Ionic liquid buffers: a new class of chemicals with potential for controlling pH in non-aqueous media†
Guang-nan
Ou
ab,
Ming-xia
Zhu
a,
Jia-rong
She
a and
You-zhu
Yuan
*a
aState Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: yzyuan@xmu.edu.cn; Fax: +86 592 2183047; Tel: +86 592 2184565
bSchool of Bioengineering, Jimei University, Xiamen 361021, China
First published on 18th October 2006
Abstract
Ionic liquids with buffering characteristics, synthesized by the reaction of [RMIM]OH base moieties with phthalic and tartaric acid, respectively, are potential reagents for controlling pH in non-aqueous media; remarkable [Base]/[Acid] molar ratio dependence of the catalytic activities has been observed in the hydrogenation of olefins with [RuCl2(PPh3)3] complex in DMF and [BMIM][BF4].
It is well documented that many chemical reactions are sensitive to pH values, that is, the yield of products and even the nature of the products may be altered appreciably if the pH changes significantly during the process of reaction. This is especially true in biochemical reactions where the pH is important to the proper metabolism and functioning of animals and plants. We can efficiently control the pH of an aqueous medium at a predetermined level by the addition of water-soluble buffer substances. Nevertheless, more reports on pH control via buffers in non-aqueous media such as organic solvents and ionic liquids (ILs) are still needed. Often the reactions in non-aqueous media have been investigated without controlling the pH of the system. The use of buffering systems involving highly hydrophobic acids and their sodium salts, and highly hydrophobic bases and their hydrochlorides, has been proposed.1 Indeed, these systems can efficiently buffer organic solvent media. Unfortunately, they were found to have only limited solubility in some of the non-polar solvents commonly used for biocatalysis. To overcome this restriction, P. J. Halling et al. developed more hydrophobic buffer systems consisting of functionalized and dendritic polybenzylethers.2 These molecules are able to buffer the ionization state of enzymes in solvents as non-polar as toluene, and can be used to evaluate the “pH profile” of biocatalysts in low-water systems. Other systems such as solid-state acid–base buffers can also be used for controlling the ionization state of the enzyme in organic solvents.3
ILs are known as designed liquids with controllable physical/chemical properties and specific functions, which have been attracting considerable interest over recent years.4 However, no attention has been given to the preparation of ILs that can maintain the apparent pH for a non-aqueous system at an optimum value. We note that the titration profiles of imidazolium hydroxides ([RMIM]OH) with organic acids such as phthalic acid (H2P) or tartaric acid (H2T) in organic solvents express a buffering-like region and the region is centered on the 1 : 1 molar ratio of [RMIM]OH and organic acid (Fig. 1). Thus, we consider that it is possible to synthesize buffer-like ILs by neutralization of aqueous solutions of [RMIM]OH with aqueous solutions of H2P or H2T in a molar ratio of 1 : 1 as illustrated in Scheme 1. Using the literature methods with a slight modification, the [RMIM]OH moieties are produced by anion exchange of the corresponding imidazolium chlorides, which are obtained by the reaction of methylimidazole with an equivalent of the appropriate alkyl chloride.5 The final yields of the target compounds range from 91.1 to 92.5%.‡ The samples were characterized using 1H NMR and electrospray ionization (ESI) mass spectrometry. In addition, we have measured the differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA), melting points, ionic conductivity, and miscibilities of the samples. All the characterization data are consistent with the expected compositions and structures. We define this kind of ILs as IL buffers. Solids are obtained at room temperature when the molar ratio of [RMIM]OH and H2P or H2T is set at 2 : 1, and these show no buffering characteristics at all.
