 40(12), 43 Search PubMed.
- A. R. Paolacci, M. Badiani, A. D’Annibale, C. Bignami, I. Fumagalli, A. Fusari, G. Lorenzini, G. Matteucci, L. Mignanego, P. Rossini, G. Schenone and G. Giovannozzi-Sermanni, Responses of plants to air pollution: biological and economic aspects, in The effects of realistic ozone exposure on the biology and productivity of peach trees and durum wheat grown in open-top chambers in central Italy, ed. G. Lorenzini and G. F. Soldatini, Pacini, Pisa, 1995, pp. 125–139 Search PubMed.
- I. Fumagalli, B. S. Gimeno, D. Velissariou, L. De Temmerman and G. Mills, Atmos. Environ., 2001, 35, 2583 CrossRef CAS.
- G. Lorenzini and C. Nali, Le piante e l’inquinamento dell’aria, Springer, Milan, 3rd edn, 2005 Search PubMed.
- C. Soda, F. Bussotti, P. Grossoni, J. Barnes, B. Mori and C. Tani, Environ. Exp. Bot., 2000, 44, 69 CrossRef CAS.
- L. Sormani, F. Sangiorgi and A. Bertolo, Responses of plants to air pollution: biological and economic aspects, in Ozone potential damage and agriculture susceptibility in Milan’s southern belt, ed. G. Lorenzini and G. F. Soldatini, Pacini, Pisa, 1995, pp. 338–345 Search PubMed.
- M. Ferretti, M. Fagnano, T. Amoriello, A. Ballarin Denti, M. Badiani, A. Buffoni, F. Bussotti, A. Castagna, S. Cieslik, A. Costantini, A. De Marco, G. Gerosa, G. Lorenzini, F. Manes, G. Merla, R. Mosello, C. Nali, E. Paoletti, B. Petriccione, S. Racalbuto, G. Rana, A. Ranieri, A. Tagliaferri, G. Vialetto and M. Vitale, Abstract for the Workshop on Critical Levels for Ozone, Obergurgl, Tyrol, Austria, 15–19 November 2005, pp. 37–42 Search PubMed.
- C. Nali, L. Crocicchi and G. Lorenzini, J. Environ. Monit., 2004, 6, 636 RSC.
- L. Kärenlämpi and L. Skärby, Critical levels for ozone in Europe: testing and finalizing the concepts, UN/ECE Workshop report, University of Kuopio, Kuopio, 1996 Search PubMed.
- A. Forlani, G. Merola and M. Fagnano, Fresenius Environ. Bull., 2005, 14, 478 CAS.
- C. Nali, C. Pucciariello and G. Lorenzini, Water, Air, Soil Pollut., 2002, 141, 337 CrossRef CAS.
- A. J. Haagen-Smit, E. F. Darley, M. Zaitlin, H. Hull and W. Noble, Pl. Physiol., 1952, 27, 18 Search PubMed.
- Chart of European cities for a sustainable development, 1994, http://www.iclei.org/europe/ac-eng.htm.
- H. E. Heggestad, Environ. Pollut., 1991, 74, 264 CrossRef CAS.
- M. L. Toncelli and G. Lorenzini, Environ. Monitor. Assess., 1999, 55, 445 Search PubMed.
- VDI, Biological measuring techniques for the determination and evaluation of the effects of air pollutants on plants (bioindication). Determination and evaluation of the phytotoxic effect of photooxidants. Method of the standardised tobacco exposure. VDI 3957, Part 6, 2003.
- G. Lorenzini, E. Triolo and A. Materazzi, Riv. Ortoflorofruttic. Ital., 1984, 68, 81 Search PubMed.
- C. Nali, Agr. Med., 1999, 129, 1 Search PubMed.
- A. C. Posthumus, Effects of gaseous air pollution in agriculture and horticulture, in Biological indicators of air pollution, ed. M. H. Unsworth and D. P. Ormrod, Butterworths, London, 1982, pp. 27–42 Search PubMed.
- M. R. Ashmore, J. N. B. Bell and C. L. Reily, Environ. Pollut., Ser. B, 1980, 1, 195 Search PubMed.
- F. Biondi, L. Mignanego and G. Schenone, Environ. Monit. Assess., 1992, 22, 73 CrossRef CAS.
- L. Mignanego, F. Biondi F. and G. Schenone, Environ. Monit. Assess., 1992, 21, 141 CrossRef CAS.
