Biochemistry of the para-sulfonato-calix[n]arenes
Florent Perret, Adina N. Lazar and Anthony W. Coleman*
Institut de Biologie et Chimie des Protéines (IBCP UMR5086), CNRS, Université Lyon 1, IFR128 BioSciences Lyon-Gerland, 7 passage du Vercors, F69367 Lyon, France. E-mail: aw.coleman@ibcp.fr; Fax: 33 472722690; Tel: 33 472722640
First published on 10th March 2006
Abstract
The biochemistry of the para-sulfonato-calix[n]arenes has shown rapid development during the past ten years, the highly diverse biomedical applications of these molecules now include anti-viral, anti-thrombotic activities, enzyme blocking and protein complexation. The future is even more promising as para-sulfonato-calix[n]arenes have, now, been shown to have potential in the diagnosis of prion-based diseases. Their innocuous nature, as far as is known at present, may open up their future use in medications.
 Florent Perret | Florent Perret was born in 1977, obtained his first degree in chemistry at the University of Lyon 1 and moved to Montpellier to obtain his MSc in bioorganic chemistry in 1999. He received his PhD in 2002 from the University of Clermont-Ferrand, under the joint supervision of Professor N. Morel-Desrosiers and Dr. A. W. Coleman, working on the thermodynamic and structural studies of the complexation of sulfonato-calix[4]arenes with amino-acids and peptides. He joined the group of Professor S. Matile at the University of Geneva for a two year post-doctoral position where he carried out research on synthetic ion channels and transport of oligo-arginine across membranes. In September 2005 he came back to Lyon for a CNRS post-doctoral position in the group of Dr. A. W. Coleman. His present research activities focus on the synthesis of new water soluble calix[n]arenes and their application in biology. His research interests include supramolecular, bioorganic and structural chemistry of molecular assemblies. |
 Adina Nicoleta Lazar | Adina Nicoleta Lazar was born in 1978 in Romania. She graduated from the Politehnica University of Bucharest as an engineer in material science in 2002 and she continued in the scientific field with a PhD in Lyon, France, in the laboratory of Assemblages Moléculaires d'Intérêt Biologique under the direction of Dr. A. W. Coleman. She has just defended her PhD thesis on Structural and Atomic Force Microscopy Characterisation of Supramolecular Assemblies. She is looking forward to continuing her research activities in the bio-physical domain. |
 Anthony William Coleman | Dr. Anthony William Coleman was born in Bradford, UK in 1953, he graduated from the University of Oxford in 1976 with a BA in Chemistry. He obtained a DPhil from the University of Sussex in 1980, where he worked, under the supervision of Professor M. J. Lappert, on the synthesis of chiral organometallic carbenes. Following post-doctoral positions at the University of Rennes and the University of Victoria, working in the area of coordination chemistry, he joined the research group of Professor Jerry L. Atwood at the University of Alabama in 1982, here he introduced calixarene chemistry into the group. In 1987 he moved to the Pharmacy Faculty of the University of Paris-Sud, joining the CNRS to develop research on the cyclodextrins, and particularly in the field of amphiphilic derivatives. In 1994, Tony Coleman moved to the Institut de Biologie et Cheimie de Protéines, Lyon where he directs the research group, Assemblages Moléculaires d'Intérêt Biologique. The current research topics of the group involve the biology and biochemistry of the calixarenes both as water soluble and amphiphilic systems; interfacial supramolecular chemistry, protein assemblies and Scanning Probe Microscopy. The group has a very strong axis towards the applications of the calixarenes in pharmaceutical and medical science. |
Introduction
The calix[n]arenes form with the crown ethers and the cyclodextrins one of the major classes of macrocyclic organic host compounds in supramolecular chemistry.1–6 They are easily prepared from the reaction of tert-butylphenol and formaldehyde, with the choice of base, solvent and reaction temperature allowing the selective synthesis of macrocycles containing four, six or eight phenolic units. Possessing distinctly different chemistries at the phenolic face and the para-aromatic position, their chemical modification, while sometimes non-trivial, is relatively straightforward. As a consequence, the range of calix[n]arene derivatives far exceeds that of other classes of host systems.Discovered in 1872 by von Bayer,7 their macrocyclic structure was ascertained in 1944 by Zinke and Ziegler.8 However, it was not until 1984, that the first water soluble calix[n]arene derivative was synthesized.9 In the same year, the first paper on the para-sulfonato-calix[n]arenes was published by Shinkai,10 these derivatives were the first having high aqueous solubility. In 1988, the first structural study of the sodium salt of para-sulfonato-calix[4]arene, SC4, was published by Atwood and one of us (AWC) (Fig. 1).11
![Structure of the sodium salt of para-sulfonato-calix[4]arene showing the bilayer structural motif.11](/image/article/2006/CC/b600720c/b600720c-f1.gif) | ||
| Fig. 1 Structure of the sodium salt of para-sulfonato-calix[4]arene showing the bilayer structural motif.11 | ||
The bilayer structure, with an intercalated hydrated layer containing the cations, along with the structures of other Group I salts of SC4 led us to christen the systems as “Organic Clays”. However this name was not settled upon without considerable reflection on the structural similarity to phospholipid bilayer structures. Still, one may wonder if this naming of the system led us away from the study of the biochemistry of the molecules and more into material properties.
