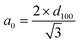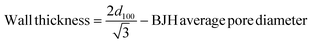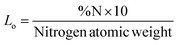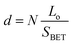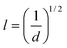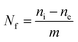Preparation of 2-mercaptobenzothiazole-derivatized mesoporous silica and removal of Hg(II) from aqueous solution†
D.
Pérez-Quintanilla
,
I.
del Hierro
,
M.
Fajardo
and
I.
Sierra
*
Departamento de Tecnología Química y Ambiental, E.S.C.E.T, Universidad Rey Juan Carlos, C/ Tulipán s/n, 28933, Móstoles, Madrid, Spain. E-mail: isabel.sierra@urjc.es
First published on 9th November 2005
Abstract
Mesoporous silicas (SBA-15 and MCM-41) have been functionalized by two different methods. Using the heterogeneous route the silylating agent, 3-chloropropyltriethoxysilane, was initially immobilized onto the mesoporous silica surface to give the chlorinated mesoporous silica Cl-SBA-15 or Cl-MCM-41. In a second reaction a multifunctionalized N,S donor compound (2-mercaptobenzothiazol) was incorporated to obtain the functionalized silicas denoted as MBT-SBA-15-Het and MBT-MCM-41-Het. Using the homogeneous route, the functionalization was achieved via the one step reaction of the mesoporous silica with an organic ligand containing the chelating functions, to give the modified mesoporous silicas denoted as MBT-SBA-15-Hom or MBT-MCM-41-Hom. The functionalized mesoporous silicas were employed as adsorbents for the regeneration of aqueous solutions at pH 6 contaminated with Hg(II) at room temperature. Results obtained indicate that mercury adsorption was higher in the mesoporus silicas prepared by the homogeneous method, and the maximum adsorption value (0.24 ± 0.02 mmol Hg(II) g−1) was obtained for MBT-SBA-15-Hom. The chemically stability in acid medium of the functionalized silicas, possibility its regeneration washing with concentrate HCl, resulting in the reuse of the adsorbent material for several cycles.
Introduction
Environmental pollution as a result of rapid technological development is a serious concern for ecology. Heavy metal ion contamination represents a significant threat to the ecosystem. Water, the most important component of the ecosystem, and its purification with adsorbents and ion-exchangers for heavy metal ion removal is an important topic for many scientific disciplines.1 Several different types of materials, among them resin, carbons, and clays, just to mention a few, have been studied and proposed for adsorption of heavy metal ions.The synthesis of adsorbents for the removal of toxic heavy metal ions from wastewater is a continuing research objective of environmental pollution control processes. Adsorbent materials like silica, alumina or modified layered materials, such as pillared clays and silicates, present pores that are generally irregularly spaced and broadly distributed in size. These materials have showed several problems like low mechanical and thermal stability and weak chemical union with the metals. These factors have encouraged the scientific community to develop new techniques to remove heavy metals from the environment.
Recently, a new family of ordered mesoporous materials has been obtained. These ordered mesoporous materials show a large BET surface area, high porosity, controllable and narrowly distributed pore sizes, and an ordered pore arrangement.2,3 The development of functionalized mesoporous materials for adsorption applications has generated a considerable interest. Among the variety of adsorption applications, the preparation of highly effective adsorbents for heavy metal ion trapping by the grafting or incorporation of ligands into mesoporous materials is clearly one of the most promising methods for environmental clean-up. In particular, materials whose surfaces have been functionalized with mercury-binding groups have shown extraordinary promise for the mitigation of mercury levels in the environment.4–16 In addition, the stability of some of these materials to acidic medium and the potential to regenerate and reuse them have been demonstrated by some authors.4 However, although some commercially available ligands as mercaptopropytrialkoxysilanes have been studied extensively for the functionalization of mesoporous silicas,4–10,12–16 studies with other non commercially available ligands are, comparatively, incomplete.17 Recently, Fryxell et al.18,19 have prepared non commercially available lanthanide and actinide-selective ligands to obtain a powerful new class of environmental sorbent materials.
2-mercaptobenzothiazol (MBT) has demonstrated capacity to remove Hg(II) from aqueous media. Terada et al.20,21 reported that MBT impregnated to silica gel can be used in preconcentration of metal ions present in water samples. More recently, Dias Filho et al.22,23 loaded MBT on a polystyrene treated clay for adsorption and preconcentration of some heavy metals from aqueous solution. However, in both cases adsorption capacity of the prepared material was not high enough to use them for environmental applications. This paper reports the application of two mesoporous silicas (SBA-15 and MCM-41) in the preparation of solid phases modified with the 2-mercaptobenzothiazol by two different approaches, (homogeneous and heterogeneous routes) and their application in the batch method for the adsorption of Hg(II) from aqueous solutions at pH 6. Chemical stability of the prepared materials was also studied to evaluate the potential of such materials in regeneration and reusability.