- F. Bussotti, P. Grossoni, C. Soda, M. Ferretti and G. Lorenzini, Acta Hortic., 1999, 496, 429 Search PubMed.
- C. Nali, M. Ferretti, M. Pellegrini and G. Lorenzini, Environ. Monit. Assess., 2001, 69, 159 CrossRef CAS.
- J. O. Nriagu, Gaseous pollutants: characterization and cycling, Wiley & Sons, New York, 1992 Search PubMed.
- G. Lorenzini, C. Nali and M. Biagioni, Environ. Monit. Assess., 1995, 34, 59 CAS.
- G. Lorenzini, M. Biagioni and C. Nali, Sci. Total Environ., 1995, 166, 193 CrossRef CAS.
- J. P. Garrec and M. Livertoux, Pollut. Atmos., 1997, 19, 78 Search PubMed.
- L. Davies, J. N. B. Bell, M. R. Ashmore and J. W. Bates, Biological monitoring in the Transmanche region, IC Consultants Ltd, Imperial College, London, 1998 Search PubMed.
- D. H. S. Richardson, Pollution Monitoring with Lichens, Richmond Publ, Co., Slough, 1993 Search PubMed.
- G. Lorenzini, U. Landi, S. Loppi and C. Nali, Environ. Monit. Assess., 2003, 82, 243 CrossRef CAS.
- A. S. Heagle, J. E. Miller and D. E. Sherril, J. Environ. Qual., 1994, 23, 613 CAS; http://www.ncl.ac.uk/airweb/ozone/clover0.htm .
- M. Fagnano, G. Merola, A. Forlani, L. Postiglione and J. Fuhrer, Water, Air, Soil Pollut., 2004, 155, 383 CrossRef CAS.
- J. Benton, J. Fuhrer, B. S. Gimeno, L. Skärby, D. Palmer-Brown, G. Ball, C. Roadknight and G. Mills, Agric., Ecosyst. Environ., 2000, 78, 19 CrossRef CAS.
- H. Harmens, G. Mills, F. Hayes and P. Williams, Air Pollution and Vegetation, UNECE-ICP Vegetation Annual Report, Centre for Ecology and Hydrology, Bangor, 2004 Search PubMed.
- G. R. Ball, D. Palmer-Brown, J. Fuhrer, L. Skärby, B. S. Gimeno and G. Mills, Ecol. Modell., 2000, 129, 153 CrossRef CAS.
- G. Mills, G. R. Ball, F. Hayes, J. Fuhrer, L. Skärby, B. S. Gimeno, L. De Temmerman and A. S. Heagle, Environ. Pollut., 2000, 109, 533 CrossRef CAS.
- V. Bermejo, B. S. Gimeno, I. Granados, J. Santamaría, J. J. Irigoyen, R. Bermelo, J. L. Porcina and G. Mills, New Phytol., 2002, 156, 43 Search PubMed.
- A. S. Heagle and L. A. Stefanski, Atmos. Environ., 2000, 34, 735 CrossRef CAS.
- B. Chevone, W. Manning, A. Varbanov and S. Krupa, Environ. Pollut., 1998, 103, 103 CrossRef CAS.
- F. Manes, F. De Santis, M. A. Giannini, C. Vazzana, F. Capogna and I. Allegrini, Sci. Total Environ., 2003, 308, 133 CrossRef CAS.
- J. Fuhrer, M. R. Ashmore, G. Mills, F. Hayes and A. W. Davison, in Establishing ozone critical levels II, ed. P. E. Karlsson, G. Selldén and H. Pleijel, Swedish Environmental Research Insitute, Gothenburg, 2003, p. 138 Search PubMed.
- W. J. Manning, B. Godzik and R. Musselman, Environ. Pollut., 2002, 119, 283 CrossRef CAS.
- P. Bungener, S. Bassin and J. Fuhrer, in Establishing ozone critical levels II, ed. P. E. Karlsson, G. Selldén and H. Pleijel, Swedish Environmental Research Institute, Gothenburg, 2003, p. 204 Search PubMed.
- S. Bassin, R. Kölliker, C. Cretton, M. Bertossa, F. Widmer, P. Bungener and J. Fuhrer, Environ. Pollut., 2004, 131, 1 CrossRef CAS.
