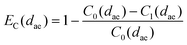Bioaerosol sampling by a personal rotating cup sampler CIP 10-M†
Peter
Görner
*,
Jean-François
Fabriès
,
Philippe
Duquenne
,
Olivier
Witschger
and
Richard
Wrobel
INRS—Institut National de Recherche et de Sécurité, PO Box 27, F-54501, Vandoeuvre-lès-Nancy, France
First published on 19th September 2005
Abstract
High concentrations of bioaerosols containing bacterial, fungal and biotoxinic matter are encountered in many workplaces, e.g. solid waste treatment plants, waste water treatment plants and sewage networks. A personal bioaerosol sampler, the CIP 10-M (M-microbiologic), has been developed to measure worker exposure to airborne biological agents. This sampler is battery operated; it is light and easy to wear and offers full work shift autonomy. It can sample much higher concentrations than biological impactors and limits the mechanical stress on the microorganisms. Biological particles are collected in 2 ml of liquid medium inside a rotating cup fitted with radial vanes to maintain an air flow rate of 10 l min−1 at a rotational speed of approximately 7000 rpm. The rotating cup is made of sterilisable material. The sampled particles follow a helicoidal trajectory as they are pushed to the surface of the liquid by centrifugal force, which creates a thin vertical liquid layer. Sterile water or another collecting liquid can be used. Three particle size selectors allow health-related aerosol fractions to be sampled according to international conventions. The sampled microbiological particles can be easily recovered for counting, incubation or further biochemical analysis, e.g., for airborne endotoxins. Its physical sampling efficiency was laboratory tested and field trials were carried out in industrial waste management conditions. The results indicate satisfactory collection efficiency, whilst experimental application has demonstrated the usefulness of the CIP 10-M personal sampler for individual bioaerosol exposure monitoring.
 Peter Görner Peter Görner | Peter Görner was born in Czechoslovakia in 1950. He studied Chemical Engineering at Technical University of Bratislava and received his PhD in Physical Chemistry from Charles University of Prague. Since then, he has been involved in aerosol research. Previously at the French National Coal Board Research Center in Paris and from 1987 at the National Research Institute on Occupational Safety and Health in Nancy, Laboratory of Aerosol Metrology. His current research interest is aerosol sampling, measurement and analysis in the workplace. |
Introduction
Bioaerosol monitoring is required in some industrial situations to assess the exposure of employees to airborne microorganisms such as bacteria, fungi or microbial toxins, e.g., endotoxin. To measure bioaerosol concentrations, new sampling techniques have recently been developed either from existing dust samplers1 or from original designs.2 When sampling microorganisms, the viability and the reproductive activity of the biological agents should be maintained. They should be easily recoverable from the sampling substrate for culture and for analytical purposes. At least, the interval between the limit of detection and the limit of saturation of both, namely the sampling and analytical methods, should cover the expected bioaerosol concentration.In general, there are three kinds of principles applied to bioaerosol sampling in order to collect and cultivate microorganisms: impaction of the particles onto nutrient agar, particle filtration by membrane filters or particle sampling into a liquid (e.g., bubblers, impingers). The agar impactors are quickly saturated in the case of higher microbiological concentrations. Desiccation of microorganisms can occur during filter sampling. Sampling microorganisms directly into a liquid provides suitable samples for biological analysis, but bubblers often have a low physical sampling efficiency and impingers can stress the microorganisms3,4 (mechanical stress, high pressure drop). Other methods of sampling directly into liquid are available,3,5e.g., wet cyclones or Venturi tubes. There is still a considerable need to develop new bioaerosol sampling methods, mainly for purposes of personal sampling.