Indeed, in 1955, Cornforth had described the novel anti-tubercular properties of a series of oligoethylene glycol derivatives of a series of compounds.12 In fact, even before these compounds were determined to be calixarenes, the study of their biochemistry had begun.
The next publication on the biochemistry of the calix[n]arenes came in 1996, when the calcium-dependent chloride ion channel blocking properties of the para-sulfonato-calix[n]arenes was discovered by Atwood and Singh.13
Two patents concerning the anti-viral and anti-thrombotic properties of, amongst other calix[n]arene derived compounds, certain para-sulfonato-calix[n]arene derivatives were published by Hwang in the early 1990's.14,15
In this Feature Article, we will focus mainly on the work undertaken, since 1994, in our research group on the biochemistry of the para-sulfonato-calix[n]arenes.
1. Synthesis
The synthesis of the para-sulfonato-calix[n]arenes† may be simply achieved in three ways: direct ipso-sulfonation of the tert-butyl-calix[n]arenes,16 sulfonation of the para-H-calix[n]arenes as first described by Shinkai10,17 or by chlorosulfonation.18,19The choice is generally determined by the nature of the macrocycle and the substituents at the phenolic face, even if direct sulfonation of para-H-calix[n]arene is the most common route. For calix[8]arene, ipso-sulfonation is the method of choice because of the extremely low solubility of para-H-calix[8]arene, in the case of calix[n]arene bearing substituents on the lower rim, chlorosulfonation is the method of choice for systems sensitive to strong acids. Fig. 2 shows the three synthetic routes for sulfonation of calix[4]arene.
![The three synthetic routes to para-sulfonato-calix[4]arene SC4.](/image/article/2006/CC/b600720c/b600720c-f2.gif) | ||
| Fig. 2 The three synthetic routes to para-sulfonato-calix[4]arene SC4. | ||
1.1. Structures
para-Sulfonato-calix[4]arene SC4 is essentially shaped as a truncated cone with hydrophilic upper and lower rims separated by a hydrophobic mid-region, hence the designation of this molecule as a bipolar amphiphile by Atwood.20 For the hexamer and octamer, they can adopt respectively 8 and 16 different conformations (roseate, cone, alternate, pinched double cone, “taco”…), depending on the solvent, the nature of the complexed guest and of the functionalisations on the lower rim.In Fig. 3 are represented three conformations of the water soluble calix[n]arenes: para-sulfonato-calix[4]arene SC4 in cone conformation, para-sulfonato-calix[6]arene SC6 in the pinched cone conformation and para-sulfonato-calix[8]arene SC8 in the double inverted cone conformation.