![Titration curve for 5.0 mL of 0.1 mol L−1 phthalic acid versus 0.1 mol L−1 [BMIM]OH in different organic solvents. The electrode is standardized with two aqueous primary standard buffer solutions. Because of the unknown liquid junction potential, the measurements of pH in non-aqueous solvents are referred to as “apparent pH”.](/image/article/2006/CC/b611810k/b611810k-f1.gif) | ||
| Fig. 1 Titration curve for 5.0 mL of 0.1 mol L−1 phthalic acid versus 0.1 mol L−1 [BMIM]OH in different organic solvents. The electrode is standardized with two aqueous primary standard buffer solutions. Because of the unknown liquid junction potential, the measurements of pH in non-aqueous solvents are referred to as “apparent pH”. | ||
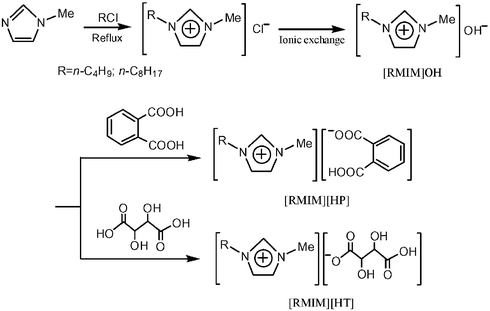 | ||
| Scheme 1 Synthesis of IL buffers. | ||
The solubility of the IL buffers in organic solvents is similar to that of conventional ILs like [BMIM][BF4] (Table 1S). They are miscible with polar solvents such as methanol, DMF, and dichloromethane and also with ILs like [BMIM][PF6] and [BMIM][BF4]. The thermal stability of the IL buffers in terms of decomposition temperature (Td) is determined by TGA. The IL buffers containing phthalate anion [HP] are more stable than that containing hydrogen tartrate [HT]. This trend indicates that the anion has a significant impact on the thermal stability of the IL buffers. The [BMIM][HT] exhibits distinct two-stage decomposition behavior, as observed in the related imidazolium salts.6 It starts to decompose at 205 °C, and 29% of the weight loss is observed up to 235 °C, which corresponds to the elimination of a CO2 moiety from this IL. Subsequently, it begins to decompose again above 235 °C, and the subsequent decomposition behavior of the residue is exactly in agreement with that of [RMIM][HP]. For a given anion, it seems that the variation in the imidazolium cation has less effect on the thermal stability of the corresponding IL buffers. The pH, dilution value, and buffer value of the IL buffers are summarized in Table 1, indicating that the behavior of the IL buffers synthesized is exactly in agreement with that of inorganic counterparts.
| IL buffer | Concentration/mol L−1 | pH | Dilution valueb | Buffer valuec |
|---|---|---|---|---|
| a Abbreviations: BMIM = 1-butyl-3-methylimidazole, OMIM = 1-octyl-3-methylimidazole, HP = hydrogen phthalate, HT = hydrogen tartrate. Data in parentheses are those of inorganic counterparts from reference 7. b Change of pH on dilution with an equal volume of water. c The buffer value is defined as the number of moles of strong base required to change the pH of one litre of solution by one unit. | ||||
| [BMIM][HP] | 0.05 | 4.01 | +0.03 (+0.052) | 0.0182 (0.016) |
| [OMIM][HP] | 0.05 | 4.01 | 0.02 | 0.0177 |
| [BMIM][HT] | 0.034 | 3.56 | 0.04 (−0.049) | 0.0307 (0.027) |
To confirm the buffering ability and exploit the usability of the ILs synthesized, a trial of hydrogenation of cyclohexene catalyzed by [RuCl2(PPh3)3]8 in non-aqueous media such as DMF and [BMIM][BF4] in the presence of such ILs with different molar ratios of [Base]/[Acid] has been carried out.§ Plots of the reaction TOF (mol–product mol–Ru−1 h−1) against the log10([Base]/[Acid]) are shown in Fig. 2. No by-products of the reactions are detected by capillary GC (SE-30, 30 m × 0.32 mm × 0.25 µm). A steep decrease in catalytic activity in terms of TOF occurs in the log10([Base]/[Acid]) range of 0.15–0.3, implying a significant effect of pH value on the reaction rate. The results are consistent with the report by F. Joó,9 where the selectivity of the aqueous–organic biphasic hydrogenation of trans-cinnamaldehyde was catalyzed by water-soluble phosphine–Ru(II) complexes. Similarly, it is implied that the likely active species [HRuCl(PPh3)3] might be favorably formed at log10([Base]/[Acid]) below 0.15, with which the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond proceeded in a higher TOF. It is also remarkable that in [BMIM][BF4] the catalytic activity does not fall to zero in log10([Base]/[Acid]) above 0.28. This observation points to somewhat more complex equilibria than a simple disappearance of [HRuCl(PPh3)3] with increasing basicity. In principle, the coordination of phthalate or tartrate cannot be ruled out either. Evidence in the hydrogenations of hexene and styrene also reveals that the catalytic activities are strongly dependent on the log10([Base]/[Acid]) in non-aqueous media as shown in Table 2. The lower reactivity of the internal olefin compared with the terminal one is analogous to what has been observed in the hydrogenation reactions by the [RuCl2(PPh3)3] complex in homogeneous catalysis.10