Experimental
Reagents and materials
Poly(ethylene glycol)-block-poly(propylene glycol)-block-polyethylene glycol (Mav = 5800; d = 1.019 × 10−3 g mL−1) and tetraethoxysilane (TEOS) 99% (M = 208.33, d = 0.933 kg m−3) were purchased from Fluka (Spain). Sodium silicate SiO2·NaOH (M = 242.23, d = 1.390 kg m−3) and cetyltrimethyammonium bromide (CTAB) (M = 364.46) was purchased from Sigma-Aldrich (Germany). Sulfuric acid 96% (M = 98.08, d = 1.840 kg m−3) was purchased from Panreac (Spain). 3-chloropropyltriethoxysilane 95% (CPTS) (M = 240.81, d = 1.007 g mL−1), triethylamine (M = 101.19, d = 0.726 g mL−1) and 2-mercaptobenzothiazol (MBT) 98% (M = 167.25), mercury from Sigma-Aldrich, were used as supplied. Organic solvents (toluene, dimethylformamide, diethyl ether, ethanol, hexane and triethylamine) were purchased from SDS (France). These solvents were distilled and dried before use according to conventional literature methods.24 Buffer solution of pH 6 was prepared by using sodium acetate and hydrochloric acid (Scharlau, Spain). Standard stock solution of Hg(II) was prepared by dissolving mercury(II) nitrate monohydrate 98% (Sigma-Aldrich) in dilute HNO3. Water (resistance 18 MΩ cm−1) used in the preparation of standard solutions was obtained from a Millipore Milli-Q-System (Waters, USA). Triton X-100 and 1,5-diphenylthiocarbazone (dithizone) were purchased from Sigma-Aldrich. Glassware was soaked in (1 + 1) HNO3 overnight and cleaned with Milli-Q water before use. All other reagents used were at least of analytical grade.Synthesis of SBA-15 mesoporous silica
A hexagonal (plane group p6mm) material (SBA-15) was prepared by using a poly(alkaline oxide) triblock copolymer surfactant in acidic media, according to the method of Zhao et al.25 4.00 g of poly(ethylene glycol)-block-poly(propylene glycol)-block-polyethylene glycol) was dissolved in 30.00 g of water and 80.00 g of 2 M HCl solution with stirring at 35 °C. Then 8.80 g of TEOS was added to that homogeneous solution with stirring at room temperature for 20 h. The solid product was recovered, washed, and air-dried at room temperature. Then it was calcinated in an atmosphere of air from room temperature to 500 °C in 8 h and heated for 6 h.Synthesis of MCM-41 mesoporous silica
A hexagonal (plane group p6mm) material (MCM-41) was prepared according to the method of Landau et al.26 using hydrothermal crystallization. A sodium silicate solution (65.45 g, 14% NaOH; 27% SiO2) was mixed with 4.20 g of 98% sulfuric acid and 140 mL of water. The mixture was stirred for 30 minutes at room temperature. CTAB (58.66 g) dissolved in 176 mL of water was added. The resulting gel was mixed with 70 mL of water (stirred at room temperature for 30 minutes). The gel was transferred to a Teflon-coated autoclave and heated at 121 °C for 144 h. A solid was produced, separated by suction filtration and dried at 100 °C for 8 h. The surfactant was removed by calcination in air at 530 °C for 6 h.Preparation of functionalized mesoporous silicas
MBT was covalently bonded to SBA-15 or MCM-41 giving mercaptobenzothiazole modified mesoporous silicas. The immobilization of these molecules on the silica support was carried out by two distinct methods (homogeneous and heterogeneous). The first stage in the preparation of functionalized mesoporous silicas by the homogeneous method was the synthesis of the silylating agent, by reaction of CPTS with MBT. The resulting compound was allowed to react with the silanol groups of activated silica, liberating the corresponding alcohol, to get the final functionalized support (Scheme 1). The second methodology used, the heterogeneous method, involves a two-step reaction of the solid support. The reaction of the activated mesoporous silica with CPTS to obtain the chlorinated silica followed by a second reaction with the appropriate amount of MBT to yield the final product (Scheme 2).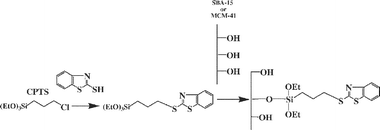 | ||
| Scheme 1 Homogeneous route for mesoporous silica functionalization. | ||
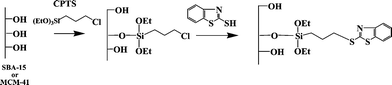 | ||
| Scheme 2 Heterogeneous route for mesoporous silica functionalization. | ||
The resulting compound, having the consistency of viscous oil (orange–brown colour), was characterized by IR, 1H NMR, 13C NMR and mass spectrometry (See electronic supplementary information†). IR (KBr pellets) show bands at ca.νmax/cm−1: 2850s (C–H), 1475s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 1600w (C
C) and 1600w (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). δH (400 MHz; CDCl3; Me4Si) 0.81 (2H, m, Si–CH2CH2CH2–S), 1.96 (2H, m, Si–CH2CH2CH2–S), 1.21 (3H, t, CH3CH2O)3Si–), 3.36 (2H, t, Si–CH2CH2CH2–S), 3.80 (2H, q, CH3CH2O)3Si–), 7.24, 7.38, 7.72 and 7.83 (4H, m, C7H4NS2). δC (400 MHz; CDCl3; Me4Si) 10.08 (Si–CH2CH2CH2–S), 18.40 ((CH3CH2O)3Si), 23.16 (Si–CH2CH2CH2–S), 36.48 (Si–CH2CH2CH2–S), 58.46 ((CH3CH2O)3Si), 120.74, 121.27, 123.92, 125.81, 134.98, 153.12 and 166.95 (C7H4NS2). m/z 368 (M+, 55%).