![Structure of the para-sulfonato-calix[n]arenes, respectively, for SC4, SC6 and SC8.](/image/article/2006/CC/b600720c/b600720c-f3.gif) | ||
| Fig. 3 Structure of the para-sulfonato-calix[n]arenes, respectively, for SC4, SC6 and SC8. | ||
1.2. Monofunctionalised para-sulfonato-calix[n]arenes
Novel para-sulfonato-calix[n]arenes bearing pendant groups such as carboxylic acids or amines at the lower rim have been synthesised by Da Silva et al.21,22 The presence of the functional groups can modify their interactions with biologically active molecules or permit their grafting onto solid surfaces. With calix[4]arenes and calix[6]arenes, the compounds were obtained in good yields by treatment of the relevant para-H-calix[n]arene with a suitable weak base, in the presence of one equivalent of the corresponding halogeno-alkane. In the case of calix[8]arene, it has previously been seen that the very low solubility of para-H-calix[8]arene prevents its use and para-tert-butyl calix[8]arene is used in the monosubstitution reaction. The corresponding sulfonate derivatives were prepared in the cases of 2-carboxymethoxy SCn-b, 2-amidomethoxy SCn-c and 2-aminoethoxy SCn-d group systems, either by sulfonation of para-H-calix[n]arene derivatives or by ipso-sulfonation of tert-butyl derivatives. The syntheses of the monofunctionalised para-sulfonato-calix[n]arenes are summarised in Fig. 4.![Synthesis of para-sulfonato-calix[n]arene derivatives. Reagents and conditions: (i) 1 equiv. alkyl bromide, base, acetonitrile, reflux, 24 h; (ii) KOH 10%, EtOH : H2O 70 ∶ 30, 90%; (iii) THF, BH3/THF complex, reflux, 4 h; (iv) 1 equiv. alkyl bromide, CsOH, THF, reflux, 1 h; (v) H2SO4 96%, 508 °C, 24 h.21,22](/image/article/2006/CC/b600720c/b600720c-f4.gif) | ||
| Fig. 4 Synthesis of para-sulfonato-calix[n]arene derivatives. Reagents and conditions: (i) 1 equiv. alkyl bromide, base, acetonitrile, reflux, 24 h; (ii) KOH 10%, EtOH : H2O 70 ∶ 30, 90%; (iii) THF, BH3/THF complex, reflux, 4 h; (iv) 1 equiv. alkyl bromide, CsOH, THF, reflux, 1 h; (v) H2SO4 96%, 508 °C, 24 h.21,22 | ||
2. Complexation of amino-acids and oligo-peptides
2.1. In solution
Both our group22–26 and that of Arena and Ungaro27 have studied, since the 1990's, the interactions of the para-sulfonato-calixarenes and their derivatives with various amino-acids, oligo-peptides and peptides. Studies have been performed both by NMR,22–25,27 microcalorimetry24,25 and RP-HPLC.26 These studies show that for SC4, the highest association constants were obtained with the basic amino-acids lysine and arginine (association constant pH 8: SC4–L-arginine: 1.5 × 103 M−1, SC4–L-lysine: 0.74 × 103 M−1,24vs. 0.63 × 102 for maximum values obtained by Arena27 for L-phenylalanine).NMR studies have yielded structural information on the different complexes and show that lateral aliphatic chains or aromatic parts of the amino-acids are embedded into the cavity of the calixarenes. Microcalorimetric titrations show that the binding process is controlled by the favourable enthalpy resulting mainly from the tight inclusion of the apolar part of the guest into the hydrophobic cavity of the host through van der Waals interactions, but also that the favourable entropy accompanying the desolvation of the charged groups upon ionic interaction plays an important role.24
In the case of para-sulfonato-calix[6]- and -[8]arenes, the same selectivity and thermodynamic behaviour are observed. For the octamer, 1 ∶ 2 stoichiometric complexes are also observed with arginine and lysine.
Bushman et al.28 have also studied the complexation of a series of amino-acids and dipeptides by para-sulfonato-calix[4]- and -[6]arenes by calorimetric titrations, and demonstrated that the size of the cavity does not influence the complexation, favoured by enthalpic contributions.
Complexation of para-sulfonato-calix[n]arenes with di- and tripeptides composed of these basic amino-acids lysine and arginine (lysyl–lysine KK, arginyl–arginine RR, lysyl–arginine KR, arginyl–lysine RK, lysyl–lysyl–lysine KKK and arginyl–arginyl–arginine RRR) has also been studied in our group, by 1H NMR and microcalorimetry.25
For SC4, only a 1 ∶ 1 stoichiometry was observed. The binding process is controlled by the favourable enthalpy resulting mainly from the tight inclusion of the apolar part of the guest into the hydrophobic cavity of the host through van der Waals interactions, but the favourable entropy accompanying the desolvation of the charged groups upon ionic interaction also plays an important role. The NMR data and the thermodynamic properties of association show that lysyl–lysine adopts a very compact folded structure upon binding by the tetrameric host (Fig. 5). The mixed dipeptides that bear a lysine residue behave like lysyl–lysine, the lysine residue being preferentially complexed in the ligand cavity (Fig. 6). Addition of a third chain perturbs this very nice arrangement and, because the entropy of binding then becomes more favourable, the affinity for the tripeptide is more important.
