Synthesis of anionic methylpalladium complexes with phosphine–sulfonate ligands and their activities for olefin polymerization†
Takuya
Kochi
,
Kenji
Yoshimura
and
Kyoko
Nozaki
*
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, Tokyo 113-8656, Japan. E-mail: nozaki@chembio.t.u-tokyo.ac.jp; Fax: +81 3 5341 7261; Tel: +81 3 5841 7261
First published on 14th November 2005
Abstract
Reaction of diarylphosphinobenzene-2-sulfonic acids with tertially amines, followed by addition of [PdMeCl(cod)], provided anionic methylpalladium(II) complexes with bidentate phosphine–sulfonate ligands, which show high activity for copolymerization of ethylene with methyl acrylate.
Olefin polymerization with late transition-metal complexes has attracted much interest1 due to their lower oxophilicity relative to early transition metals, and many catalysts have been developed to polymerize various olefins and to copolymerize non-polar olefins with carbon monoxide2 or with polar olefins.3 In order to achieve higher activity and further understanding of the reaction mechanism, structurally-characterized organometallic complexes have been isolated in many cases before the polymerization. In the case of palladium catalysts with bidentate ligands, the corresponding monomethyl complexes4 have generally been recognized as convenient catalyst precursors, because simple generation (Scheme 1) of a vacant site at the cis-position to the methyl group, followed by coordination of an olefin, would readily initiate the coordination polymerization. These methylpalladium complexes have also been frequently used for mechanistic investigation of the copolymerization.
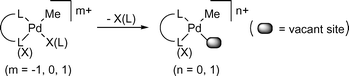 | ||
| Scheme 1 Generation of a vacant site on monomethyl palladium complexes with bidentate ligands. | ||
In the past decade, significant efforts have been devoted to developing late transition-metal catalysts capable of producing highly functionalized polymers by copolymerization of non-polar olefins with readily-available polar olefins. For example, Brookhart's diimine palladium complexes can copolymerize ethylene or propylene with polar vinyl monomers such as methyl acrylate.3c,d The copolymers produced were highly branched and methyl acrylate was incorporated only at the chain end.
In 2002, Pugh and coworkers have developed palladium catalysts which can produce unique polymers such as a highly linear copolymer of ethylene and alkyl acrylates5a and a non-perfectly alternating copolymer of ethylene and CO.5b In their reports, the palladium catalysts were formed in situ by combination of diarylphosphinobenzene-2-sulfonic acid with Pd(dba)2 or Pd(OAc)2. Very recently, Rieger et al. reported the first examples of structurally-characterized palladium complexes with bidentate phosphine–sulfonate ligands, which gave higher catalytic activity and a higher number of extra ethylene insertions in the non-perfectly alternating copolymerization of ethylene and CO.6 To improve the catalyst system, our group has concurrently attempted to synthesize palladium complexes with diarylphosphinobenzene-2-sulfonic acids. Herein we report the first synthesis of methylpalladium(II) complexes with bidentate phosphine–sulfonate ligands. Preliminary results for the copolymerization of ethylene and methyl acrylate are also described.
Treatment of diphenylphosphinobenzene-2-sulfonic acid 1a with diisopropylethylamine in dichloromethane, followed by addition of PdMeCl(cod), afforded the anionic methylpalladium complex 2a in 94% yield (Scheme 2).7 Similarly, the reaction of o-methoxyphenyl substituted ligand 1b gave methylpalladium complex 2b in 92% yield. When triethylamine was used instead of diisopropylethylamine for the reaction of 1a, the corresponding methylpalladium complex 2a′ was also obtained in 89% yield.