C bond proceeded in a higher TOF. It is also remarkable that in [BMIM][BF4] the catalytic activity does not fall to zero in log10([Base]/[Acid]) above 0.28. This observation points to somewhat more complex equilibria than a simple disappearance of [HRuCl(PPh3)3] with increasing basicity. In principle, the coordination of phthalate or tartrate cannot be ruled out either. Evidence in the hydrogenations of hexene and styrene also reveals that the catalytic activities are strongly dependent on the log10([Base]/[Acid]) in non-aqueous media as shown in Table 2. The lower reactivity of the internal olefin compared with the terminal one is analogous to what has been observed in the hydrogenation reactions by the [RuCl2(PPh3)3] complex in homogeneous catalysis.10
![Base–acid molar ratio effect on catalytic hydrogenation of cyclohexene in DMF and [BMIM][BF4]. Reaction conditions: 12.0 mg of [RuCl2(PPh3)3]; 50 mg of IL buffer with different molar ratios of [BMIM]OH and H2P; 2.00 mL of solvent; 1.00 mL of olefin; hydrogen pressure = 2.5 MPa; stirring speed = 800 rpm; temperature = 50 °C; time = 5 h.](/image/article/2006/CC/b611810k/b611810k-f2.gif) | ||
| Fig. 2 Base–acid molar ratio effect on catalytic hydrogenation of cyclohexene in DMF and [BMIM][BF4]. Reaction conditions: 12.0 mg of [RuCl2(PPh3)3]; 50 mg of IL buffer with different molar ratios of [BMIM]OH and H2P; 2.00 mL of solvent; 1.00 mL of olefin; hydrogen pressure = 2.5 MPa; stirring speed = 800 rpm; temperature = 50 °C; time = 5 h. | ||
| Substrate | Log10([Base]/[Acid]) = 0 | Log10([Base]/[Acid]) = 0.315 | ||
|---|---|---|---|---|
| Conversion/% b | TOF/h−1b | Conversion/%c | TOF/h−1c | |
| a Hydrogen pressure = 0.5 MPa. Other conditions are the same as in Fig. 2. b Reaction time = 30 min. c Reaction time = 60 min. | ||||
| Hexene | 97.8 | 1251 | 58.5 | 374 |
| Styrene | 55.3 | 769 | 40.6 | 282 |
| Cyclohexene | 2.3 | 36 | 0.7 | 6 |
The influence of the molar ratio of [Base]/[Acid] on the proton species during the hydrogenation of [RuCl2(PPh3)3] has been measured in the solutions by 1H NMR. The 1H NMR spectrum of the reaction solution buffered by [BMIM][HP] at log10([Base]/[Acid]) 0.0 in DMF shows a quartet at −17.8 ppm, which is assignable to the proton of Ru–H (Table 3), indicating the formation of the hydride species [HRuCl(PPh3)3].11 When the catalytic solution is buffered by [BMIM][HP] at log10([Base]/[Acid]) 0.315, the proton signal at −17.8 ppm is absent but a new signal appears at −28.3 ppm as a triplet. The assignment of the new resonance needs further identification. The 1H NMR measurements in [BMIM][BF4] are somewhat in agreement with those in DMF, but the spin-couplings are more complicated. Nevertheless, the results indicated that the hydride species [HRuCl(PPh3)3] formed by the hydrogenation of [RuCl2(PPh3)3] at log10([Base]/[Acid]) lower than 0.15 would be key and active for the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.
| Solvent | Log10([Base]/[Acid]) = 0.0 | Log10([Base]/[Acid]) = 0.315 | ||
|---|---|---|---|---|
| δ H (ppm) | J HP (Hz) | δ H (ppm) | J HP (Hz) | |
| DMF | −17.8 (qrt) | 24 | −28.3 (t) | 25 |
| [BMIM][BF4] | −19.0 (s) | not resolved | −28.5 (s) | not resolved |
| −32.4 (s) | not resolved | |||
| −33.1 (s) | not resolved | |||
In summary, the base moieties [RMIM]OH readily react with acids H2P and H2T, respectively, to form a kind of ILs with buffering characteristics, which have not been reported in the literature. They are potential reagents for buffering pH in non-aqueous media. A remarkable [Base]/[Acid] molar ratio dependence of the catalytic activities has been found in the hydrogenation of olefins with [RuCl2(PPh3)3] complex in DMF and [BMIM][BF4].
We acknowledge financial support from the NSFC (20021002, 20473065 and 20433030), the Key Project of Fujian Province (2005HZ01-3), the RFDP and the Key Project of the Chinese Ministry of Education (20050384011 and 106099). We thank Dr X. L. Liao, Dr Y. Liu and Dr Z. Q. Ou for their technical support in the measurements of NMR, ESI-MS, and TGA–DSC.
Notes and references
- (a) G. Carrea and S. Riva, Angew. Chem., Int. Ed., 2000, 39, 2226 CrossRef CAS; (b) K. Xu and A. M. Klibanov, J. Am. Chem. Soc., 1996, 118, 9815 CrossRef CAS; (c) N. Harper, M. Dolman, B. D. Moore and P. J. Halling, Enzyme Microb. Technol., 2001, 29, 413 CrossRef CAS; (d) A. D. Blackwood, L. J. Curran, B. D. Moore and P. J. Halling, Biochim. Biophys. Acta, 1994, 1206, 161 CAS.