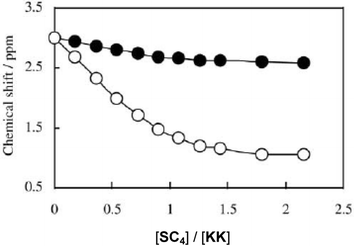 | ||
| Fig. 5 Chemical shift changes experienced by the lysine ε1 (○) and ε2 (●) protons of KK in the presence of increasing amounts of SC4. | ||
 | ||
| Fig. 6 Chemical shift changes experienced by the lysine ε protons (○) and the arginine δ protons (♦) of KR (a) and of RK (b) in the presence of increasing amounts of SC4. | ||
![Solid state bilayer stacking of the para-sulfonato-calix[4]arene/lysine complex showing the lysine spanning the bilayer (in dark green).30](/image/article/2006/CC/b600720c/b600720c-f7.gif) | ||
| Fig. 7 Solid state bilayer stacking of the para-sulfonato-calix[4]arene/lysine complex showing the lysine spanning the bilayer (in dark green).30 | ||
![The bilayer structure of (R,S)-histidine and para-sulfonato-calix[4]arene, the histidine is shown in green for clarity.31](/image/article/2006/CC/b600720c/b600720c-f8.gif) | ||
| Fig. 8 The bilayer structure of (R,S)-histidine and para-sulfonato-calix[4]arene, the histidine is shown in green for clarity.31 | ||
With para-sulfonato-calix[6]arene SC6 both 1 ∶ 1 and 1 ∶ 2 complexes are observed. The hexameric host binds two lysyl–lysine guests in a non-cooperative manner, which suggests that it adopts in solution a conformation of the 1,2,3-alternate type, as observed in the solid state. The thermodynamic data and the dissymmetry of the NMR spectra suggest that only the central side chain of the tripeptide is turned towards the interior of the partial cone. A very rigid structure is then obtained by stacking of the three guanidinium groups of the guest over the phenyl units of the hexameric host, which possibly gives rise to important π–π contributions. The complexation of RRR by SC6 appears in fact to be a remarkable case of tight binding for which the important favourable enthalpy change is almost compensated by an unfavourable entropy change, yielding an affinity that is weaker than for KKK. Complex behaviour, associated with higher order stoichiometry and probably aggregation, occurs in the case of the para-sulfonato-calix[8]arene, SC8.
The complexation properties in solution of the mono-functionalised derivatives with regard to amino-acids have also been investigated by Da Silva et al.22 The association constants show a 1 ∶ 1 stoichiometry and the pendant group at the lower rim strongly modifies their interactions with amino-acids as compared to the parent para-sulfonato-calix[n]arene. While generally, cationic amino-acids lysine and arginine bind strongly to all derivatives, the binding of the other amino-acids is dependant on the nature of the pendant arms and particularly, pendant functions lead to strong binding with aspartic acid, serine and tryptophan. Moreover, 1H NMR studies suggest that the monosubstitution leads to a cone conformation for the calix[4]arene derivative, and a “taco” conformation for calix[6]arene and calix[8]arene derivatives. The results are summarized in Table 1.