Design of the CIP 10 microbiological aerosol sampler
The CIP 10 personal respirable aerosol sampler (ARELCO, France) based on the rotating filter cup was designed by Courbon,6 and is widely used in occupational hygiene for the assessment of individual exposure of workers to respirable aerosols. Particle-size selectors for the thoracic aerosol fraction7,8 and the inhalable aerosol fraction9 were designed to satisfy the sampling requirements for health-related aerosol fractions in accordance with the CEN,10 ACGIH11 and ISO12 sampling criteria. The rotating cup is equipped with a porous polyurethane foam filter to collect sampled particles. Recently, a specific rotating cup was designed and tested in order to sample microbiological agents in the air.13 Even though some authors use the polyurethane foam for bioaerosol sampling,1,14 in our case the foam filter in the rotating cup was replaced by a layer of collecting liquid (2–2.5 ml). To maintain the liquid inside the rotating cup, an upper ring was added to the cup (Fig. 1, 2). To induce an air flow through the device, radial grooves were cut in the upper side of the ring. The rotation of the cup at about 7000 rpm inside its housing maintains the flow rate at 10 l min−1. The airborne microorganisms are aspirated through an air inlet and enter the rotating cup axially (Fig. 2). Then, the particles are driven by centrifugal force toward the liquid collection surface, maintained in vertical position in the cup due to centrifugal force. Particles follow a helicoidal trajectory and they are deposited at a smooth angle into the sampling liquid. The configuration of the CIP 10-M air flow causes minimal stress to the microorganisms. There is neither mechanical impaction nor pressure drop shock. After the sampling period, the microorganism-laden liquid is recovered for subsequent cultivation or analysis. | ||
| Fig. 1 View of the open and the closed CIP 10-M sampler. | ||
 | ||
| Fig. 2 Schematic of the rotating cup collection stage of the CIP 10-M. | ||
The physical sampling efficiency of the CIP 10-M personal bioaerosol sampler was evaluated under controlled laboratory conditions and the field sampling of airborne microorganisms was carried out in various occupational situations.
Particle size dependent sampling efficiency of the CIP 10-M
The particle collection efficiency of the rotating cup, equipped with the collecting liquid (pyrogen free sterile water), was measured in laboratory conditions as a function of particle size at a flow rate of 10 l min−1. The experimental equipment was a horizontal low-speed wind tunnel described by Fabriès et al.15 A polydisperse test aerosol composed of glass microspheres (Ballotini® B3000) or latex particles (Rhodopass® A080) was used. The particle sizing was achieved by the API time-of-flight particle sizer (Aerosizer®, Amherst Instruments Process Inc., Hadley, MA, USA) or Coulter Multisizer® (Coulter Electronics Ltd., Lutton, UK). Both efficiency measurement methods have been described by Fabriès et al.16 and Görner et al.17The rotating cup collection efficiency (EC) for a given particle aerodynamic diameter (dae) is given by the ratio of the collected particle concentration (CC) and the concentration entering the rotating cup housing (C0):
The resulting collection efficiency as a function of particle size is reported in Fig. 3 for both experimental aerosols and both particle sizing methods. The physical collection efficiency of the sampler is >50% for particles >1.8 μm in aerodynamic diameter, and >95% for particles >2.8 μm. The collection efficiency decreases with decreasing particle size and reaches ≈20% for particles smaller than 1 μm. This collection efficiency is similar to those of many single-stage microbiological impactors.16
 | ||
| Fig. 3 Particle size-dependent collection efficiency of the CIP 10-M bioaerosol sampler. | ||
The CIP 10-M can be equipped with three different particle selectors making possible the sampling of health-related aerosol fractions (inhalable, thoracic and respirable) following standardized international sampling conventions. In this case, the efficiency of the chosen sampling head follows the conventional penetration curve of some of the aerosol fractions, laid down in the international standards.10–12 The overall sampling efficiency of the sampler is a combination of the selection and the collection efficiency. In the case of this study, the CIP 10-M was used in industrial environments without any selector. The aerosol was aspirated directly to the sampling cup by a circular orifice inlet (Fig. 1). The model of Grinshpun et al.18 for particle aspiration efficiency shows that sampling through this orifice at a flow rate of 10 l min−1 ensures an aspiration efficiency close to 1 for particles with an aerodynamic diameter <20 μm (Fig. 4). Hence, the collection efficiency reported in Fig. 3 can be considered to be the overall sampling efficiency of the sampler for particle sizes up to 20 μm.