- L. Brako and J. L. Zarucchi, Catalogue of the flowering plants and gymnosperms of Peru, Monograph systematic botany of Missouri Gard 45, Missouri Botanical Garden, St. Louis, MO, 1993 Search PubMed.
- A. G. Gentile, W. A. Feder, R. E. Young and Z. Santner, J. Am. Soc. Hort. Sci., 1971, 96, 94 Search PubMed.
- P. L. Nimis, G. Bot. Ital., 1991, 125, 126 Search PubMed.
- S. P. Cline and W. G. Burkman, The Role Of Quality Assurance In Ecological Programs, in Air Pollution and Forest Decline, Proc. 14th Int. Meeting for Specialists in Air Pollution Effects on Forest Ecosystems, Interlaken, Switzerland, 2–8 October 1988, ed. J. B. Bucher and I. Bucher-Wallin, 1989, p. 361–365 Search PubMed.
- F. Bussotti, M. Schaub, A. Cozzi, N. Kräuchi, M. Ferretti, K. Novak and J. M. Skelly, Environ. Pollut., 2003, 125, 81 CrossRef CAS.
- R. T. Sherwood, C. C. Berg, M. R. Hoover and K. E. Zeiders, Phytopathol., 1983, 73, 173 Search PubMed.
- S. Coren and J. S. Girgus, Seeing is deceiving: the psychology of visual illusions, Wiley, New York, 1978 Search PubMed.
- L. Mortensen, Effects of gaseous air pollution in agriculture and horticulture, in The use of indicator plants for photochemical oxidants in Denmark, ed. M. H. Unsworth and D. P. Ormrod, Butterworths, London, 1982, pp. 466–467 Search PubMed.
- M. Della Mea, G. L. Calzoni and N. Bagni, Fresenius Environ. Bull., 1997, 6, 475 CAS.
- G. Lorenzini, C. Nali, M. R. Dota and F. Martorana, Environ. Monitor. Assess., 2000, 62, 175 Search PubMed.
- G. lorenzini and C. Nali, J. Biol. Educ., 2004, 38, 158 Search PubMed.
- G. Dollard, D. Fowler, R. I. Smith, A.-G. Hjellbrekke, K. Uhse and M. Vallasse, Water, Air, Soil Pollut., 1995, 85, 1949 CrossRef CAS.
- C. Nali, C. Pucciariello and G. Lorenzini, Agric., Ecosyst. Environ., 2002, 90, 277 CrossRef CAS.
- S. V. Krupa, W. J. Manning and M. Nosal, Environ. Pollut., 1993, 81, 137 CrossRef CAS.
- I. Filella, J. Peñuelas and A. Ribas, Environ. Pollut., 2005, 134, 145 CrossRef CAS.
- G. Lorenzini, Piante vascolari come bioindicatori della qualità dell’aria (inquinamento da ozono) proposte normative, in Atti Workshop ‘Biomonitoraggio della qualità dell’aria sul territorio nazionale’, ANPA, Roma, 1999, pp. 201–217 Search PubMed.
- R. G. Derwent, Ozone in the United Kingdom, Department of Environment, London, 1987 Search PubMed.
- M. Coyle and A. Vipond, Transboundary Air Pollution: Acidification, Eutrophication and Ground-Level Ozone in the UK, CEH, Edinburgh, 2001, http://www.nbu.ac.uk/negtap/ Search PubMed.
- G. Lorenzini and C. Nali, Recent Res. Dev. Plant Biol., 2002, 2, 67 Search PubMed.
- P. Bungener, G. R. Balls, S. Nussbaum, M. Geissmann, A. Grub and J. Fuhrer, New Phytol., 1999, 142, 271 Search PubMed.
- C. Nali, E. Paoletti, R. Marabottini, G. Della Rocca, G. Lorenzini, A. R. Paolacci, M. Ciaffi and M. Badiani, Atmos. Environ., 2004, 38, 2247 CrossRef CAS.
- M. Iriti, L. Belli, C. Nali, G. Lorenzini, G. Gerosa and F. Faoro, Environ. Pollut., 2005 DOI:10.1016/j.envpol.2005.08.046.
- UN/ECE, Manual on the methodologies and criteria for modelling and mapping critical loads and levels and air pollution effects, risks and trends. rev. 2004, http://www.icpmapping.org.
Footnotes |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| ‡ The complete national dataset of ozone biomonitoring experience is available at http://www.biomonitoraggio.org. |
| This journal is © The Royal Society of Chemistry 2006 |