- M. Dolman, P. J. Halling and B. D. Moore, Biotechnol. Bioeng., 1997, 55, 278 CrossRef CAS.
- (a) N. Fontes, N. Harper, P. J. Halling and S. Barreiros, Biotechnol. Bioeng., 2003, 82, 802 CrossRef CAS; (b) N. Harper, M. Dolman, B. D. Moore and P. J. Halling, Chem.–Eur. J., 2000, 6, 1923 CrossRef CAS; (c) M. Quiros, M. C. Parker and N. J. Turner, J. Org. Chem., 2001, 66, 5074 CrossRef CAS; (d) E. Zacharis, B. D. Moore and P. J. Halling, J. Am. Chem. Soc., 1997, 119, 12396 CrossRef CAS.
- Examples of recent reviews: (a) D. R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905 RSC; (b) J. S. Lee, Chem. Commun., 2006, 1049 RSC; (c) J. Muzart, Adv. Synth. Catal., 2006, 348, 275 CrossRef CAS; (d) C. Baudequin, D. Bregeon, J. Levillain, F. Guilen, J. C. Plaquevent and A. C. Gaumont, Tetrahedron: Asymmetry, 2005, 16, 3921 CrossRef CAS; (e) J. S. Wilkes, J. Mol. Catal. A: Chem., 2004, 214, 11 CrossRef CAS; (f) C. Bauequin, J. Baudoux, J. Levillain, D. Cahard, A. C. Gaumont and J. C. Plaquevent, Tetrahedron: Asymmetry, 2003, 14, 3081 CrossRef CAS; (g) S. T. Handy, Chem.–Eur. J., 2003, 9, 2938 CrossRef CAS; (h) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS; (i) F. Endres, ChemPhysChem, 2002, 3, 144 CrossRef CAS; (j) J. S. Wilkes, Green Chem., 2002, 4, 73 RSC; (k) D. B. Zhao, M. Wu, Y. Kou and E. Z. Min, Catal. Today, 2002, 74, 157 CrossRef CAS; (l) H. Olivier-Bourbigou and L. Magna, J. Mol. Catal. A: Chem., 2002, 182(183), 419 CrossRef.
- (a) S. K. Poole, P. H. Shetty and C. F. Poole, Anal. Chim. Acta, 1989, 218, 241 CrossRef CAS; (b) K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398 CrossRef CAS.
- Z. B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2004, 10, 6581 CrossRef CAS.
- R. G. Bates, Determination of pH: theory and practice, Wiley, New York, 2nd edn, 1973 Search PubMed.
- T. A. Stephenson and G. Wilkinson, J. Inorg. Nucl. Chem., 1965, 28, 945.
- F. Joó, J. Kovács, A. C. Bényei and A. Kathó, Angew. Chem., Int. Ed., 1998, 37, 969 CrossRef CAS.
- P. S. Hallman, B. R. McGarvey and G. Wilkinson, J. Chem. Soc. A, 1968, 3143 RSC.
- R. Abbel, K. Abdur-Rashid, M. Faatz, A. Hadzovic, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2005, 127, 1870 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization data of IL buffers, including 1H NMR, ESI-MS, TGA–DSC; the determination of melting points, ionic conductivity, miscibilities, buffer regions and other physical chemistry properties of the samples. See DOI: 10.1039/b611810k |
| ‡ General procedure for the synthesis of ionic liquid buffers: An aqueous solution of [RMIM]OH was prepared by passing the corresponding imidazolium halide ([RMIM]X) through a column filled with anion-exchange resin, as described in the literature.5 The aqueous [RMIM]OH was then neutralized with equimolar H2A (phthalic acid or tartaric acid) in a beaker and the pH of the solution was adjusted to the desired value by adding the aqueous solution of H2A. The solution was evaporated at 50 °C under reduced pressure to give a viscous liquid, which was then vacuum-dried at 50 °C for 18 h to afford the ionic liquid buffer product. |
| § Typical procedures of catalytic hydrogenation: 12.0 mg of [RuCl2(PPh3)3], 50 mg of ionic liquid with desired pH value (adjusted by varying the molar ratio of [BMIM]OH and H2P), 2.00 mL of solvent, and 1.00 mL of olefin were introduced into a stainless steel autoclave. The autoclave was flushed three times consecutively with high purity hydrogen. The autoclave was then filled with hydrogen to the desired pressure. The reaction mixture was stirred at 800 rpm at 50 °C for the required time. The reaction was quenched by putting the reactor in an ice–water bath. The reactants in the upper layer and the catalyst in the lower layer were separated by decantation. The organic phase was analyzed with a gas chromatograph equipped with an FID and a capillary column (SE-30, 30 m × 0.32 mm × 0.25 µm). |
| This journal is © The Royal Society of Chemistry 2006 |