 | ||
| Fig. 4 Aspiration efficiency according to the Grinshpun18 model. Orifice of diameter 7.7 mm, flow rate of 10 l min−1, vertical position, horizontal wind speed of 0.15 m s−1. | ||
Measurement of bacteria in the urban-waste sorting industry
Three methods were selected for bioaerosol sampling in a waste treatment plant: (i) Single stage impactor MAS 100 (Merck®), flow rate of 100 l min−1, (Fig. 5A). (ii) Aerosol filtration through membrane filters, flow rate of 2 l min−1, (Fig. 5B). (iii) CIP 10-M rotating cup bioaerosol sampler, flow rate of 10 l min−1, (Fig. 5B, C). | ||
| Fig. 5 A. MAS 100 single-stage biological impactor. B. Worker with the 37 mm sampling cassette and the CIP 10-M bioaerosol sampler. C. Filling the CIP 10-M rotating cup with collecting liquid. | ||
The first technique impacts the microorganisms directly onto a nutrient agar ∅ = 90 mm ready for culture. For the aerosol filtration sampling we used 37 mm polycarbonate filters (Nuclepore®) with 0.8 μm pores, inserted into polystyrene cassettes (Millipore®) with an air inlet of ∅ = 4 mm. The microorganisms were recovered from the filter into pyrogen free sterile water by means of an ultrasonic generator. In the case of sampling with the CIP 10-M, the rotating cup was filled with pyrogen free sterile water where the microorganisms were directly sampled. After recovery of the collecting liquid, the inside part of the rotating cup was rinsed twice and the rinsing water was added to the sample. The liquid samples were then filtered through 47 mm cellulose ester filters with 0.45 μm pores. The filters were put onto the nutrient agar for culture. The agar Petri dishes were incubated at 22 °C or 37 °C for 5 days.
The sampling time was 150 minutes except with the single-stage impactor where the short sampling times of 1 min and 2 min 30 s were used because of impactor saturation problems. Indeed, the limit of detection (LD) and the limit of saturation (LS) are the parameters to be taken into account when using each sampling and analytical method. For the methods using microbial culture and the counting of colony forming units (CFU), the detection and the saturation limits can be estimated as follows. When a single colony is detected on the agar, in accordance with the discrete Poisson law, the upper limit of the 98% confidence interval is 3, (there is a probability of 98% that the colony number varies from 0 to 3). Taking the volume of sampled air into consideration, it is possible to calculate the lowest measurable concentration CLD. The maximum measurable concentration CMM is unlimited in the case of the filter and CIP 10-M methods because of the possibility of sample dilution. For the biological impactor, which samples directly onto the agar, dilution is not possible. The limit of saturation is estimated from the observation that a maximum of 400 colonies can grow on the 90 mm Petri dish with no mutual contact among them. The number of colony forming units (CFU) is then estimated by using the correction of Hinds19 due to the multiple impact in front of each acceleration orifice. The limits of detection and saturation of the cultivable microorganisms for all the methods used are given in Table 1.
| Sampler | Flow rate/l min−1 | Sampled time/min | Sampled volume/l | C LD/CFU m−3 | C MM/CFU m−3 |
|---|---|---|---|---|---|
| CIP 10-M | 10 | 150 | 1500 | 20 | Unlimited |
| 37 mm Cassette | 2 | 150 | 300 | 125 | Unlimited |
| MAS 100 | 100 | 1 | 100 | 30 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| 2.5 | 250 | 12 | 6900 |
The bioaerosol measurements were performed in a small sorting room where two workers sorted the recyclable materials from the waste. Three CIP 10-M and two sampling cassettes (C37-2) were used simultaneously at a fixed point for 150 minutes. Halfway through, the rotating cups of all three CIP 10-M were changed, and the CIP 10-M sampling times were split into two equal periods. The cassette sampling continued with no interruption. Ten 1 min and ten 2.5 min samples were taken with the MAS 100 single-stage biological impactor. The samples were transported immediately to the laboratory to recover the microorganisms and to start the culture. The bacterial colonies were counted after five days of cultivation on the soy trypticase agar with an antifungal additive (actidione), at 22 °C or 37 °C. The results of the cultivable bacteria concentrations measured are reported in Fig. 6 in CFU per cubic metre of sampled air.