N). δH (400 MHz; CDCl3; Me4Si) 0.81 (2H, m, Si–CH2CH2CH2–S), 1.96 (2H, m, Si–CH2CH2CH2–S), 1.21 (3H, t, CH3CH2O)3Si–), 3.36 (2H, t, Si–CH2CH2CH2–S), 3.80 (2H, q, CH3CH2O)3Si–), 7.24, 7.38, 7.72 and 7.83 (4H, m, C7H4NS2). δC (400 MHz; CDCl3; Me4Si) 10.08 (Si–CH2CH2CH2–S), 18.40 ((CH3CH2O)3Si), 23.16 (Si–CH2CH2CH2–S), 36.48 (Si–CH2CH2CH2–S), 58.46 ((CH3CH2O)3Si), 120.74, 121.27, 123.92, 125.81, 134.98, 153.12 and 166.95 (C7H4NS2). m/z 368 (M+, 55%).
5.00 g of the MBT derivative was reacted with 5.00 g of activated SBA-15 or MCM-41 (5 h at 160 °C under high vacuum) in 50 mL of dry toluene with mechanical stirring (48 h under reflux conditions under a nitrogen atmosphere). The resulting modified mesoporous silica (L-Sil-Hom: MBT-SBA-15-Hom or MBT-MCM-41-Hom) was filtered off and washed with toluene (2 × 30 mL), ethanol (2 × 30 mL) and diethyl ether (2 × 30 mL). Finally, the product was heated for 4 h at 110 °C under vacuum.
Characterization
1H NMR and 13C NMR spectra were recorded using a Varian-Mercury Plus (400 MHz) spectrometer using CDCl3 as solvent and reference. Mass spectra were recorded on a VG Autospec spectrometer in the Aragon Materials Institute (Zaragoza, Spain). X-ray diffraction (XRD) patter of the silicas were obtained on a Phillips diffractometer model PW3040/00 X’Pert MPD/MRD at 45 KV and 40 mA, using a wavelength Cu Kα (λ = 1.5418 Å). N2 gas adsorption-desorption isotherms were performed using a Micromeritics TriStar 3000 analyzer. Infrared spectra were recorded on a Nicolet-550 FT-IR spectrophotometer or on a Mattson Infinity FT-IR spectrophotometer in the region 4000 to 400 cm−1 by using spectra quality KBr powder or CsI plates. Proton-decoupled 29Si MAS NMR spectra were recorded on a Varian-Infinity Plus spectrometer at 400 MHz. Elemental analysis (%C and %N) was performed in the Investigation Service of the Universidad Autónoma de Madrid (Spain). The thermal stability of the modified mesoporous silicas was studied by using a Setsys 18 A (Setaram) termogravimetric analyzer.Chemical stability of the functionalized mesoporous silicas
To determinate the chemical stability of the materials, acidic and basic solutions were prepared (1, 3 and 6 M HCl or NaOH). The functionalized mesoporous silicas were shaken in these solutions for 24 h, filtered off through Whatman No 44 filter paper, washed with Milli-Q water and finally dried at 110 °C. After this treatment, the amount of attached molecules onto the mesoporous silica surface was determined by elemental analysis.Hg(II) adsorption on the functionalized mesoporous silicas
A batchwise process was employed in the adsorption of Hg(II) on the modified mesoporous silicas from aqueous solution of controlled pH at 25 °C. The adsorption procedures were repeated with untreated SBA-15 and MCM-41, but under these conditions adsorption was negligible (0.005 ± 0.002 mmol of Hg(II) g−1 of SBA-15 and 0.06 ± 0.02 mmol of Hg(II) g−1 of MCM-41).A sample of 0.2 g of MBT-SBA-15-Het, MBT-SBA-15-Hom, MBT-MCM-41-Het or MBT-MCM-41-Hom was suspended in 30 mL of aqueous solution of pH 6. Then 5 mL of Hg(II) 0.1M solution were added and the mixture was maintained under mechanical stirring for 4 h at 25 °C to attain equilibrium. After equilibration, the mixture was filtered off through Whatman No 44 filter paper, which was washed with abundant Milli-Q water. Finally, the total volume of the filtrate was completed to 100 mL with Milli-Q water by using a volumetric flask. An aliquot of 1 mL was diluted to a final volume of 50 mL with Milli-Q water. The unbound Hg(II) ions present in this solution were subjected to colorimetric analysis.
Determination of Hg(II)
Hg(II) was determined colourimetrically using a Varian Cary 50 UV-Vis spectrophotometer.27 In order to prepare the calibration curves, 5 mL of Hg(II) 0.1 M solution were added to 30 mL of aqueous solution of pH 6. The mixture was maintained under mechanical stirring for 4 h at 25 °C and then was filtered through Whatman No 44 paper, which was washed abundantly with Milli-Q water. Finally, the total volume of the filtrate was completed to 100 mL with Milli-Q water. Different aliquots of this solution were diluted to a final volume of 50 mL with Milli-Q water to achieve standard solutions with an Hg(II) concentration between 0.025–0.150 mM.1 mL of each standard solution or 1 mL of the sample solution was added to small beakers and their pH was adjusted to an optimum value (pH 2) for the formation of the mercury–dithizone complex by using 5 mL of 1 M HCl. A saturated solution of dithizone in Triton X-100 (7 mL) was then added, and the solutions were allowed to equilibrate for a few minutes before recording the absorbance at 492 nm. Metal dithizonates formed under these conditions were stable for at least 45 min.