| Lys | Arg | Asp | Trp | Ser | |
|---|---|---|---|---|---|
| a Numbers in brackets represent standard deviations. | |||||
| SC4 | 1356 (349) | 1546 (721) | 512 (24) | 263 (30) | 120 (14) |
| SC4-b | 887 (74) | 800 (60) | 2852 (1137) | 178 (13) | 3555 (1859) |
| SC4-c | 578 (52) | 840 (137) | 2250 (1400) | 211 (42) | 540 (232) |
| SC4-d | 202 (30) | 447 (115) | 5620 (2618) | 205 (30) | 420 (20) |
| SC6 | 2200 (276) | 3090 (1024) | 354 (38) | 1448 (293) | 112 (38) |
| SC6-b | 1714 (176) | 1475 (108) | 3201(1306) | 433 (86) | 168 (14) |
| SC6-c | 1254 (112) | 2018 (153) | 2516 (1026) | 505 (31) | 235 (21) |
| SC6-d | 980 (160) | 673 (31) | 5423 (2291) | 2975 (464) | 178 (18) |
| SC8 | 4288 (1543) | 10083 (6705) | 614 (68) | 3465 (1156) | 320 (32) |
| SC8-b | 2097 (730) | 3879 (1914) | ND* | 1984 (564) | ND* |
| SC8-c | 669 (69) | 425 (200) | ND* | 200 (24) | ND* |
| SC8-d | 967 (102) | 812 (134) | ND* | 423 (45) | ND* |
All the results concerning the ability of functionalised calix[n]arenes, amongst those para-sulfonato-calix[n]arenes, to form complexes, to act as extractants in liquid–liquid extraction and even as carriers in the transport of different biological amine compounds (amino-acids, peptides...) have been recently reviewed by Mutihac et al.29
2.2. In the solid state
Complementary to the solution studies, solid states investigations have been performed to study complexation of amino-acids by para-sulfonato-calix[n]arenes. The first structure was carried out by Seltki et al.,30 and showed a host : guest stoichiometry of 1 ∶ 2 between para-sulfonato-calix[4]arene and lysine (Fig. 7). The packing presents a bilayer structure in which we can observe two hydrophobic bilayers separated by a chiral hydrophilic layer containing one lysine molecule embedded in the cavity of the calixarene, the second lysine spans the bilayer.Other crystal structures have been obtained by Raston et al. with racemic amino-acids31 (alanine, phenylalanine, histidine) and chiral amino-acids (S-alanine, S-histidine and S-tyrosine).32 Their packing present the same typical bilayer structure, with the amino-acid included inside of the cavity of the host (Fig. 8).
Recently, in our laboratory, Lazar et al.33 have determined the solid state structure of para-sulfonato-calix|4]arene with arginine, which is characterised by a novel zigzag bilayer of calixarene molecules, six molecules of arginine, each having different conformations and an infinite water channel (Fig. 9).
![Solid state zig-zag bilayer backing of the para-sulfonato-calix[4]arene/arginine complex and view of the water channel.33](/image/article/2006/CC/b600720c/b600720c-f9.gif) | ||
| Fig. 9 Solid state zig-zag bilayer backing of the para-sulfonato-calix[4]arene/arginine complex and view of the water channel.33 | ||
 | ||
| Fig. 10 Partial packing diagram of SC6 showing the bi-layer type arrangement of calixarenes and the L-leucine molecules (green) that occupy interstitial spaces in the extended structure.34 | ||
 | ||
| Fig. 11 View of the crystal packing of SC6 with arginine, unpublished results.35 | ||
Four of the arginine molecules have their lateral chains included into the molecular cavities of the calix molecules and the other two occupy independent sites in the cage. The orientation of each host molecule, as well as the conformation of the arginine guest, depend strongly on these interactions. As a result of the different polar environments, no identical interactions occur for the four inclusion complexes.
For 6- and 8-membered sulfonato-calixarenes, fewer solid state studies have been done. Only recently, Atwood and Raston34 published the first solid state structure of the para-sulfonato-calix[6]arene/amino-acid complex in which SC6 is organised in the “double cone” or “ taco” conformation and has multi-guest capability binding either L- or D-leucine in a single crystal in a bi-layer type arrangement from a racemic mixture of the amino-acid (Fig. 10).
We have resolved recently in our group the crystal structure of para-sulfonato-calix[6]arene/arginine complex. The crystal cell and packing are different from that previously observed by Raston, even if para-sulfonato-calix[6]arene is still in its “taco” form, arginine molecules are not embedded in the cavities but behave as linkers between two calixarenes (Fig. 11).35
2.3. Methanolysis of N-acetyl-L-amino-acids
Concerning the complexation of amino-acids, di- and tripeptides by para-sulfonato-calix[n]arenes, we can also refer to another study published by Goto et al.36 in which they claimed that specific catalysis of para-sulfonato-calix[n]arenes was observed in the alcoholysis of N-acetyl-L-amino-acids in methanol (particularly histidine, lysine and arginine). 1H NMR experiments they have done, support the formation of an inclusion complex with basic amino-acids and the results indicated that the catalytic activity of para-sulfonato-calix[n]arenes originates from its forming a complex with specific substrates, similar to enzymatic reactions.It is also worth noting that, in a recent crystallographic study on the complexation of para-sulfonato-calix[4]arene with histidine, we have observed the inverse phenomena, meaning that in the presence of methanol, we observed the esterification of the carboxylic acid group of histidine in the presence of the calixarene. In the crystal structure we can observe that three of the four complexed histidines present in the cell packing are esterified (Fig. 12).37
