Interplay between lead carboxylate and Ti or Zr isopropoxides in solution routes to perovskites: synthesis, molecular structures and reactivity of single source non-oxo Pb–Zr and Pb–Ti carboxylatoalkoxides supported by 2-ethylhexanoate ligands
Anne Brethona, Liliane G. Hubert-Pfalzgraf*a and Jean-Claude Daranb
aUniversité C. Bernard Lyon1, IRC, 2 Avenue A. Einstein, 69 626, Villeurbanne Cedex, France
bLCC, 205 route de Narbonne, 31077, Toulouse, France. E-mail: Hubert@.univ-lyon1.fr
First published on 3rd November 2005
Abstract
The reactions between Ti(OiPr)4 and Zr2(OiPr)8(HOiPr)2, respectively, and lead 2-ethylhexanoate Pb(O2CC7H15)2 have been investigated at rt and by heating. The initial mixed-metal species, characterized by single-crystal X-Ray diffraction, were adducts namely Pb4Zr4(µ-O2CR′)8(µ-OR)6(µ3-OR)2(OR)8(OHR)21 and Pb2Ti4(µ-O2CR′)4(µ-OR)6(µ3-OR)2(OR)82 (R′ = CHCH(Et)C2H4Me, R = iPr) independently of the stoichiometry used. They are the first Pb–Ti and Pb–Zr non-oxo carboxylatoalkoxides reported. 1 is also the first Pb–Zr species based on an alkoxide-carboxylate ligand set matching the PbZrO3 stoichiometry. Both structures are centrosymmetric with six-coordinate transition metals, as required for the perovskite, and are based on triangular M2Pb cores (M = Zr, Ti). The lead centers display quite high coordination numbers, six and seven. The thermal and hydrolytic condensation reactions of 1 and 2 were investigated. Heat treatment of 2 and elimination of the volatiles under vacuum afforded Pb2Ti3(µ4-O)(µ3-O)(µ-O2CC7H15)2(µ-OiPr)6(OiPr)43 resulting from extrusion of Ti(OiPr)4 and scrambling of carboxylate ligands. Characterization of the various compounds was achieved by elemental analysis, FT-IR, 1H and 207Pb NMR.
Introduction
The PbZrxTi1−xO3 (PZT) ceramic remains one of the most studied ferroelectric due to the diversity of its applications, actuators, sensors, piezoelectric devices to name but a few.1 Chemical routes such as MOCVD (Metal Organic Chemical Vapour Deposition),2 sol–gel processing3 or MOD (Metal Organic Deposition)4 have been used for access to coatings. The usual solution routes are based on easily accessible lead carboxylates and metal alkoxides using various solvents and/or additives such as diols or β-diketones for instance for improving stability or allowing patterning.5 Lead acetate and/or its hydrate was thus often associated to various alkoxides in 2-methoxyethanol.3 Long-chain carboxylates such as 2-ethylhexanoates are the choice precursors for MOD.4 Reactions between lead 2-ethylhexanoate and n-butoxides of group 4 metals are a system of easy commercial access which has the advantage to avoid the drawback of the use of 2-methoxyethanol.6 Reproducibility for industrial processes requires the use of solutions with a minimum of preparation as well as a better understanding of relationships between structure–processing–properties. Structurally characterized mixed-metal Pb–Ti and Pb–Zr heterometallics are generally based on ethoxide or isopropoxide- and acetate ligand sets.1b,7 Typical compounds are Pb2Ti4(µ-O)2(µ-OAc)2(OEt)148 and Pb2Zr4(µ-O)2(µ-OAc)4(OEt)129 for the ethoxide but Pb2Ti2(µ4-O)(µ-OAc)2(OiPr)8 and PbZr3(µ4-O)(µ-OAc)2(OiPr)10 for the isopropoxide derivatives.10a Studies on alkoxide routes and isolation of Pb2Ti2(µ4-O)(OiPr)10, Pb4Zr2(OiPr)16, Pb3M(µ4-O)(OiPr)8 (M = Ti, Zr) and PbZr(OtBu)6 have confirmed the influence of the metal and of the OR ligand on the stoichiometry of the Pb–Ti or Pb–Zr species.11 They have also confirmed the difficulty to accede to Pb–Zr species of 1 : 1 stoichiometry. Studies devoted to the influence of the carboxylate ligands remain scarce and limited to the Pb–Ti system.10bWe wish to report herein the study of the molecular constitutions of solutions of lead 2-ethylhexanoate and titanium or zirconium isopropoxides. The first Pb–Ti and Pb–Zr mixed-metal carboxylatoalkoxides whose formulae correspond to simple adducts were isolated and structurally characterized as Pb4Zr4(µ-O2CR′)8(µ-OR)6(µ3-OR)2(OR)8(OHR)21 and Pb2Ti4(µ-O2CR′)4(µ-OR)6(µ3-OR)2(OR)82 (R′ = CHCH(Et)C2H4Me, R = iPr). Their thermal and hydrolytic transformations were investigated. An oxo species of unusual stoichiometry Pb2Ti3(µ4-O)(µ3-O)(µ-O2CC7H15)2(µ-OiPr)6(OiPr)43, was isolated. The various compounds were characterized by elemental analysis, FT-IR, multinuclear NMR (1H and 207Pb) and for the powders derived from hydrolysis by TGA and XRD.
Results and discussion
Reactions between lead 2-ethylhexanoate and zirconium or titanium isopropoxides at rt
The highly viscous, long chain lead carboxylate, Pb(O2CC7H15)2 reacts almost immediately at rt in toluene with zirconium or titanium isopropoxides. However, differences in reactivity were observed. Whereas Zr2(OiPr)8(HOiPr)2 reacts with [Pb(O2CC7H15)2]m giving a species of 1 : 1 stoichiometry, shown to be Pb4Zr4(O2CC7H15)8(OiPr)16(HOiPr)21 (eqn (1)), a species of 1 : 2 stoichiometry Pb2Ti4(O2CC7H15)4(OiPr)162 was obtained with Ti(OiPr)4 (eqn (2); R′ = C7H15).12 No formation of ester was detected. 1 was isolated in 61% yield by crystallisation in isopropanol. 2 was obtained with either one or two equivalents of Ti(OiPr)4 reacting with the lead carboxylate although the yield was improved, up to 76%, in the later case.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb(O2CR′)2 + 2 Pb(O2CR′)2 + 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr2(OiPr)8(HOiPr)2
→ Pb4Zr4(O2CR′)8(OiPr)16(HOiPr)2 + 2 Zr2(OiPr)8(HOiPr)2
→ Pb4Zr4(O2CR′)8(OiPr)16(HOiPr)2 + 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PriOH PriOH | (1) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb(O2CR′)2 + 4 Pb(O2CR′)2 + 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti(OiPr)4
→ Pb2Ti4(O2CR′)4(OiPr)16 Ti(OiPr)4
→ Pb2Ti4(O2CR′)4(OiPr)16 | (2) |
Their spectroscopic data are collected in Table 1. The FT-IR spectra of 1 show absorption bands at 1562, 1422 and 1410 cm−1 attributed to the νas and νs stretching vibrations of the CO2 moiety, respectively. The νasCO2 stretching vibrations are shifted to higher frequencies with respect to those of lead 2-ethylhexanoate (1519 cm−1). Similar features are observed for 2. The differences ΔνasCO2 − νsCO2, in the range 125–155 cm−1, suggest that the 2-ethylhexanoate ligands are bridging or bridging-chelating for both compounds.13 The νMOR absorptions are observed between 600 and 400 cm−1. An additional feature for the spectra of 1 is the absorption band at 3390 cm−1 suggesting the presence of alcohol in the metal coordination sphere.