 | ||
| Scheme 2 Preparation of anionic methylpalladium(II) complexes with phosphine–sulfonate ligands. | ||
The structure of 2a′ was determined by X-ray crystallography‡ to reveal that the phosphine–sulfonate ligand coordinates to palladium in a bidentate fashion, similar to Rieger's dicyclopentadienylethoxy palladium complex (Fig. 1). The palladium complex adopts a square planar geometry and the methyl group is located at the trans-position to the oxygen atom of the sulfonate. It is interesting that a chloride ion is bound to the palladium to form an anionic methylpalladium complex, considering simple loss of the chloride ion would give a neutral complex. The synthesis of 2a can also be performed in acetonitrile, meaning that acetonitrile did not replace the chloride ion on the palladium. Anionic methylpalladium(II) complexes are relatively rare, and as far as we know, all of the previously reported examples possess a dianionic ligand and a methyl group on palladium.8 The nitrogen atom of the ammonium ion is in close contact with the chloride (3.27 Å) and the ammonium hydrogen should be located at a reasonable position for a hydrogen bond with the chloride ligand.
 | ||
| Fig. 1 Structure of complex 2a′. Hydrogen atoms are omitted for clarity. Thermal ellipsoids are shown at the 50% probability level. Selected bond lengths (Å) and angles (Å): Pd(1)–C(1) 2.051(4); Pd(1)–O(1) 2.184(3); Pd(1)–P(1) 2.2195(12); Pd(1)–Cl(1) 2.3997(12); Cl(1)⋯N(1) 3.268(5). | ||
Notable temperature dependence was observed in the 1H NMR spectra of complex 2b. At room temperature the two methoxy groups of 2b gave one sharp singlet at 3.58 ppm, which split into two broad signals at 3.49 and 3.66 ppm on cooling at −60 °C. This behavior can be accounted for by considering the conformational interchange of the aryl groups which are arranged in an unsymmetric structure as disclosed by the crystallographic study on complex 2a′ (Scheme 3).
 | ||
| Scheme 3 Conformational interchange of the aryl groups of 2b. | ||
Copolymerization of ethylene and methyl acrylate was then investigated with catalyst 2 (Table 1). When the copolymerization was carried out at 80 °C with NaBArf4, 2b showed higher activity than 2a, as is consistent with Pugh's result (entry 2, 3).5a,9,10 Screening of additives such as NaBArf4 and AgOTf was performed to examine the effectiveness in removing the chloride ion from the palladium metal of 2b. Effect of the additives on the copolymerization was quite notable. For example, the catalytic activity of the copolymerization with NaBArf4 at 80 °C (entry 2) was twice as high as that reported by Pugh with Pd(dba)2 and 1b in the same reaction conditions,5a while the reactions with AgOTf or no additive showed much lower activities (entry 6, 8). However, the highest comonomer incorporation was observed with no additive, and particularly, the reaction at 80 °C provided the copolymer with 16% of methyl acrylate incorporated in the polymer chain (entry 8). An increase in reaction temperature generally leads to an enhancement in catalytic activity and a reduction of polymer molecular weight, but no gain or a decrease in the activity was observed at high temperature possibly due to decomposition of the catalyst (entry 1, 6, 8). The highest molecular weight (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) was obtained in the reaction with no additive at 50 °C for 72 h. Addition of galvinoxyl had little influence on the activity of the reaction with no additive at 80 °C, while significantly reduced activity (4 g mmol−1 h−1) was observed for the reaction with NaBArf4. Espinet and co-workers have reported that galvinoxyl not only serves as a radical trap but can react with a Pd–H bond.11 The observed reduction of activity with NaBArf4 can be attributed to the frequent chain transfer reaction via
β-hydride elimination and generation of a Pd–H bond, relative to those in the reaction with no additive.