![Crystal packing of the histidine para-sulfonato-calix[4]arene complex showing the esterification of histidine (in dark green) in the presence of methanol (non-esterified histidine in light green), unpublished results.37](/image/article/2006/CC/b600720c/b600720c-f12.gif) | ||
| Fig. 12 Crystal packing of the histidine para-sulfonato-calix[4]arene complex showing the esterification of histidine (in dark green) in the presence of methanol (non-esterified histidine in light green), unpublished results.37 | ||
3. Complexation of other small bioactive molecules
Calixarenes have been widely used for the detection of molecules of pharmaceutical interest. For example the detection of steroids (cortisone, prenisolone…) in organic solvents has been investigated by NMR in the presence of resorcinarenes.3.1. Steroids
A preliminary investigation of the complexation of the three para-sulfonato-calix[n]arenes with testosterone has been carried out by Millership et al. by RP-HPLC.38 Stability constants of the host–guest complexes have been determined at three different pH’s and indicated that the association is dependant on the size of the calixarenes and the pH of solution. In our group, we have used electrospray mass spectroscopy to study non-covalent inclusion complexes between these water-soluble calixarenes, testosterone and two other steroids (progesterone and estradiol).39 Titration experiments have demonstrated differences with regard to the selectivity of each para-sulfonato-calix[n]arenes against steroids; SC8 interacts more strongly with estradiol and SC6 with progesterone. Competition experiments confirmed the selectivity of the complexation (Fig. 13). | ||
| Fig. 13 ES-MS spectra of the complex formed between SC6, progesterone, testosterone and estradiol at a molar ratio of 20 ∶ 1 ∶ 1 ∶ 1 at orifice voltages of 20 V and 50 V.39 | ||
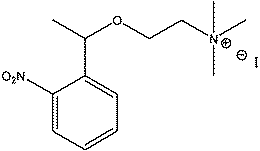 | ||
| Fig. 14 Structure of the cholinergic ligand A, studied by Specht et al.42 | ||
3.2. Acetylcholine
Another molecule of pharmaceutical interest, the neurotransmitter acetylcholine, shows high affinity for para-sulfonato-calix[n]arenes.Lehn et al.40 have shown that para-sulfonato-calix[4]- and -[6]arene formed very stable complexes with acetylcholine and quaternary ammonium cations. 1H NMR titrations gave association constants up to 4 × 105 M−1. These high affinities for choline and acetylcholine are comparable to those observed for biological recognition sites. They have also determined the crystal structures of the [choline-SC4]. Two complexes have been observed, each one having the choline N-terminal inserted inside the cavity of the receptor.
These results have led other groups to continue studies on these complexes. For example, Takashi41 has developed a new fluorometric method for the detection of the neurotransmitter acetylcholine (10−4M), using a dansylcholine(DANch) complex with para-sulfonato-calix[8]arene. The effects of other synaptic neurotransmitters on the fluorescence of the DANCh complex were examined for dopamine, histamine, ATP, GABA, glycine, L-glutamic acid, and L-aspartic acid. Among the neurotransmitters studied, acetylcholine was most effective in changing the fluorescence of the DANCh complex with SC8.
More recently, in 2005, Specht et al.42 reported the investigation of para-sulfonato-calix[n]arenes as synthetic receptors for photolabile cholinergic ligand A, a photolytic precursor of choline (Fig. 14).
Results from NMR studies have shown that SC4, SC6 and SC8 molecules formed 1 ∶ 1 complexes with A, having similar binding potential to that observed with the cholinergic enzymes acetylcholinesterase and butyrylcholinesterase. Further studies have suggested that SC8 forms a ditopic complex by binding concomitantly to both the cationic choline moiety and the aromatic photolabile group of A, whereas SC4 and SC6 form monotopic complexes with A. At neutral pH, SC4 specifically binds the cationic choline moiety, while, at acidic pH, it complexes unselectively both the cationic choline moiety and the aromatic group of A. Their results have shown that para-sulfonato-calix[n]arenes are versatile artificial receptors which bind in various ways to the bifunctional photolabile cholinergic ligand A, depending on their size, geometry, and state of protonation.
Before this work, they had also found that para-sulfonato-calix[4]arene binds strongly “caged” cholinergic ligands forming host–guest complexes with binding constants less than 100 µM.43 Photoirradiation of the host–guest complexes gave the desired fragmentation reactions releasing the cholinergic ligands and the kinetics of photorelease of cholinergic ligands from the host–guest complex is similar to that obtained with caged cholinergic ligands alone.