| 1H NMR (CDCl3) | IR (cm−1) | IR | ||||
|---|---|---|---|---|---|---|
| Compound | T/°C | CH(carb) | OCH(iPr) | CH3 (iPr) | νsCO2, νasCO2 | νMOR |
| For 1: νOH 3390 cm−1. | ||||||
| 1 | 25 | 2.06 (m, 16 H) | 5.15 (sept, J = 6 Hz), 4.59 (sept, J = 6 Hz), 4.28 (sept, J = 6 Hz), 4.05 (br) (4 : 4 : 8 : 2, 18H) | 1.18–1.20 (d, 108 H, J = 6 Hz) | 1562s, 1533sh, 1410s | 644sh, 559s, 462s |
| −20 | 2.06 (m, 16 H) | 5.03, 4.54, 4.26, 4.03 (sept, J = 6 Hz) (4 : 4 : 8 : 2, 18H) | 1.18–1.20 (d, 108 H, J = 6 Hz) | |||
| 2 | 25 | 2.05 (m, 8H) | 5.42, 4.86, 4.45 (sept, J = 6 Hz) (4 : 8 : 4, 16H) | 1.3–1.35 (d, 96 H, J = 6 Hz) | 1562s, 1535sh, 1415s | 594s, 545m, 520m, 476m |
| −58 | 2.07 (m, 8H) | 5.72 (sept, J = 6 Hz), 4.85 (sept, J = 6 Hz), 4.68 (sept, J = 6 Hz) (4 : 8 : 4, 16H) | 1.3–1.42 (d, 96H, J = 6 Hz) | |||
| 3 | 25 | 2.1 m (2H) | 5.38 (sept, J = 6 Hz), 5.05 (sept, J = 6 Hz), 4.86 (sept, J = 6 Hz), 4.75 (sept, J = 6 Hz) (2 : 2 : 4 : 2, 10 H) | 1.25–1.21 (d, 60 H, J = 6 Hz) | 1582vs, 1560sh, 1535sh, 1421s | 603vs, 549s, 500sh, 471m |
The proton NMR spectra of 1 and 2 in CDCl3 confirm the presence of both isopropoxide and carboxylate ligands as well as their relative stoichiometry. The carboxylate resonances are quite broad and mostly uninformative. For 1, the methine groups of the isopropoxides appear at rt as three well resolved septets at 5.15, 4.59, 4.28 ppm and a broad peak at 4.05 ppm, respectively, with a 4 : 4 : 8 : 2 integration ratio. These spectra are independent of the dilution accounting thus for a single molecular species. No additional resonances are observed at low temperature but the broad peak resolved into a septet, suggesting that it could correspond to isopropanol. 207Pb NMR has also been used since it can provide insights into lead coordination chemistry.14,15 The spectrum of 1 in toluene shows two main peaks, a broad one centered at 2735 ppm (Δν1/2 = 1268 Hz) and a sharper one at 2653 ppm (Δν1/2 = 397 Hz) in a 1 : 1 ratio. These chemical shifts suggest quite high coordination numbers for the lead centers. 1H NMR spectra of 2 display peaks with an OR/O2CR′ ratio of four. They resemble to those of 1 and account for at least three types of magnetically non-equivalent isopropoxide ligands with CH signals at 5.42, 4.86 and 4.45 ppm, respectively, in a 4 : 8 : 4 ratio.
| Symmetry transformation used to generate equivalent atoms:i −x + 1, −y + 1, −z + 1. | |||
|---|---|---|---|
| O1–1Zr1 | 2.190(8) | O14–Zr2 | 2.171(7) |
| O12–Zr1 | 1.934(8) | O21–Zr2 | 2.206(8) |
| O13–Zr1 | 1.920(7) | O22–Zr2 | 1.946(8) |
| O24–Zr1 | 2.239(7) | O23–Zr2 | 1.938(8) |
| O31–Zr1 | 2.082(7) | O24–Zr2 | 2.230(7) |
| O14–Zr1 | 2.189(7) | O32–Zr2 | 2.079(7) |
| Zr1–Zr2 | 3.527(1) | ||
| Pb3–Zr1 | 3.782(1) | ||
| Pb3–Zr2 | 3.771(1) | O41–Pb4 | 2.615(8) |
| Pb3–Pb4 | 4.191(1) | O41–Pb4 | 2.597(8) |
| Pb4–Pb4i | 4.257 | O43–Pb4 | 2.604(8) |
| O31–Pb3 | 2.399(6) | O44–Pb4 | 2.463(8) |
| O42–Pb3 | 2.343(8) | O32–Pb3 | 2.386(7) |
| O41–Pb3 | 2.882(8) | O45–Pb4 | 2.403(8) |
| O24–Pb3 | 2.909(7) | O46–Pb4 | 2.460(8) |
| O43–Pb3 | 2.847(7) | O46–Pb4# | 2.679(8) |
| O47–Pb4 | 2.78(1) | ||
| Zr2–O14–Zr1 | 108.0(3) | ||
| Zr2–O24–Zr1 | 104.2(3) | ||
| Zr1–O31–Pb3 | 114.9(3) | ||
| Zr2–O32–Pb3 | 115.1(3) | ||
| O42–Pb3–O32 | 82.2(3) | O42–Pb3–O24 | 127.7(2) |
| O42–Pb3–O31 | 92.6(3) | O32–Pb3–O24 | 65.1(2) |
| O32–Pb3–O31 | 109.3(2) | O31–Pb3–O24 | 64.3(2) |
| O42–Pb3–O41 | 48.0(3) | O41–Pb3–O24 | 158.6(2) |
| O32–Pb3–O41 | 126.1(3) | O43–Pb3–O31 | 156.1(3) |
| O31–Pb3–O41 | 94.3(2) | ||
| O13–Zr1–O12 | 99.0(3) | O31–Zr1–O11 | 167.4(3) |
| O13–Zr1–O31 | 98.6(3) | O14–Zr1–O11 | 83.4(3) |
| O12–Zr1–O31 | 97.5(3) | O13–Zr1–O24 | 165.5(3) |
| O13–Zr1–O14 | 94.9(3) | O12–Zr1–O24 | 95.1(3) |
| O12–Zr1–O14 | 163.9(3) | O31–Zr1–O24 | 82.8(3) |
| O31–Zr1–O14 | 88.4(3) | O14–Zr1–O24 | 70.7(3) |
| O13–Zr1–O11 | 91.7(3) | O11–Zr1–O24 | 85.5(3) |
| O12–Zr1–O11 | 88.0(3) | ||
| O23–Zr2–O22 | 100.8(4) | O22–Zr2–O21 | 89.9(3) |
| O23–Zr2–O32 | 99.9(3) | O32–Zr2–O21 | 167.2(3) |
| O22–Zr2–O32 | 97.8(3) | O14–Zr2–O21 | 83.7(3) |
| O23–Zr2–O14 | 162.0(3) | O23–Zr2–O24 | 92.1(3) |
| O22–Zr2–O14 | 95.4(3) | O22–Zr2–O24 | 166.4(3) |
| O32–Zr2–O14 | 85.5(3) | O32–Zr2–O24 | 83.9(3) |