Results and discussion
Fig. 1 and 2 show the XRD patterns for mesoporous silicas. The SBA-15 displayed a well-resolved pattern at low 2θ with a very sharp (100) diffraction peak at 0.96° and two weak peaks (110 and 200) at 1.60° and 1.85°, respectively, that can be indexed as a hexagonal lattice with d-spacing values of 92.03, 55.68 and 48.60 Å, respectively. A unit cell parameter, a0, of 106.27 Å was obtained using the following equation:In the case of MCM-41, we can also see a very sharp (100) diffraction peak at 2.43° and three additional high order peaks (110, 200 and 210) with lower intensities at 4.17, 4.75 and 6.28°, respectively. d-spacing values for these XRD peaks were 36.32, 21.15, 18.60 and 14.07 Å, respectively. a0 was equal to 41.94 Å.
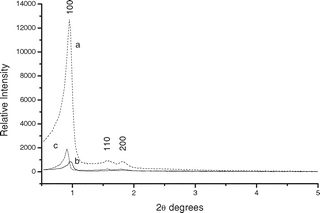 | ||
| Fig. 1 XRD patterns of (a) SBA-15, (b) MBT-SBA-15-Het and (c) MBT-SBA-15-Hom. | ||
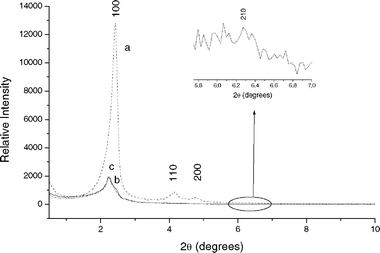 | ||
| Fig. 2 XRD patterns of (a) MCM-41, (b) MBT-MCM-41-Het and (c) MBT-MCM-41-Hom. | ||
For functionalized silicas, MBT-SBA-15-Het, MBT-SBA-15-Hom, MBT-MCM-41-Het and MBT-MCM-41-Hom, a considerable decrease in the XRD peaks intensity was observed. It should be noted that the intensity decrease in the (100) peak for functionalized silicas provides further evidence of grafting occurring mainly inside the mesopore channels. Collectively, the XRD patter of the functionalized silicas also suggest not only a significant degree of short range ordering of the structure and well-formed hexagonal pore arrays of the samples, but also the maintenance of the structural orderness of the synthesized materials after functionalization.
Fig. 3 and 4 show the nitrogen adsorption-desorption isotherms for mesoporous silicas. For bare SBA-15, MBT-SBA-15-Hom and MBT-SBA-15-Het (Fig. 3) isotherms are type IV according to the IUPAC classification and have a H1 hysteresis loop that is representative of mesopores. The volume adsorbed for all isotherms sharply increased at a relative pressure (P/P0) of approximately 0.5, representing capillary condensation of nitrogen within the uniform mesopore structure. The inflection position shifted slightly toward lower relative pressures and the volume of nitrogen adsorbed decrease with functionalization. On the other hand, the hysteresis loop of the isotherm of MCM-41 is smoother than that of SBA-15, and different than that of the functionalized MCM-41, because in MBT-MCM-41-Hom and MBT-MCM-41-Het the hysteresis cycle disappears (isotherms type I according to IUPAC, Fig. 4). These differences can be attributed to a decrease in the pore volume after functionalization. We can also observe that the inflection position shifted slightly toward lower relative pressures and the volume of nitrogen adsorbed decrease with functionalization.
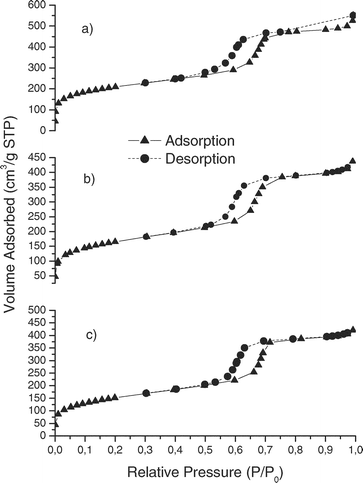 | ||
| Fig. 3 Nitrogen adsorption isotherms of (a) SBA-15, (b) MBT-SBA-15-Het and (c) MBT-SBA-15-Hom. | ||
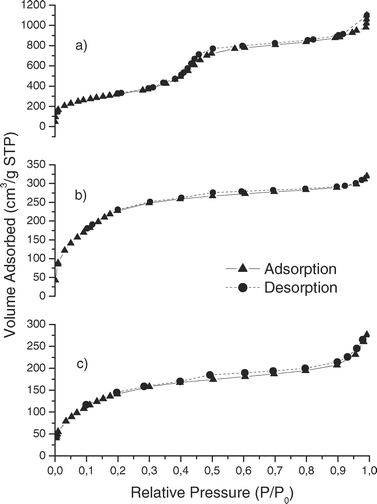 | ||
| Fig. 4 Nitrogen adsorption isotherms of (a) MCM-41, (b) MBT-MCM-41-Het and (c) MBT-MCM-41-Hom. | ||
Table 1 shows the physical parameters of nitrogen isotherms, such the BET surface area (SBET) and BJH average pore diameter for mesoporous silicas. As expected, the SBET for the SBA-15 is lower (740 m2 g−1) than for the MCM-41 (1173 m2 g−1), whereas the BJH average pore diameter is higher (71.9 and 34.0 Å, respectively). The wall thickness for SBA-15 and MCM-41 (34.37 Å and 7.94 Å, respectively) was calculated using the following equation:
| Sample | BET specific surface area/m2 g−1 | Pore diameter(BJH)/Å |
|---|---|---|
| SBA-15 | 740.24 | 71.9 |
| MBT-SBA-15-Het | 590.83 | 61.6 |
| MBT-SBA-15-Hom | 577.53 | 58.0 |
| MCM-41 | 1172.52 | 34.0 |
| MBT-MCM-41-Het | 884.88 | 17.0 |
| MBT-MCM-41-Hom | 553.26 | 13.9 |
Fig. 5 and 6 show the IR patterns of mesoporous silicas between 4000–400 cm−1. Functionalized mesoporous silicas presented characteristic bands for aliphatic C–H stretching vibrations for pendant alkyl chains around 3000–2800 cm−1 and bands at ca. 1600 and 1475 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the attached MBT group.28,29
C stretching vibrations of the attached MBT group.28,29
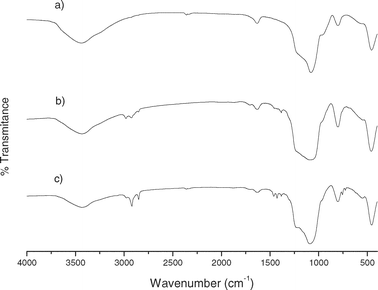 | ||
| Fig. 5 FTIR spectra of (a) activated MCM-41, (b) MBT-MCM-41-Het and (c) MBT-MCM-41-Hom. | ||
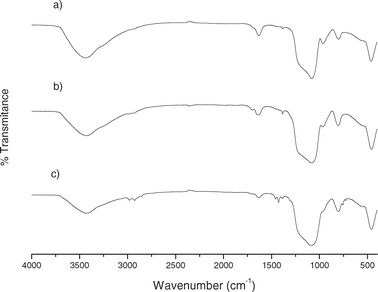 | ||
| Fig. 6 FTIR spectra of (a) activated SBA-15, (b) MBT-SBA-15-Het and (c) MBT-SBA-15-Hom. | ||
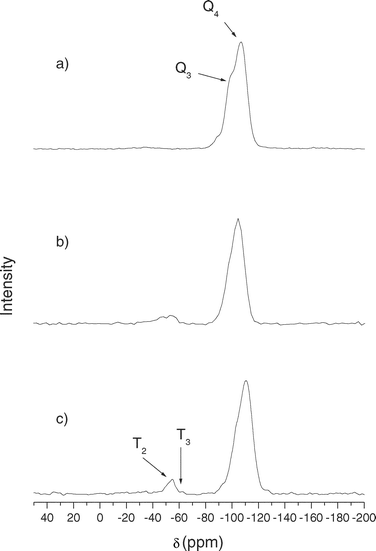
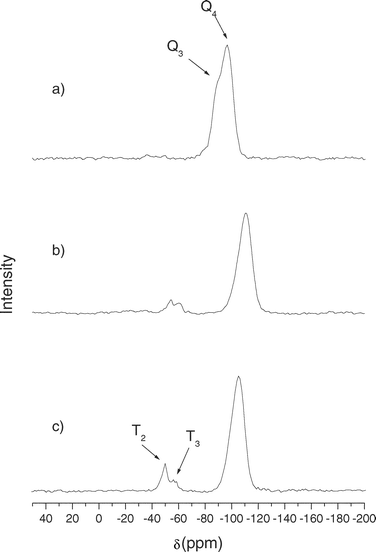
Fig. 7 and 8 show the 29Si MAS-NMR spectra of mesoporous silicas. Bare silica (SBA-15 and MCM-41) shows mainly two peaks at −105 and −95 ppm which are assigned to Q4 framework silica sites ((SiO)4Si) and Q3 silanol sites ((SiO)3SiOH), respectively.10,11 Clearly, Q4 is the dominant peak in both spectra because it is the most abundant site. Functionalized silicas present a large decrease in the intensity of the Q3 signal, which verified the anchoring of the functional groups to Si–OH. In addition, two new peaks appeared at −45 and −60 ppm, which are assigned to T2 ((SiO)2SiOH–R) and T3 ((SiO)3Si–R) sites, respectively.
It is possible to calculate the amount of attached molecules onto the mesoporous silica surface (Lo) from the percentage of nitrogen in the functionalized mesoporous silicas, calculated by elemental analysis, using the following expression:
| Nitrogena (% weight) | Nitrogen/mmol g−1 | Carbona (% weight) | Carbon/mmol g−1 | L o b/mmol g−1 | C/Nc | |
|---|---|---|---|---|---|---|
| a Average of two determinations ± standard deviation. b L o = milimoles of ligand per gram of functionalized silica = (% nitrogen × 10)/N atomic weight. c C/N = mmol g−1 C/mmol g−1 N. | ||||||
| MBT-SBA-15-Het | 0.24 ± 0.01 | 0.17 | 3.60 ± 0.01 | 3.00 | 0.17 | 17.2 |
| MBT-SBA-15-Hom | 1.27 ± 0.08 | 0.91 | 13.86 ± 0.09 | 11.55 | 0.91 | 12.7 |
| MBT-MCM-41-Het | 0.37 ± 0.01 | 0.26 | 9.12 ± 0.04 | 7.60 | 0.26 | 28.9 |
| MBT-MCM-41-Hom | 1.60 ± 0.03 | 1.14 | 16.94 ± 0.02 | 14.11 | 1.14 | 12.4 |
The presence of three alkoxy groups in the CPTS and silylating agent means that they could react with the silica surface via one, two or all alkoxy groups. However, detailed NMR characterization of silane binding to silica surfaces carried out by Maciel et al.30–32 debunked the myth that trifunctional silanes bind to the surface in a bidentate or tridentate fashion. In fact, the C/N molar ratio obtained in homogeneous route (12.7 for MBT-SBA-15-Hom and 12.4 for MBT-MCM-41-Hom) indicates a preferably 1 ∶ 1 stoichiometry, between the silanol groups on the silica surface and the silylating agent. However, via the heterogeneous route, the C/N ratio is higher than expected (17.2 for MBT-SBA-15-Het and 28.9 for MBT-MCM-41-Het), due to the presence of remaining unreacted chloropropyl groups on the silica surface. In this case, the reaction of the chlorinated silica with MBT is not completed, even when working in excess of chelating ligand.