 | ||
| Fig. 6 Cultivable bacteria concentration in the waste sorting room. The CIP 10-M sampling times were split into two consecutive periods (1 and 2). The cassette sampling lasted for the entire time (period 1 + 2). The CLD is the limit of detection of colony forming unit counting for the CIP 10-M microbiological sampler and the filter cassette (C37-2) sampler, respectively. | ||
The number of CFU counted is greater at the incubation temperature of 22 °C than at 37 °C. This indicates that the microbiological flora is composed mainly of environmental bacteria.
The results of the impactor measurements are not reported in Fig. 6. All the impactor samples were saturated. It can be seen from Fig. 6 that the sorting room concentrations were higher than the maximum concentration measurable by the impactor.
A fraction of the samples were kept for epifluorescence microscopic observation. The microorganisms were stained by DAPI fluorochrome (4′,6-diamidino-2-phenylindole) lit by an ultra violet lamp and counted on a black field using an epifluorescence microscope (magnification 1000×). This procedure enables the counting of all the microbial cells with no restriction concerning viability or reproduction ability. The total cell concentration (cell m−3) is reported in Fig. 7. It is about one hundred times greater than the concentration of cultivable bacteria.
 | ||
| Fig. 7 Total microbial cell concentration in the waste sorting room. Counted by epifluorescence optical microscopy. The CLD is the limit of detection of total cell counting for the CIP 10-M microbiological sampler and the filter cassette (C37-2) sampler, respectively. | ||
The results show that the concentrations stemming from both sampling methods are close. This is true in the case of cultivable bacteria and also in the case of the total cells.
Measurement of fungi in a waste-water treatment plant
The sampling of cultivable fungi was carried out in a waste-water treatment plant by all three sampling methods. The only change was using a 25 mm filter cassette at 1 l min−1 instead of the 37 mm cassette at 2 l min−1. The samples were taken in five different locations: EXT, outside the plant; BIO, close to biofilters; LAM, close to lamella filters; PRE, slush press; COL, waste-water collector. The samples were recovered as described in the previous paragraph. Two specific culture media were used to cultivate the fungi, namely malt agar and DG 18 agar. The colonies of fungi were counted after ten days of incubation at a temperature of 30 °C. The fungi concentrations measured are reported in Fig. 8. | ||
| Fig. 8 Concentrations of airborne fungi measured at a waste-water treatment plant. CLD are the detection limit levels for the sampling methods used. CFU counted after ten days of incubation. | ||
The fungi concentrations at all the locations measured in the plant were relatively small. They varied mostly from 102 to 103 CFU m−3. The concentrations measured by the single-stage biological impactor appeared to be slightly higher. This is probably due to the direct deposit of the spores on the agar. Fungi spores are known to be hydrophobic. In the case of other two methods, this can cause some losses during recovery of the samples by means of water.
Measurement of endotoxins in the waste collection hall of an urban-waste incinerator
Endotoxins are the lipopolysaccharides present in the cell membranes of Gram negative bacteria. They can have a harmful effect on the human body, e.g., an allergic effect.Stationary air sampling was performed in order to assess the concentration of airborne endotoxins in a reception hall of an urban-waste incinerator. Samples were collected during 100 minutes by air filtration through polycarbonate (PC) filters (Nuclepore®), glass fibre (GF) filters (Whatman®) and by a CIP 10-M sampler. The PC and GF filters were checked for endotoxin contamination. The results of blank measurements of filter endotoxin content show less than 0.025 EU per PC filter and less than 1.0 EU per GF filter. The 37 mm cassettes with a sampling orifice of 4 mm, operated at a flow rate of 2 l min−1 were used as filter holders.