| O23–Zr2–O21 | 88.5(3) | O14–Zr2–O24 | 71.2(3) |
| O21–Zr2–O24 | 86.2(3) | ||
| Symmetry code to generate equivalent atoms:i x, −y + 1/2, z. | |||
|---|---|---|---|
| Pb1–O31 | 2.331(5) | ||
| Pb1–O33 | 2.366(5) | ||
| Pb1–O32 | 2.395(5) | ||
| Pb1–O34 | 2.803(6) | ||
| Pb1–O34i | 2.909(7) | ||
| Pb1–O24 | 2.849(6) | ||
| Ti1–O13 | 1.795(5) | ||
| Ti1–O12 | 1.804(5) | Ti2–O23 | 1.785(5) |
| Ti1–O31 | 1.973(5) | Ti2–O22 | 1.805(4) |
| Ti1–O14 | 2.021(5) | Ti2–O32 | 1.963(5) |
| Ti1–O11 | 2.081(5) | Ti2–O14 | 2.071(4) |
| Ti1–O24 | 2.137(5) | Ti2–O24 | 2.075(5) |
| Ti2–O21 | 2.096(5) | ||
| Ti1–Ti2 | 3.308(2) | ||
| Pb1–Ti2 | 3.658(2) | ||
| Pb1–Ti2 | 3.630(2) | ||
| Pb1–Pb1i | 4.617(2) | ||
| O31–Pb1–O33 | 92.1(2) | O32–Pb1–O34 | 125.4(2) |
| O31–Pb1–O32 | 106.5(2) | O31–Pb1–O34 | 109.9(2) |
| O33–Pb1–O32 | 85.2(2) | O33–Pb1–O34 | 121.2(2) |
| O31–Pb1–O34 | 104.2(2) | O32–Pb1–O34 | 133.1(2) |
| O33–Pb1–O34 | 49.5(2) | ||
| O13–Ti1–O12 | 98.8(2) | O11–Ti1–O24 | 87.9(2) |
| O13–Ti1–O31 | 95.1(2) | ||
| O12–Ti1–O31 | 95.7(2) | O23–Ti2–O22 | 97.2(2) |
| O13–Ti1–O14 | 163.8(2) | O23–Ti2–O32 | 96.1(2) |
| O12–Ti1–O14 | 96.4(2) | O22–Ti2–O32 | 96.9(2) |
| O31–Ti1–O14 | 89.2(2) | O23–Ti2–O14 | 95.3(2) |
| O13–Ti1–O11 | 87.5(2) | O22–Ti2–O14 | 166.2(2) |
| O12–Ti1–O11 | 90.5(2) | O32–Ti2–O14 | 87.3(2) |
| O31–Ti1–O11 | 172.7(2) | O23–Ti2–O24 | 166.8(2) |
| O14–Ti1–O11 | 86.5(2) | O22–Ti2–O24 | 95.4(2) |
| O13–Ti1–O24 | 93.3(2) | O32–Ti2–O24 | 86.0(2) |
| O12–Ti1–O24 | 167.8(2) | O14–Ti2–O24 | 71.7(2) |
| O31–Ti1–O24 | 85.3(2) | O23–Ti2–O21 | 88.4(2) |
| O14–Ti1–O24 | 71.5(2) | O22–Ti2–O21 | 90.1(2) |
| O32–Ti2–O21 | 171.1(2) | ||
| O14–Ti2–O21 | 84.7(2) | ||
| Ti2–O32–Pb1 | 113.8(2) | ||
| Ti1–O14–Ti2 | 107.8(2) | ||
| Ti2–O24–Ti1 | 103.5(2) | ||
| Ti1–O31–Pb1 | 114.8(2) | ||
![ORTEP view of 1 with atom labelling for O, Zr and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [1 −
x, 1 −
y, 1 −
z].](/image/article/2006/DT/b513142c/b513142c-f1.gif) | ||
| Fig. 1 ORTEP view of 1 with atom labelling for O, Zr and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [1 − x, 1 − y, 1 − z]. | ||
![ORTEP view of 2 with atom labelling for O, Ti and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [x, −y + 1/2, z].](/image/article/2006/DT/b513142c/b513142c-f2.gif) | ||
| Fig. 2 ORTEP view of 2 with atom labelling for O, Ti and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [x, −y + 1/2, z]. | ||
The basic heterometallic building block (BB) of 1 is a triangular PbZr2(OR)4(µ-OR)3(µ3-OR)(µ-O2CR′) unit. Two of them are assembled via a Pb2(O2CR′)4(OHR)2 moiety (Pb–Pb distances of 4.22 Å av.) into a centrosymmetric Pb4Zr4 array. All zirconium centers are six-coordinate. The lead atoms belonging to the BB, Pb3, are six-coordinate whereas those who ensure the junctions between the BB via a Pb2O2 ring, Pb4, are seven coordinate as often observed for lead in a carboxylate ligands environment. All lead centers have a highly distorted stereochemistry. The surrounding of Pb3 corresponds to a trigonal prism whereas that of Pb4 corresponds to a pentagonal bipyramid with O43 and O47 in the axial positions. Distortions are due to the various intracyclic angles [O32–Pb3–O31 of 109.3(2) and 68.2(3)° for O46i–Pb4–O46 for instance] as well as to the small bite angles [48.0(3)–53.0(3)°] of the bridging-chelating carboxylates. The lone pairs on lead appear stereochemically inactive with no obvious vacancy in the first coordination sphere. 1 can thus be considered as holodirected.16 Although the hydrogen' could not be located, analysis of the M–OR bond lengths on Zr and Pb centers suggest that isopropanol is linked to Pb4 with a bond distance of 2.78(1) Å. Large variations are observed for the Pb–O bond distances [2.343(8)–2.909(7) Å], the longest one corresponding to the Pb–µ3-OR linkages. Zr–O bond distances spread over the range 1.920(7)–2.230(7) Å. The Zr⋯Zr distance with a value of 3.527(1) Å is slightly longer than for Zr2(OiPr)8(iPrOH)2.17 The Zr–O–C angles of the terminal isopropoxides are large [163.3(1)–171.4(9)°] as common for zirconium alkoxides.18 A short contact [Pb4⋯C45 of 2.81(1) Å] is observed with one of the C of the carboxylate ring.