500) was obtained in the reaction with no additive at 50 °C for 72 h. Addition of galvinoxyl had little influence on the activity of the reaction with no additive at 80 °C, while significantly reduced activity (4 g mmol−1 h−1) was observed for the reaction with NaBArf4. Espinet and co-workers have reported that galvinoxyl not only serves as a radical trap but can react with a Pd–H bond.11 The observed reduction of activity with NaBArf4 can be attributed to the frequent chain transfer reaction via
β-hydride elimination and generation of a Pd–H bond, relative to those in the reaction with no additive.
| Entry | Catalyst | Additive | Temp/°C | Yield/g | Activity/g mmol−1 h−1 | TOF/h−1b | Methyl acrylate incorporation (mol%)c | M n | M w/Mn |
|---|---|---|---|---|---|---|---|---|---|
| a Conditions: 0.01 mmol of catalyst 2; ethylene pressure, 3.0 MPa; 2.5 mL of methyl acrylate; solvent, 2.5 mL of toluene; reaction time, 15 h. b TOF (turnover frequency): mol olefin/(mol Pd·h). c Determined by 1H NMR spectroscopy. d Copolymerization was carried out for 72 h. | |||||||||
| 1 | 2b | NaBArf4 (0.01 mmol) | 90 | 2.59 | 17 | 490 | 13 | 3200 | 1.64 |
| 2 | 2b | NaBArf4 (0.01 mmol) | 80 | 2.61 | 17 | 490 | 13 | 4100 | 1.87 |
| 3 | 2a | NaBArf4 (0.01 mmol) | 80 | 0.94 | 6.3 | 200 | 7 | 2700 | 1.46 |
| 4 | 2b | NaBArf4 (0.01 mmol) | 70 | 2.00 | 13 | 400 | 9 | 6200 | 1.54 |
| 5 | 2b | NaBArf4 (0.01 mmol) | 60 | 1.40 | 9.3 | 290 | 8 | 6100 | 2.44 |
| 6 | 2b | AgOTf (0.02 mmol) | 80 | 0.49 | 3.3 | 90 | 14 | 2400 | 1.54 |
| 7 | 2b | AgOTf (0.02 mmol) | 60 | 0.96 | 6.4 | 180 | 14 | 4500 | 1.61 |
| 8 | 2b | None | 80 | 0.24 | 1.6 | 43 | 16 | 4500 | 1.61 |
| 9 | 2b | None | 70 | 0.30 | 2.0 | 55 | 15 | 6800 | 1.61 |
| 10 | 2b | None | 50 | 0.19 | 1.3 | 35 | 14 | 8500 | 2.11 |
| 11 | 2b d | None | 50 | 0.57 | 0.8 | 22 | 13 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.79 |
In conclusion, the first syntheses of methylpalladium(II) complexes with phosphine–sulfonate bidentate ligands were achieved. An X-ray structure of 2a′ revealed that the complexes were rare examples of anionic methylpalladium(II) complexes. We further examined the copolymerization of ethylene and methyl acrylate with the catalysts and found interesting effects of additives on the catalytic activity, the molecular weight and the degree of methyl acrylate incorporation. These preliminary copolymerization results indicate that further examination of additives, ligands, bases used to prepare the catalysts, and reaction conditions would lead to an improved catalyst system for production of the unique highly linear copolymer of ethylene and methyl acrylate.
Acknowledgements
We are grateful to Prof. Takayuki Kawashima and Dr Makoto Yamashita (the University of Tokyo) for the X-ray crystal analysis.References
-
(a) G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428 CrossRef CAS
; (b) S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169 CrossRef CAS
; (c) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS
.
- C. Bianchini and A. Meli, Coord. Chem. Rev., 2002, 225, 35 CrossRef CAS
.
-
(a) L. S. Boffa and B. M. Novak, Chem. Rev., 2000, 100, 1479 CrossRef CAS
; (b) M. J. Yanjarappa and S. Sivaram, Prog. Polym. Sci., 2002, 27, 1347 CrossRef CAS
; (c) L. K. Johnson, S. Mecking and M. Brookhart, J. Am. Chem. Soc., 1996, 118, 267 CrossRef CAS
; (d) S. Mecking, L. K. Johnson, L. Wang and M. Brookhart, J. Am. Chem. Soc., 1998, 120, 888 CrossRef CAS
; (e) C. Carlini, M. Martinelli, A. M. R. Galletti and G. Sbrana, Macromol. Chem. Phys., 2002, 203, 1606 CrossRef CAS
; (f) R. T. Stibrany, D. N. Schulz, S. Kacker, A. O. Patil, L. S. Baugh, S. P. Rucker, S. Zushma, E. Berluche and J. A. Sissano, Macromolecules, 2003, 36, 8584 CrossRef CAS
; (g) X.-F. Li, Y.-G. Li, Y.-S. Li, Y.-X. Chen and N.-H. Hu, Organometallics, 2005, 24, 2502 CrossRef CAS
.