Taking into account Lo and SBET of the mesoporous silicas, the average surface density, d, of the attached molecules and the average intermolecular distance, l, can be calculated by applying the following equations:33
where N is Avogadro’s number. As expected (Table 3) in mesoporous silicas functionalized by the homogenous method d is higher and l is lower than in mesoporous silicas functionalized by the heterogeneous method, due to the better functionalization degree obtained with the homogeneous method. Also we can observe that values of d and l for the functionalized SBA-15 are higher and lower, respectively, than values observed for the functionalized MCM-41. These results confirm a higher efficiency in the functionalization of the SBA-15, probably due to its higher pore diameter.
Thermal stability of the modified mesoporous silicas has been established by thermogravimetric analysis. The TGA profiles indicate a comparable stability of the new materials, independently of the synthetic method employed. As can be seen in Fig. 9 and 10, the TGA curves of the different modified materials prepared (MBT-SBA-15-Het, MBT-MCM-41-Het, MBT-SBA-15-Hom and MBT-SBA-15-Hom) are essentially identical. They show that degradation process occurs between 275–600 °C and the weight loss is about 16–25%, due to the breakage of pendant groups anchored on the silica surface (exothermic process). The mass loss observed for modified silicas via homogeneous method (MBT-SBA-15-Hom and MBT-MCM-41-Hom) is in agreement with the amount of ligand covalently bound to the support (Table 2), calculated by elemental analysis. However, for the samples prepared by the heterogeneous route (MBT-SBA-15-Het and MBT-MCM-41-Het), a higher weight loss than expected value is observed. An explanation for this is the presence on the silica surface of unreacted chloropropyl pendant chains. The thermal stability of these samples is according with previous results shown in the literature for other functionalized mesoporous silicas.1,34,35
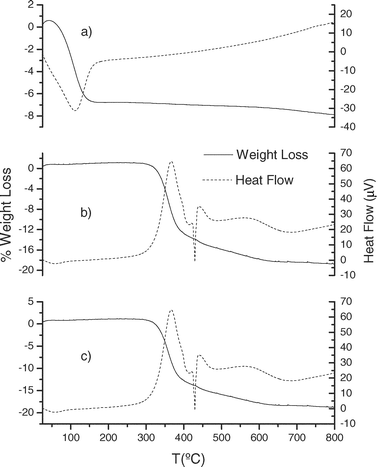 | ||
| Fig. 9 Thermogravimetric curves and heat flow of (a) activated SBA-15, (b) MBT-SBA-15-Het and (c) MBT-SBA-15-Hom. | ||
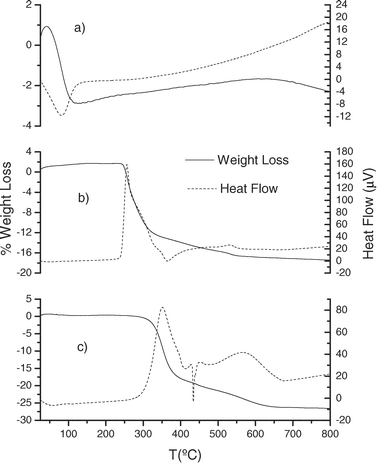 | ||
| Fig. 10 Thermogravimetric curves and heat flow of (a) activated MCM-41, (b) MBT-MCM-41-Het and (c) MBT-SBA-15-Hom. | ||
The chemical stability of the functionalized mesoporous silicas was examinated in acidic and basic solutions. Each sample was mixed with 1, 3 and 6 M HCl or NaOH solution and kept under agitation at ambient temperature during 24 h. The change in the functionalization degree was calculated by elemental analysis of the samples before and after the chemical treatment. As can be seen in Table 4, after acid treatment (6 M HCl) the percentage of nitrogen in the functionalized mesoporous silicas only experience a slightly decrease. The high stability exhibited by the attached organofunctional group presumably is due to the –(CH2)3– group which binds the MBT to the mesoporous silica surface. It has been shown that when the length of the hydrocarbon bridge was more than two methylene groups, the rupture of Si–C bond did not occur in mineral acid medium, since longer chains no longer have a functional handle that can undergo beta-elimination of the Si cation.36,37 On the other hand, functionalized mesoporous silicas were not stable in alkali solutions due to the breaking of the Si–O–Si bonds by hydroxide ions attack.26
| Nitrogena (% weight) | Nitrogen/mmol g−1 | Carbona (% weight) | Carbon/mmol g−1 | L o b/mmol g−1 | C/Nc | |
|---|---|---|---|---|---|---|
| a Average of two determinations ± standard deviation. b L o = milimoles of ligand per gram of functionalized mesoporous silica = (% nitrogen × 10)/M. atomic nitrogen. c C/N = mmol g−1 C/mmol g−1 N. | ||||||
| MBT-SBA-15-Het | 0.17 ± 0.00 | 0.12 | 2.62 ± 0.07 | 2.18 | 0.12 | 17.6 |
| MBT-SBA-15-Hom | 1.22 ± 0.01 | 0.87 | 10.72 ± 0.03 | 8.93 | 0.87 | 10.3 |
| MBT-MCM-41-Het | 0.33 ± 0.00 | 0.24 | 6.05 ± 0.01 | 5.04 | 0.24 | 21.2 |
| MBT-MCM-41-Hom | 1.68 ± 0.01 | 1.20 | 14.22 ± 0.04 | 11.85 | 1.20 | 9.9 |
The capacity of the modified silicas to extract Hg(II) ions from aqueous solutions at pH 6 was determined in triplicate, using a batchwise process. The amount of mercury loaded by the synthesized materials was determined by means of the equation:
Following this procedure, the capacity to remove mercury by different modified mesoporous silicas (MBT-SBA-15-Het, MBT-MCM-41-Het, MBT-SBA-15-Hom and MBT-MCM-41-Hom) was determined by Nf calculation from aqueous solution, we have done the studies of adsorption of mercury in buffer a pH 6 because at this pH the adsorption is maximum. At pH greater than 6, metal hydrolysis and breakage of the covalent bond between surface hydroxyls groups and the silylating agent may occur; also free silanol groups become active at higher pH and can contribute also in the metal adsorption process.