The structure of 2 is also centrosymmetric and based on triangular Ti2Pb(O2CR′)(OR)8 units similar to those observed for 1. The main difference with 1 lies in the connection between the BB. For 2, the BB are linked by their lead atoms via bridging-chelating µ,η2-carboxylate ligands and its overall stoichiometry is thus that of the BB. This results in a slightly asymmetrical bridge [2.803(6) and 2.909(7) Å] and in six-coordinate lead centers due to Pb–µ3-OR bonds. The Ti–O bond distances range from 1.785(5) to 2.137(5) Å and vary in the order Ti–OR(t) < Ti-µ-OR < Ti–O2CR′ as expected. The M⋯M distance [3.308(1) Å] is shorter than in the case of zirconium. The Pb–O bond lengths vary from 2.331(5) to 2.909(7) Å, the longest ones corresponding to the carboxylate and µ3-OR bridges. The Ti–O–C angles of the terminal OR are smaller than for 1 as usually observed.10
Triangular BB are quite common for heterometallics based on a MM′2 stoichiometry although they are often capped by two triply bridged ligands.19 A large number of heterometallic species involving zirconium are based on the [Zr2(OR)9]− moiety.18,20 An alternative description of the structures of 1 and 2 is based on the [M2(µ-OR)3(µ3-OR)(µ-O2CR′)(OR)4]− moiety trapping Pb2(O2CR′)3(ROH)+ or Pb(O2CR′)+ units, respectively. In contrast with the Pb–M heterometallic species based on acetates,10 the 2-ethylhexanoate ligands display two types of coordination modes, bridging and bridging-chelating. The related Pb–O bond distances are quite long as compared to usual Pb–O(carboxylate) ones. No solid state data are available for lead 2-ethylhexanoate but the lead carboxylates are reported as oligomers due to the assembling behavior of the O2CR′ ligands and as illustrated by lead succinate [Pb(O4C4H4)]∞ for instance.21 Metal alkoxides depolymerize metal acetates giving heterometallic acetatooxoalkoxides.10,22 Depolymerization of lead 2-ethylhexanoate is also achieved here, the difference in the frameworks of 1 and 2 reflecting the lower reactivity of Zr2(OiPr)8(iPrOH)2 as compared to Ti(OiPr)4 for formation of MM′ species.6,18,23 Reactions between anhydrous lead acetate, insoluble, and group 4 alkoxides afforded only oxo heterometallic species even for reactions achieved at rt.10 The acetate ligands usually bridge the two types of metals but the presence of oxo ligands implies more structural reorganization than here, especially in the case of zirconium isopropoxide.10a The solubility of lead 2-ethylhexanoate in non-polar media allows formation of a mixed-metal Pb–Zr species without complete breakdown of the chelating-bridging Pb carboxylate array. Another observation is that for heterometallics involving lead acetate, lead displays stereochemically active lone pairs and lower coordination numbers. It is noteworthy that adducts 1 and 2 were isolated in high yields despite the presence of water (∼0.5%) in commercial lead 2-ethylhexanoate.
Single-crystal data of 2-ethylhexanoate derivatives, often considered as metallic soaps, remain very scarce since the long chain carboxylates favor disorder and the obtaining crystals of poor quality.24 These limitations are valid here, especially for compound 1, but the solid state structures are supported by the 1H and 207Pb NMR spectra. The latter displaying two signals with a 1 : 1 integration ratio for 1 suggest that its solid state structure is retained in solution. 207Pb NMR data on seven-coordinated lead species are scarce. A chemical shift of 1667 ppm has been reported for heptacoordinate lead in Pb6(O2CiPr)12.15 The data obtained for 1 indicate only a difference of 100 ppm for the six- and seven fold-coordinate lead centers. The 1H NMR data of 1 and 2 suggest that the chemical shifts of the µ3-OR and µ-OR(MM) ligands are similar or that the quite long Pb–µ3-OR bonds are broken in solution, reducing the coordination number of the corresponding lead atoms to five in solution. Compounds 1, 2 and 3 (see below) are, to the best of our knowledge, the first examples of mixed-metal species with 2-ethylhexanoate ligands.
Reactivity studies of the Pb–Zr and Pb–Ti species
The obtaining of homogeneous solutions by hydrolysis allows some 1H NMR monitoring in CDCl3. Hydrolysis was immediate for 1 and 2. The resulting spectra were characterized in the CH region of the OiPr ligands by decrease of the initial peaks, apparition of new sets of resonances as well as apparition (for 2) or increase (for 1) of the peak of isopropanol. The latter shifts to higher frequencies with the extent of hydrolysis suggesting exchange between coordinated and free alcohol molecules. The first hydrolytic steps are likely to be intramolecular as observed for BaZr4(OR)18.25 and to proceed at the electrophilic metals, zirconium or titanium and on their bridging alkoxide ligands.26 This should affect the µ3-OR and transform the [PbM2(µ3-OR)(µ-OR)(µ-OR)2(µ-O2CR′)(OR)4]+ building blocks units into [PbM2(µ3-O)(µ-OR)2(µ-O2CR′)(OR)4]+ ones. This is confirmed by the development of species having two types of OR ligands in a 8 : 4 integration ratio in both cases, namely 4.90, 4.70 and 5.38, 5.68 ppm for 1a (M = Zr) and 2a (M = Ti), respectively. This transformation would leaves the transition metals M five-coordinate, quite unlikely for zirconium with OiPr ligands. However, the chemical shift of isopropanol suggests coordination to the transition metals of some of the alcohol molecules eliminated by hydrolysis. Eqn (3) and (4) summarizes these first hydrolytic steps whereas Scheme 1 represents structures of 1a and 2a based on six-coordinate transition metals in agreement with the 1H NMR data. All attempts to isolate 1a or 2a in crystalline form were unsuccessful.