The collecting liquid used in the CIP 10-M microbiological sampler was 2 ml of pyrogen free sterile water (B. Braun Medical, France). Three PC filter cassettes, three GF filter cassettes and three CIP 10-M samplers were fixed at a horizontal ramp and run simultaneously for 100 minutes. The samplers were analysed within 24 hours after sampling. The filters were placed in pyrogen free sterile water inside a conical 50 ml polypropylene tube (Greiner-Bio-One), vortexed for 1 minute at 2500 rpm, then ultrasonicated for 20 minutes at 47 kHz and 60 °C and again vortexed. In the case of the GF filters, the extract was filtered in sterile conditions through a polyestersulfone (PES) filter (Milles-GP, Millipore, USA). The CIP 10-M rotating cups were rinsed twice in order to recover the whole of the sample. Endotoxins were quantified with the Limulus Amaebocyte Lysate (LAL) assay.20 The LAL-Kinetic-QCL™ analysing kit (Cambrex, France) was used. The reaction of the endotoxins with the LAL reagent water on a microtitration plate was monitored with a photometer Elx 808 IU (Biotek, France), at a wavelength of 405 nm. The calibration endotoxin was the Escherichia coli 055:B5 endotoxin. All the handling operations were done in endotoxin-free vessels under a microbial security cabinet.
The airborne endotoxin concentration (CE) in endotoxin units per cubic metre (EU m−3) was calculated from the following equation:
| CE = (e × vo)/V |
| V = (Q × t)/1000 |
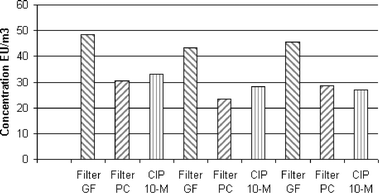 | ||
| Fig. 9 Airborne endotoxin concentrations in the waste collection hall of an urban-waste incinerator measured by three different sampling methods. All samples were taken simultaneously for 100 minutes. | ||
The endotoxin concentration level varies from about 20 to 50 EU m−3. This level can be encountered in similar types of industry.21 The results from the glass fibre samples (GF) are systematically higher than those from polycarbonate (PC) filters. The results from the CIP 10-M are situated in between, closer to the PC filters results. Since the analytical treatment is rigorously the same for all the samples, these differences could stem from the recovery rate of endotoxins from different collecting substrates. It would appear that endotoxin-borne substances adhere better to the polycarbonate surface than to the glass fibres. In the case of the CIP 10-M, the rotating cup is rinsed with no wiping or ultrasonic treatment. It is possible that some residual deposit remained on the inner-wall surface of the rotating cup.
Discussion
During this study, pyrogen free sterile water was used as sampling liquid. A small amount of non-biocide detergent can be added to the liquid to facilitate the recovery of hydrophobic spores from the sampling cup. In the case of hot and dry atmospheric conditions, non volatile sampling liquids could be used in order to prevent its evaporation.The sampling liquid can not be spilt from the rotating cup neither during the sampling (high centrifugal force) nor during the sample transportation (cup closed by a tight lid). The only delicate period is between the filling of the cup and the switching-on of the device. It is recommended to equip the monitored person with the device already switched-on.
All parts of the sampler in contact with sampled bioaerosol are easily sterilisable, including the particle selectors. It is recommended to use sampling cups that have been previously sterilised and packed in the laboratory. Selectors of health-related aerosol fractions were not used in this study because of the comparison of the CIP 10-M with not specifically selective devices.
Conclusion
A new personal bioaerosol sampler, the CIP 10-M has been designed. Its physical sampling efficiency meets the conventional aerosol sampling criteria. Field trials have demonstrated a fair microbiological efficiency in comparison with existing microbiological sampling devices. The liquid collection substrate (in our case sterile pyrogen free water) allows high microbial concentrations to be measured with no saturation. The CIP 10-M provides a biological performance comparable to that of bioimpactors, but allows the measurement of higher concentrations. When sampling fungi the performance is slightly lower, probably due to losses of hydrophobic spores during recovery of the sampling liquid. The CIP 10-M microbiological sampler is also suitable for collecting samples for endotoxin analysis.The CIP 10-M personal bioaerosol sampler is compact, and is easy to transport, assemble, clean and sterilize. Its internal battery gives it an operating autonomy of more than 24 hours. It can be operated with inhalable, thoracic or respirable aerosol selectors to measure the exposure of workers to the health-related aerosol fractions laid down in the international standards.