-
(a) M. Brookhart, F. C. Rix, J. M. DeSimone and J. C. Barborak, J. Am. Chem. Soc., 1992, 114, 5894 CrossRef CAS
; (b) F. C. Rix, M. Brookhart and P. S. White, J. Am. Chem. Soc., 1996, 118, 4746 CrossRef CAS
; (c) K. Nozaki, N. Sato and H. Takaya, J. Am. Chem. Soc., 1995, 117, 9911 CrossRef CAS
; (d) K. Nozaki, N. Sato, Y. Tonomura, M. Yasutomi, H. Takaya, T. Hiyama, T. Matsubara and N. Koga, J. Am. Chem. Soc., 1997, 119, 12779 CrossRef CAS
; (e) G. Verspui, F. Schanssema and R. A. Sheldon, Angew. Chem., Int. Ed., 2000, 39, 804 CrossRef CAS
; (f) C. S. Schulz, J. Ledford, J. M. DeSimone and M. Brookhart, J. Am. Chem. Soc., 2000, 122, 6351 CrossRef CAS
.
-
(a) E. Drent, R. van Dijk, R. van Ginkel, B. van Oort and R. I. Pugh, Chem. Commun., 2002, 744 RSC
; (b) E. Drent, R. van Dijk, R. van Ginkel, B. van Oort and R. I. Pugh, Chem. Commun., 2002, 964 RSC
.
- A. K. Hearley, R. J. Nowack and B. Rieger, Organometallics, 2005, 24, 2755 CrossRef CAS
.
- Rieger et al. reported that a stoichiometric reaction between PdMeCl(cod) and a diarylphosphinobenzene-2-sulfonic acid derivative did not provide any methylpalladium complex with the phophine–sulfonate ligand. See ref. 6.
-
(a) R. J. Crutchley, J. Powell, R. Faggiani and C. J. L. Lock, Inorg. Chim. Acta, 1977, 24, L15 CrossRef CAS
; (b) T. Marx, L. Wesemann, S. Dehnen and I. Pantenburg, Chem. Eur. J., 2001, 7, 3025 CrossRef CAS
; (c) L. F. Groux, T. Weiss, D. N. Reddy, P. A. Chase, W. E. Piers, T. Ziegler, M. Parvez and J. Benet-Buchholz, J. Am. Chem. Soc., 2005, 127, 1854 CrossRef CAS
.
- The 1H NMR spectrum of the volatile materials collected by vaccum transfer of the crude mixture showed no byproduct such as oligomers of ethylene and/or methyl acrylate.
- The activity of the reaction with 2b for 3 h was 29 g mmol−1 h−1. The copolymerization reaction slows down as it proceeds and the amounts of the remaining monomers decrease.
- A. C. Alvéniz, P. Espinet, R. López-Fernández and A. Sen, J. Am. Chem. Soc., 2002, 124, 11278 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data for 2a, 2a′ and 2b; X-Ray data for 2a′. See DOI: 10.1039/b512452m |
| ‡ Crystal data for 2a′: C25H33ClNO3PPdS, M = 600.40, monoclinic, a = 14.186(6) Å, b = 10.679(5) Å, c = 17.678(8) Å, β = 92.549(2)°, V = 2675.5(19) Å3, T = 120(2) K, space group P21/c, Z = 4, µ(Mo-Kα) = 0.958 mm−1, 14896 reflections measured, 4364 unique (Rint = 0.0255) which were used in all calculations. The final wR(F2) was 0.1208 (all data). CCDC reference number 283231. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b512452m |
| This journal is © The Royal Society of Chemistry 2006 |