As can be seen in Table 5, in both cases, mercury adsorption was higher in the mesoporous silicas prepared by the homogeneous method (MBT-SBA-15-Hom and MBT-MCM-41-Hom). The higher amount of molecules with basic properties anchored by the homogenous route justified the better values obtained versus the heterogeneous route (Table 2). The maximum adsorption value (0.24 ± 0.02 mmol Hg(II) g−1) was obtained for MBT-SBA-15-Hom. Comparing these results with the Nf obtained for a amorphous silica (MS-3040, SBET = 400 m2 g−1) functionalized with the same ligand by both methods, MBT-MS-3040-Het and MBT-MS-3040-Hom (unpublished data), it is clear that the use of mesoporous silica, with higher surface area, controllable pore size and narrow pore size distribution) improve the Hg(II) adsorption from aqueous media. These results also clearly improve the Nf values obtained by other authors using impregnated silica gel with MBT (0.012 mmol of Hg(II) adsorbed per gram of material)21 or polystyrene treated clays impregnated with MBT (0.013 and 0.021 mmol of Hg(II) adsorbed per gram of material).22,23
| Preparation | N f a |
|---|---|
| a Adsorption capacity (mmol of Hg(II) adsorbed per gram of functionalized slica). Average of three determination ± standard deviation. | |
| MBT-MS-3040-Het | 0.08 ± 0.01 |
| MBT-MS-3040-Hom | 0.13 ± 0.02 |
| MBT-SBA-15-Het | 0.10 ± 0.01 |
| MBT-SBA-15-Hom | 0.24 ± 0.02 |
| MBT-MCM-41-Het | 0.13 ± 0.01 |
| MBT-MCM-41-Hom | 0.21 ± 0.02 |
Reported Hg(II) adsorption data in aqueous media using functionalized mesoporous silica are scarce and the literature of the field is difficult to understand or even confusing if the reader is not up to date. Usually, the functionalization of the supports is achieved by one step reaction of silanol groups with 3-mercaptopropyltrialkoxysilane and 3-aminopropyltrialkoxysilane. On the other hand, when mesoporous silica has been modified using a new ligand, commercially unavailable, the heterogeneous method has been preferably used. In this case, the alkylamine, alkylchlorine or alkylthiol monolayer covalently bound to the support reacts with a chelating ligand in a two step procedure.17 The range of data reported for surface coverage oscillates between the 0.49 mmol g−1 for HMS-T15 modified with 3-aminoproyltrimethoxisilane38 and 5.2 mmol g−1 for MCM-41 modified with 3-mercaptoproyltrimethoxisilane.4 The loading capacity data published are again quite varied, with a maximum Nf reported of 3.06 mmol Hg per gram of mesoporous silica obtained by Chen et al.10 for MCM-41 coated with ≡Si–(CH2)3SH and a minimum of 0.38 mmol Hg per gram of mesoporous silica obtained by Liu et al.13 for SBA-15 coated with ≡Si–(CH2)3NH2. Using MCM-41 functionalized by the heterogeneous method with a commercially unavailable ligand, 1-allyl-3-propylthiourea, Antochshuk and Jaroniec17 reported a maximum adsorption capacity of 1.5 mmol Hg per gram of functionalized mesoporous silica. Nevertheless, it is clear the influence of the physical characteristics of the silica support, the ligand nature grafted onto the silica and the functionalization method on the metal uptake capacity of these materials.
Conclusions
The mercaptobenzothiazole ligand, with sulfur and nitrogen donor atoms, is a suitable reagent for the preparation of surface monolayers by functionalization of well ordered mesoporous materials (SBA-15 and MCM-41). The synthetic methods used, homogeneous and heterogeneous methods, yield different types of materials with different Hg(II) adsorption capacity. The surface coverage of covalently bonded donor ligands and the Hg(II) adsorption capacity show by the materials prepared via the homogeneous route is higher than those prepared via the heterogeneous one. This fact suggests a better applicability of such mesoporous silica supports to extract Hg(II) from aqueous solutions. Also these materials are potentially useful in chemical separation process of metals classified as borderline or hard acids.Acknowledgements
The authors acknowledge the Projects GCO-2003-20 and PPR-2004-48 from the Universidad Rey Juan Carlos and the CAM Projects 07N/011/2002 and GR/MAT/0929/2004 for financial support.References
- B. Lee, Y. Kim, H. Lee and J. Yi, Microporous Mesoporous Mater., 2001, 50, 77 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard and S. B. McCullen, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS.