1 + 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O → Pb4Zr4(µ3-O)2(µ-O2CR′)8(OR)12(ROH)x (1a) + (6 −
x) H2O → Pb4Zr4(µ3-O)2(µ-O2CR′)8(OR)12(ROH)x (1a) + (6 −
x)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ROH ROH | (3) |
2 + 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O → Pb2Ti4(µ3-O)2(µ-O2CR′)4(OR)12(ROH)x (2a) + (4 −
x) H2O → Pb2Ti4(µ3-O)2(µ-O2CR′)4(OR)12(ROH)x (2a) + (4 −
x)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ROH ROH | (4) |
 | ||
| Scheme 1 | ||
1a and 2a were also detected, in small amounts (∼5%) in the reaction medium. Their presence, while no ester was detected, is most likely due to hydrolysis by the traces of water in commercial lead 2-ethylhexanoate.12
Hydrolysis at higher hydrolysis ratio is likely to affect µ-OR(PbM) and terminal OR ligands, the condensation becoming intermolecular and leading to more drastic structural modifications. For instance, hydrolysis at h = 8 of 1 gives spectra displaying the peak of free isopropanol, two main septuplets at 4.68 and 4.28 ppm and numerous smaller broad peaks (up to eight of similar area spreading over the range 6.1–4.20 ppm at −20 °C) belonging to a same species as shown by dilution experiments. These observations account for formation of oligomers due to extensive condensation with a large number of magnetically non-equivalent OR ligands. The last hydrolytic steps involve elimination of some 2-ethylhexanoic acid (FT-IR evidence). Elimination of acid is also confirmed by the obtaining of a powder analyzing as PbZrO(O2CR′)(ROH) by hydrolysis at rt of 1 by an excess of water.
The PbZrO3 (PZ) perovskite is one of least stable and thus difficult to obtain lead based perovskite.6 Hydrolysis of 1, whose stoichiometry matches that of PZ, was thus also achieved with an excess of water (h = 70) in isopropanol. FT-IR data of the amorphous powder obtained show residual carboxylate ligands (νasCO2 1540, νsCO2 1409 cm−1) as expected for differential hydrolysis.7 Its TGA and DTA patterns display several strong exothermic peaks for combustion of the residual organics which occurs in quite mild conditions (250–450 °C). A total weight loss of 37% suggests that the powder has a composition PbZrO(O2CR′)(ROH) (theoretical loss 37.8%). Annealing of the powder under air afforded a crystalline material. Crystallization starts around 450 °C and was analyzed in terms of formation of pyrochlore.6 Crystallization of the PZ perovskite starts around 550 °C and a pure perovskite phase is obtained at 600 °C. Thus the use of a single-source precursor (SSP) allows to avoid segregation and gives access to crystalline PZ at ∼100 °C lower than by using mixtures in which no homogeneity at a molecular level is achieved.6 Hydrolysis of 2 affords PbTi3O7 as well as red lead oxide as observed for the previously reported PbTi2 species.10a
Condensation of the Pb2Ti4 species, 2, is also faster than that of the Pb4Zr4 one 1 since no more evolution of 2 was observed after heating for 6 h at 120 °C. After removal of the volatiles of that reaction medium under vacuum (60 °C/10−3 mm Hg), compound 3 could be crystallized in ∼25% by adding isopropanol to the crude product. Elemental analyses account for a Pb/Ti ratio of 2 : 3. This change in the Pb/Ti stoichiometry is confirmed by isolation of titanium isopropoxide by fractional distillation of the volatiles. The FT-IR spectrum of 3 accounts for an oxo species as illustrated by absorption bands at 837, 784, 704 cm−1. Its 1H NMR spectra indicate a 10 : 2 ratio for the OiPr:O2CR′ ligands and four signals in the methine region at 5.38, 5.05, 4.86 and 4.35 ppm in a 2 : 2 : 4 : 2 ratio. The 207Pb NMR data show two sharp peaks of comparable intensities at 3005 and 2682 ppm.
Despite the unusual M2M′3 stoichiometry, the structure of compound 3, Pb2Ti3(µ4-O)(µ3-O)(µ-O2CC7H15)2(µ-OiPr)6(OiPr)4 appears quite symmetrical (Fig. 3). Selected bond distances and angles are collected in Table 4. 3 can be seen as a Pb2Ti2O2(µ-O2CR′)2(OR)6 species having two types of oxo ligands namely a central µ4 one O2 and a peripheral one O1 which acts as ligand toward a Ti(OiPr)4 moiety leading to a five-coordinate Ti. Such coordination of an alkoxide by a peripheral oxo ligand has been observed for Pb6Nb4O4(OEt)24.27 This µ3-oxo ligand O1 has a quite regular planar trigonal geometry. In contrast, the stereochemistry of the tetragonal oxo ligand is distorted with an increase in the Ti2⋯Ti2 distance up to 3.608 Å (as compared to 2), as well as an opening of the Ti2O2Ti2 angle [up to 136.2(4)°] for accommodation of the two bridging carboxylates. The Ti–O bond distances spread over the range 1.791(7) to 2.112(6) Å with the ranking Ti–OR < Ti-µ3O ≈ Ti-µOR< Ti-µ4-O < Ti-µO2CR′. The Pb–O bond distances are longer [2.161(4)–2.555(6) Å] and vary along Pb-µ3O < Pb-µ4O < Pb–OR but they are shorter than for 1 and 2 due to the lower coordination numbers of lead. The Pb–OR–Ti bridges are quite asymmetrical. In contrast with 1 and 2, the lone pairs of the five-coordinate tetragonal pyramidal lead centers are stereochemically active. Compound 3 can also be seen as the association of two PbO units, Ti(OR)4 and Ti2(O2CR′)2(OR)6.