References
- L. C. Kenny, J. D. Stancliffe, B. Crook, S. Stagg, W. D. Griffiths, I. W. Stewart and S. J. Futter, Am. Ind. Hyg. Assoc. J., 1999, 59, 831–841.
- T. Reponen, K. Willeke, S. A. Grinshpun, A. Nevalainen, in Aerosol Measurement - Principles, Techniques and Applications, ed. P. A. Baron and K. Willeke, Wiley Interscience, Chichester, 2nd edn, 2001, pp. 751–777 Search PubMed.
- S. A. Grinshpun, K. Willeke, V. Ulevicius, A. Juozaitis, S. Terzieva, J. Donelly, G. N. Stelma and K. P. Brenner, Aerosol Sci. Technol., 1997, 26, 326–342 CAS.
- W. H. Lin and C. S. Li, Aerosol Sci. Technol., 1999, 30, 109–118 CAS.
- W. Licht, Air Pollution Control Engineering - Basic calculations for particulate collection, Marcel Dekker Inc., New York, USA, 2nd edn, 1988 Search PubMed.
- P. Courbon, R. Wrobel and J. F. Fabriès, Ann. Occup. Hyg., 1988, 32, 129–143 CrossRef CAS.
- P. Görner, J. F. Fabriès and R. Wrobel, J. Aerosol Sci., 1994, 25, S487–S488 CrossRef.
- J. F. Fabriès, P. Görner, E. Kauffer, R. Wrobel and J. C. Vigneron, Ann. Occup. Hyg., 1998, 42, 453–465 CrossRef CAS.
- P. Görner, R. Wrobel, F. Roger and J. F. Fabriès, J. Aerosol Sci., 1999, 30, S893–S894 CrossRef.
- CEN EN 481, Workplace atmospheres: Specification for conventions for measurement of suspended matter in workplace atmospheres, Comité Européen de Normalisation, Brussels, 1993 Search PubMed.
- ACGIH, Threshold limit values for chemical substances and physical agents and biological exposure indices, American Conference of Governmental Industrial Hygienists, Cincinnati, OH, 1994–95 Search PubMed.
- ISO IS 7708, Air quality—Particle size fractions definitions for the health-related sampling, International Standards Organization, Geneva, 1995 Search PubMed.
- Patent no 03 02323 (2003) Capteur individuel portatif pour la mesure de l’aérosol microbiologique. Patent pending 26 February 2003.
- J. Guillot, M. Berthelemy, B. Polack, V. Laine, P. Lacube, R. Chermette and P. Roux, J. Eukaryotic Microbiol., 1999, 46(5), S100–S101 Search PubMed.
- J. F. Fabriès, B. Carton and R. Wrobel, Staub-Reinhalt. Luft, 1984, 44, 405–409 Search PubMed.
- J. F. Fabriès, R. Wrobel, P. Görner and G. Greff-Mirguet, J. Aerosol Sci., 2001, 32, S333–S334.
- P. Görner, R. Wrobel and J. F. Fabriès, J. Aerosol Sci., 2000, 31, S268–S269.
- (a) S. A. Grinshpun, K. Willeke and S. Kalatoor, Atmos. Environ., 1993, 27A, 1459–1470 CrossRef CAS; (b) S. A. Grinshpun, K. Willeke and S. Kalatoor, Atmos. Environ., 1994, 28, 375 CrossRef.
- W. C. Hinds, Aerosol Technology. Properties, Behaviour, and Measurement of Airborne Particles, John Wiley&Sons, Chichester, UK, 2nd edn., 1999, ch. 19: “Bioaerosols” Search PubMed.
- CEN EN 14031, Workplace atmospheres – Determination of airborne endotoxin, Comité Européen de Normalisation, Brussels, 2003 Search PubMed.
- J. Thorn, L. Beijer, T. Jonsson and R. Rylander, Ann. Occup. Hyg., 2002, 46, 549–554 CrossRef CAS.
Footnote |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| This journal is © The Royal Society of Chemistry 2006 |