- L. Mercier and Y. J. Pinnavaia, Adv. Mater., 1997, 9, 500 CrossRef CAS.
- B. J. Liu, X. Feng, G. E. Fryxell, L.-Q. Wang, A. Y. Kim and M. Gong, Chem. Eng. Technol., 1998, 21(1), 97 CrossRef CAS.
- B. J. Liu, X. Feng, G. E. Fryxell, L.-Q. Wan, A. Y Kim and M. Gong, Adv. Mater., 1998, 10(2), 161 CrossRef CAS.
- L. Mercier and T. J. Pinnavaia, Environ. Sci. Technol., 1998, 32, 2749 CrossRef CAS.
- J. Brown, L. Mercier and T. J. Pinnavaia, Chem. Commun., 1999, 69 RSC.
- X. Chen, X. Feng, J. Liu, G. E. Fryxell and M. Gong, Sep. Sci. Technol., 1999, 34(6&7), 1121 CrossRef CAS.
- P. Price, J. Clark and D. Macquarrie, J. Chem. Soc., Dalton Trans., 1999, 101 Search PubMed.
- J. Brown, R. Richer and L. Mercier, Microporous Mesoporous Mater., 2000, 37, 41 CrossRef.
- A. M. Liu, K. Hidajat, S. Kaki and D. Y. Zhao, Chem. Commun., 2000, 1145 Search PubMed.
- R. I. Nooney, M. Kayanaraman, G. Kennedy and E. J. Maginn, Langmuir, 2001, 17, 528 CrossRef CAS.
- A. Bibby and L. Mercier, Chem. Mater., 2002, 14, 1591 CrossRef CAS.
- H.-J. Im, C. E. Barnes, S. Daí and Z. Xue, Microporous Mesoporous Mater., 2004, 70, 57 CrossRef CAS.
- V. Antochshuk and M. Jaroniec, Chem. Commun., 2002, 258 Search PubMed.
- G. E. Fryxell, H. Wu, Y. Lin, W. J. Shaw, J. C. Birnbaum, J. C. Linehan, Z. Nie, K. Kemmer and S. Kelly, J. Mater. Chem., 2004, 14, 3356 RSC.
- G. E. Fryxell, Y. Lin, S. Fiskum, J. C. Girnbaum, H. Wu, K. Kemner and S. Kelly, Environ. Sci. Technol., 2005, 39(5), 1324 CrossRef CAS.
- K. Terada, K. Morimoto and T. Kiba, Bull. Chem. Soc. Jpn., 1980, 53, 1605.
- K. Terada, K. Matsumoto and H. Kimura, Anal. Chim. Acta, 1983, 153, 237 CrossRef CAS.
- N. L. Dias Filho, P. L. Wagner and Y. Gushikem, Talanta, 1995, 42, 1031 CrossRef.
- N. L. Dias Filho, Y. Gushikem and P. L. Wagner, Anal. Chim. Acta, 1995, 306, 167 CrossRef.
- D. D. Perrin, W. L. Armaredo and D. R. Perrin, Purification of laboratory chemicals, Pergamon Press, Oxford, 2nd edn, 1980 Search PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120(24), 6024 CrossRef CAS.
- M. V. Landau, S. P. Varkey, M. Herskowitz, O. Regev, S. Pevzner, T. Sen and Z. Luz, Microporous Mesoporous Mater., 1999, 33, 149 CrossRef CAS.
- R. P. Paradkar and R. R. Wiliams, Anal. Chem., 1994, 66, 2752 CrossRef.
- E. Pretsch, T. Clerc, J. Seibl and W. Simon, Tablas para la elucidación estructural de compuestos orgánicos por métodos espectroscópicos, Alhambra, Madrid, 1991 Search PubMed.
- D. Pavia, G. Lampman and G. Kriz, Introduction to Spectroscopy, Harcourt College Publishers, Fort Worth, Texas, USA, 2001 Search PubMed.
- D. W. Sindorf and G. E. Maciel, J. Am. Chem. Soc., 1981, 103, 4263 CrossRef CAS.
- D. W. Sindorf and G. E. Maciel, J. Am. Chem. Soc., 1983, 105, 3767 CrossRef CAS.
- R. W. Linton, M. L. Miller, G. E. Maciel and B. L. Hawkins, Surf. Interface Anal., 1985, 7(4), 196 CrossRef CAS.
- N. L. Dias Filho, Colloids Surf., A, 1998, 144, 219 CrossRef CAS.
- D. J. Macquarrie, D. B. Jackson, J. E. G. Mdoe and H. J. Clark, New J. Chem., 1999, 23, 539 RSC.
- L. Bois, A. Bonhommé, A. Ribes, B. Pais, G. Raffin and F. Tessier, Colloids Surf., A, 2003, 221, 221 CrossRef CAS.
- P. Roumeliotis, A. A. Kurganov and V. A. Davankov, J. Chromatogr., 1983, 266, 439 CrossRef CAS.
- G. V. Kudryavtsev, D. V. Milchenko, S. Z. Bernadyuk, T. E. Vertinskaya and G. V. Lisichkin, Theor. Exp. Chem. USSR, 1987, 23(6), 711 Search PubMed.
- Y. Kim, B. Lee and J. Yi, Sep. Sci. Technol., 2004, 39(6), 1427 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: IR, 1H NMR, 13C NMR and mass spectra. See DOI: 10.1039/b507983g |
| This journal is © The Royal Society of Chemistry 2006 |