| Ti1–O3 | 1.791(7) | O1–Ti1 | 1.873(7) |
| Ti1–O8 | 1.959(6) | O6–Ti2 | 1.805(6) |
| O4–Ti2 | 2.093(6) | O9–Ti2 | 2.112(6) |
| O5–Ti2 | 1.894(6) | O2–Ti2 | 1.944(3) |
| O8–Ti1 | 1.959(6) | O7–Ti2 | 1.896(6) |
| O5–Pb1 | 2.506(6) | O2–Pb1 | 2.462(4) |
| O8–Pb1 | 2.548(6) | O7–Pb1 | 2.555(6) |
| O1–Pb1 | 2.161(4) | ||
| Ti2–Ti2 | 3.608(2) | ||
| Ti1–Pb1 | 3.498(2) | ||
| Ti2–Pb1 | 3.478(2) | ||
| Ti2–Pb1 | 3.497(2) | ||
| Ti2–O5–Pb1 | 103.6(2) | Ti2–O2–Pb1 | 104.46(9) |
| Ti1–O8–Pb1 | 101.0(2) | Ti2–O2–Pb1 | 103.64(9) |
| Ti1–O1–Pb1 | 120.1(2) | Ti2–O2–Pb1 | 104.46(9) |
| Pb1–O1–Pb1 | 119.8(3) | Pb1–O2–Pb1 | 98.8(2) |
| Ti2–O2–Ti2 | 136.2(4) | Ti2–O7–Pb1 | 102.6(2) |
| O3–Ti1–O3 | 105.3(4) | O6–Ti2–O2 | 169.6(3) |
| O3–Ti1–O1 | 127.4(2) | O5–Ti2–O2 | 87.8(2) |
| O3–Ti1–O1 | 127.4(2) | O7–Ti2–O2 | 88.3(2) |
| O3–Ti1–O8 | 97.5(3) | O6–Ti2–O4 | 86.0(3) |
| O3–Ti1–O8 | 97.1(3) | O5–Ti2–O4 | 172.2(3) |
| O1–Ti1–O8 | 77.9(2) | O7–Ti2–O4 | 89.4(3) |
| O1–Ti1–O8 | 77.9(2) | O6–Ti2–O9 | 85.7(3) |
| O8–Ti1–O8 | 155.8(3) | O5–Ti2–O9 | 89.1(3) |
| O6–Ti2–O5 | 98.7(3) | O7–Ti2–O9 | 172.5(3) |
| O6–Ti2–O7 | 99.0(3) | O2–Ti2–O9 | 86.3(2) |
| O5–Ti2–O7 | 95.9(3) | O4–Ti2–O9 | 85.1(3) |
| O1–Pb1–O2 | 70.7(2) | ||
| O1–Pb1–O5 | 94.1(2) | ||
| O2–Pb1–O5 | 64.8(1) | ||
| O1–Pb1–O8 | 60.9(2) | ||
| O2–Pb1–O8 | 131.5(2) | ||
| O5–Pb1–O8 | 114.5(2) | ||
| O1–Pb1–O7 | 93.4(2) | ||
| O2–Pb1–O7 | 64.5(1) | ||
| O5–Pb1–O7 | 122.2(2) | ||
| O8–Pb1–O7 | 118.8(2) | ||
![ORTEP view of 3 with atom labelling for O, Ti and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [−x, y, −z + 1/2].](/image/article/2006/DT/b513142c/b513142c-f3.gif) | ||
| Fig. 3 ORTEP view of 3 with atom labelling for O, Ti and Pb atoms (ellipsoids at 30% probability). C atoms are represented as sphere of arbitrary radius. H atoms as well as the disordered C atoms are omitted for clarity. Symmetry code i: [−x, y, −z + 1/2]. | ||
The formation of 3 results from a drastic reorganization of compound 2 by elimination of Ti(OR)4 as summarized by eqn (5). Spectroscopic data (1H NMR, FT-IR) of the crude product obtained after heating of 2 before elimination of volatiles gave evidence for traces of titanium isopropoxide. However, the passage from a Pb2Ti4 stoichiometry to a Pb2Ti3 one is favored by the treatment under vacuum and the volatility of Ti(OiPr)4. The 2-ethylhexanoate ligands linked to lead appear more labile than those on titanium. This lability and the tendency to form oxo species was also observed by long heating of Pb(O2CR′)2 during its synthesis from lead oxide. 1H and 207Pb NMR spectra account for the retention of the solid state structure of 3 by dissolution in non-polar media.
 | (5) |
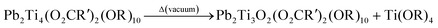 | (6) |
Experimental
All manipulations were performed under argon using Schlenk tubes and vacuum line techniques with solvents purified by standard methods. Lead 2-ethylhexanoate (Strem) was used as received. Ti(OiPr)4 (Aldrich) was purified by distillation and Zr2(OiPr)8(iPrOH)2 was synthesized as reported.17 Hydrolyses were achieved at rt in THF or in the parent alcohol. Water was added via the same solvent. The resulting powders were separated by filtration. 1H and 207Pb NMR spectra (52.3 MHz) were recorded on a Bruker AC-250 spectrometer. Lead chemical shifts are given with respect to Pb(NO3)2 as external reference. IR spectra were run on a Paragon 500 FT-IR spectrometer, they were obtained as Nujol mulls for the air-sensitive species, as KBr pellets for the hydrolyzed ones. Analytical data were obtained from the Centre de Microanalyses du CNRS. TGA/DTA data were collected on a Setaram 92 system in air with a thermal ramp of 5 °C min−1. XRD were obtained with a Siemens D 500 diffractometer (Cu-Kα radiation). Spectroscopic data (NMR, FT-IR) are collected in Table 1.Synthesis of Pb4Zr4(O2CC7H15)8(OiPr)16(HOiPr)2 (1)
[Zr(OiPr)4(HOiPr)]2 (1.67 g, 4.31 mmol) in toluene was added to Pb(O2CC7H15)2 (2.13 g, 4.31 mmol) in 10 ml of toluene. The medium was stirred at rt for 10 h. Elimination of the volatiles in vacuo gave an oil. Addition of isopropanol gave 1 as colorless crystals at −10 °C [2.25 g, 61%/Pb(O2CC7H15)2]. 1 was poorly soluble in isopropanol, more soluble in toluene or hexane. Anal. Calc. For C118H248O34Pb4Zr4: (3260.52) C, 41.63; H, 7.34, Pb, 24.34; Zr, 10.72. Found: C, 41.85; H, 7.45; Pb, 25.03; Zr, 11.20%. 207Pb{1H} NMR (toluene, ppm): 2735 (broad), 2653 (1 : 1).Synthesis of Pb2Ti4(O2CC7H15)4(OiPr)162
The same procedure applied to Ti(OiPr)4 (2.87 ml, 9.66 mmol), Pb(O2CC7H15)2 (2.38 g, 4.83 mmol) in 20 ml of toluene and addition of iPrOH–hexane (1 2 in volume) gave 2 (3.92 g, 76%/Pb(O2CC7H15)2). 2 was poorly soluble in hexane, more soluble in toluene and isopropanol. Anal. Calc. For C80H174O24Pb2Ti4 (2120.12): C, 45.19; H, 8.25; Pb; 19.46; Ti, 9.01. Found: C, 45.54; H, 8.32; Pb, 20.82; Ti, 9.45%. 207Pb{1H} NMR (toluene, ppm): 2944Synthesis of Pb2Ti3O2(O2CC7H15)2(OiPr)103
Ti(OiPr)4 (1.82 ml, 6.12 mmol) was added to Pb(O2CC7H15)2 (1.51 g, 3.06 mmol). After heating at 120 °C for 6 h, the yellow oil was distilled (60 °C/10−3 mm Hg) to remove the by-products. Addition of 6 ml of isopropanol to the residue gave a solid 3 at −20 °C (0.75 g, 0.51 mmol, 25%/Ti, 33%/Pb). 3 was soluble in isopropanol, THF and hydrocarbons. Anal. Calc. for C46H100O16Ti3Pb2 (1463.30): C, 37.65; H, 6.87; Pb, 28.24; Ti, 9.79, Found C 38.05, H, 6.93, Pb 28.72, Ti, 9.83%. 207Pb{1H} NMR (toluene, ppm): 3005 (65 Hz), 2682 (85 Hz) (1 : 1).Crystal structure determination of 1, 2 and 3
Single crystals of 1 and 3 were obtained from isopropanol, those of 2 were obtained in isopropanol–hexane. They were mounted under inert perfluoropolyether on a glass fiber and cooled in the cryostream of the diffractometer. Data were collected at 180(2) K using the monochromatic Mo-Kα radiation. The structures were solved by direct methods (SIR97)28 and refined by least-squares on F2 using SHELXL-97.29 All H atoms attached to carbon were introduced in idealized positions [d(CH) = 0.96 Å] and treated as riding models. In 3, some of the carbon atoms of the alkoxide ligands presented large ellipsoids and disordered models to better fit the electron density were applied using the available tools (PART and DFIX) in SHELXL-97. In 2, owing to the low number of data, the C atoms were refined isotropically. As in 3, some of the alkyl chains were disordered and treated accordingly. In 1, the anisotropic thermal parameters for C atoms are very large but no disordered models could be defined. The drawings were done with ORTEP-32.30 Crystal data and refinement parameters are shown in Table 5.| 1 | 2 | 3 | |
|---|---|---|---|
| a R1 = ∑‖Fo| − |Fc‖/∑|Fo|, wR2 = [∑[w(Fo2 − Fc2)2]/∑[w(Fc2)2]]1/2. | |||
| Empirical formula | C108H224O34Pb4Zr4 | C80H168O24Pb2Ti4 | C46H96O16Pb2Ti3 |
| Mr | 3260.52 | 2120.03 | 1463.3 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) | C2/c |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| a/Å | 11.377(1) | 11.3722(6) | 21.663(2) |
| b/Å | 13.118(1) | 12.4435(6) | 14.231(1) |
| c/Å | 26.190(3) | 19.303(1) | 22.737(2) |
| α/° | 85.559(8) | 91.624(4) | 90 |
| β/° | 83.667(7) | 96.393(4) | 116.342(8) |
| γ/° | 76.410(8) | 109.728(5) | 90 |
| V/Å3 | 3771.0(6) | 2549.0(2) | 6281.7(9) |
| Z | 1 | 1 | 4 |
| Diffractometer | Oxford X-CALIBUR CCD | Stoe IPDS | Oxford X-CALIBUR CCD |
| µ(Mo-Kα)/mm−1 | 4.772 | 3.650 | 5.762. |
| No. of unique reflections (Rint) | 10765(0.0674) | 6680 (0.0848) | 4824 (0.0485) |
| Absorption correction | Analytical | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Data/restraints/parameters | 10765/772/704 | 6680/480/544 | 4824/15/219 |
| Goodness-of-fit on F2 | 1.069 | 1.059 | 1.081 |
| R1, wR2 [I > 2σ(I)]a | 0.0438, 0.1165 | 0.0554, 0.1484 | 0.0432, 0.1156 |
| R1, wR2 (all data) | 0.0568, 0.1225 | 0.0637, 0.1586 | 0.0553, 0.1216 |
CCDC reference numbers 230776–230778 for 2, 3 and 1, respectively.
For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513142c
Conclusion
The molecular constitution of solutions of Ti and Zr alkoxides and lead carboxylates is function of the alkoxide as well as of the carboxylate ligands. The first non-oxo Ti–Pb and Zr–Pb carboxylatoalkoxides have being obtained at rt with 2-ethylhexanoate as carboxylate ligands. heir stoichiometry is 1 : 1 in the case of zirconium but 1 : 2 for titanium, independently of the ratio between the reagents. Compound 1, Pb4Zr4(µ-O2CR′)8(µ-OR)6(µ3-OR)2(OR)8(OHR)2, is thus the first Pb–Zr carboxylatoalkoxide of 1 : 1 stoichiometry reported and thus matching the formula of the PbZrO3 (PZ) ceramic, this stoichiometry being not accessible with acetate as ligands. Evaluation of their condensation by hydrolysis and by heating shows that formation of extensive arrays is faster by hydrolysis. The volatility of Ti(OiPr)4 can promote a change in the stoichiometry of the Pb2Ti4 species by heating giving the Pb2Ti3(µ4-O)(µ3-O)(µ-O2CC7H15)2(µ-OiPr)6(OiPr)4 oxo species 3. Compounds 1, 2 and 3 are, to the best of our knowledge, the first structurally characterized mixed-metals species with 2-ethylhexanoate ligands. The Pb–Zr single source precursor favors access to PZ at low temperature.Acknowledgements
We are grateful to Protavic for financial support.References
- (a) N. Setter, J. Eur. Ceram. Soc., 2001, 21, 1279 CrossRef CAS; (b) C. D. Chandler, C. Roger and J. M. Hampden-Smith, Chem. Rev., 1993, 93, 1205–1241 CrossRef CAS; (c) S. T. Swartz and V. E. Wood, Condens. Matter News, 1992, 1, 4–28 Search PubMed.
- C. H. Peng and S. B. Desu, J. Am. Ceram. Soc., 1994, 77, 1799 CAS; T. Katoyama, M. Fujimato, M. Shimizu and T. Shiosaki, J. Cryst. Growth, 1991, 115, 289 CrossRef.
- C. E. Lakeman and D. Payne, J. Am. Ceram. Soc., 1992, 75, 309; T. J. Boyle, D. Dimos, R. W. Schwartz, T. M. Alam, M. B. Sinclair and C. D. Buchheit, J. Mater. Res., 1997, 12, 1022–1030 CrossRef CAS; R. W. Schwartz, J. A. Voigt, B. A. Tutle, D. A. Payne, T. L. Reichert and R. S. Dasalla, J. Mater. Res., 1997, 12, 444–456 CrossRef CAS; T. Fukui, C. Sakurai and M. Okuyama, J. Mater. Res., 1992, 7, 791–794 CrossRef CAS.
- P. Gaucher, J. Hector, J. C. Kurfiss, in Science and Technology of Electroceramic Thin Films, ed. O. Auciello and R. Waser, Kluwer, Dordrecht, Netherlands, 1995, p. 147 Search PubMed; S. Morlens, L. Ortega, B. Rousseau, S. Phok, J. L. Deschanvres, P. Chaudouet and P. Odier, Mater. Sci. Eng. B, 2003, 104, 185 Search PubMed; A. A. Avey and R. H. Hill, J. Am. Chem. Soc., 1996, 118, 237–238 CrossRef.
- M. L. Calzada, F. Carmona, R. Sirera and B. Jimenez, J. Am. Ceram. Soc., 1995, 78, 1802 CrossRef CAS; R. F. Zhang, J. Ma, M. B. Kong, Y. S. Chen and T. S. Zhang, Mater. Let., 2002, 55, 388–393 Search PubMed; M. L. Calzada, A. Gonzalez, R. Poyato and L. Pardo, J. Mater. Chem., 1993, 13, 1451 Search PubMed.
- S. P. Faure, O. Chaux and P. Gaucher, J. Phys. IV, 1998, 8(Pr 9-6), 1929–37 Search PubMed; S. P. Faure, P. Barboux, P. Gaucher and J. Livage, J. Mater. Chem., 1992, 2, 713–717 RSC.
- L. G. Hubert-Pfalzgraf, J. Mater. Chem., 2004, 14, 3113–3123 RSC; L. G. Hubert-Pfalzgraf, Inorg. Chem. Commun., 2003, 6, 102–120 CrossRef CAS , and references therein.
- H. K. Chae, D. A. Payne, Z. Xu and L. Ma, Chem. Mater., 1994, 6, 1589–1592 CrossRef CAS.
- L. Ma and D. A. Payne, Chem. Mater., 1994, 6, 875–877 CrossRef CAS.
- (a) L. G. Hubert-Pfalzgraf, S. Daniele, R. Papiernik, M. C. Massiani, B. Septe, J. Vaissermann and J. C. Daran, J. Mater. Chem., 1997, 7, 753–762 RSC , and references therein; (b) L. Spiccia, B. O. West and Q. Zhang, Polyhedron, 1998, 17, 1851–1861 CrossRef CAS.
- J. Teff, J. C. Huffman and K. G. Caulton, Inorg. Chem., 1996, 35, 2981–2987 CrossRef CAS; D. J. Teff and K. G. Caulton, Inorg. Chem., 1998, 37, 2554–2562 CrossRef CAS.
- Although commercial lead 2-ethylhexanoate contains ∼0.5% in weight of water, it will be written as Pb(O2CC7H15)2 for clarity.
- G. B. Deacon and R. J. Philipps, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS.
- J. J. Dechter, Prog. Inorg. Chem., 1982, 29, 285 CAS; R. K. Harris, J. J. Kennedy and W. McFarland, NMR and the Periodic Table, ed. R. K. Harris and B. E. Mann, Academic Press, New York, 1978 Search PubMed.
- G. D. Fallon, L. Spiccia, B. O. West and Q. Zhang, Polyhedron, 1997, 16, 19–23 CrossRef CAS.
- L. Shinoni-Livny, I. P. Glusker and C. W. Bock, Inorg. Chem., 1998, 37, 1853 CrossRef CAS.
- B. A. Vaarstra, J. C. Huffman, P. S. Gradeff, L. G. Hubert-Pfalzgraf, J. C. Daran, S. Parraud, K. Yunlu and K. G. Caulton, Inorg. Chem., 1990, 29, 3126 CrossRef.
- D. C. Bradley, R. C. Mehrotra, I. P. Rothwell, A. Singh, Alkoxo and Aryloxo Derivatives of Metals, Academic Press, London, 2001 Search PubMed; N. Y. Turova, E. P. Turevskaya, V. G. Kessler, M. I. Yanovskaya, The Chemistry of Metal Alkoxides, Kluwer, London, 2002 Search PubMed.
- K. G. Caulton and L. G. Hubert-Pfalzgraf, Chem. Rev., 1990, 90, 969–995 CrossRef CAS.
- M. Veith and S. Mathur, Polyhedron, 1998, 17, 1005–1034 CrossRef CAS.
- M. R. St, J. Foreman, M. J. Plater and J. M. S. Skakle, J. Chem. Soc., Dalton Trans., 2001, 1987–1903 Search PubMed.
- S. Boulmaaz, R. Papiernik, L. G. Hubert-Pfalzgraf, B. Septe and J. Vaissermann, J. Mater. Chem., 1997, 7, 2053–61 RSC; L. G. Hubert-Pfalzgraf, Polyhedron, 1994, 13, 1181–1195 CrossRef.
- S. Daniele, L. G. Hubert-Pfalzgraf, J. C. Daran and S. Halut, Polyhedron, 1994, 13, 927–932 CrossRef CAS; B. Malic, I. Arcon, A. Kodrc and M. Kosev, J. Sol–Gel Sci. Technol., 1999, 16, 135–141 CrossRef CAS.
- L. Junran, H. Chunhui, L. Biaoguo, X. Guangxian and X. Gaodeng, Huaxue Xuebao, 1990, 11, 226; S. T. Warzeska, M. Zonnenveld, R. van Gorkum, W. J. Muizebelt, E. Bouwman and J. Reedijk, Prog. Org. Coat., 2002, 44, 243–248 CrossRef CAS.
- B. A. Vaarstra, J. C. Huffman, W. E. Streib and K. G. Caulton, Inorg. Chem., 1991, 30, 3068–72 CrossRef.
- F. Biechel, J. Dubuc and M. Henry, New J. Chem., 2004, 28, 764–69 RSC.
- R. Papiernik, L. G. Hubert-Pfalzgraf, J. C. Daran and Y. Jeannin, J. Chem. Soc., Chem. Commun., 1990, 965–66 RSC.
- A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori and R. Spagn, SIR97 a program for automatic solution of crystal structures by direct methods, J. Appl. Crystallogr., 1999, 32, 115 CrossRef CAS.
- G. M. Sheldrick, SHELXL97: Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed.
- L. J. Farrugia, ORTEP-32 for Windows, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