3.3. Drug solubilisation
Yang et al.44–46 have studied recently the solubilisation by para-sulfonato-calix[n]arenes in acidic aqueous solution of three practically insoluble drugs, nifedipine (an important calcium channel blocker that is used extensively for the clinical management of a number of cardiovascular diseases), furosemide (a high ceiling diuretic) and niclosamide (an anthelmintic drug that is active against most tapeworms) (Fig. 15). | ||
| Fig. 15 Structures of the drugs studied by Yang et al.44–46 | ||
They demonstrated that the molecular size of the calix[n]arene significantly influences the increase in the solubility of the drugs, from which results probably the incorporation of the aromatic groups of the drugs into the cavities of the calixarenes. As most of synthetic drugs are not soluble in water, these results are interesting for their solubilisation in aqueous media.
3.4. ATP hydrolysis
Yao et al.47 also reported on the catalytic effect of para-sulfonato-calix[4]arene on the hydrolysis of ATP.Studies were carried out using HPLC and also by using laser photolysis and pulse radiolysis for investigating the supramolecular interaction between water-soluble calix[4]arene and ATP. The results of laser photolysis and pulse radiolysis are consistent with the supramolecular catalysis speculated from the HPLC experiment and they claimed that this supramolecular interaction is crucial for the catalysis effect of para-sulfonato-calix[4]arene on hydrolysis of ATP.
4. Complexation with proteins
4.1. Bovine serum albumin
The complexation of Bovine Serum Albumin (BSA), an arginine- and lysine-rich protein, with para-sulfonato-calix[n]arenes has been demonstrated by our group48 by means of ElectroSpray Ionization Mass Spectrometry (ESI-MS), Dynamic Light Scattering and Atomic Force Microscopy. It has been shown that with para-sulfonato-calix[4]arene, one strong and two weaker binding sites are detected (Fig. 16). | ||
| Fig. 16 View of mass spectra of the complexation of BSA with SC4 at molar ratio of 1 ∶ 3 BSA : Calixarene after deconvolution. At this molar ratio, BSA is present as the uncomplexed form and the three complexed forms with one, two and three molecule of SC4. | ||
The effects on the structure of thin films formed by surface deposition of BSA show that the para-sulfonato-calix[n]arenes act to reticulate the films and produce essentially planar systems.
In a more recent publication,49 we have used ESI-MS in order to determine the association constants (KA) and binding stoichiometries for parent para-sulfonato-calix[n]arenes and their derivatives with BSA. KA values were determined by titration experiments using a constant concentration of protein (Fig. 17). It has been shown for the tetramer, SC4 and SC4-d interact strongly with BSA showing 3 non-equivalent binding sites (Table 2).
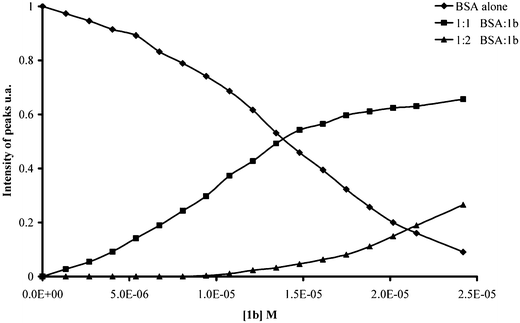 | ||
| Fig. 17 Plot of intensity of peaks of BSA versus the increasing concentration of SC4-b. | ||
| KA1 (×105 M−1) | KA2 (×105 M−1) | KA3 (×105 M−1) | R2 | |
|---|---|---|---|---|
| SC4 | 7.69 | 3.85 | 0.33 | 0.987 |
| SC4-b | 1.15 | 0.87 | — | 0.973 |
| SC4-c | 1.72 | 2.33 | — | 0.998 |
| SC4-d | 1.69 | 2.94 | 0.60 | 0.998 |
| SC6 | 0.40 | — | — | 0.965 |
| SC6-b | 0.29 | — | — | 0.972 |
| SC6-c | 0.48 | 2.04 | — | 0.994 |
| SC6-d | 2.08 | 0.48 | — | 0.940 |
| SC8 | 0.07 | — | — | 0.945 |
| SC8-b | 0.26 | — | — | 0.985 |
| SC8-c | 0.14 | — | — | 0.970 |
| SC8-d | 0.19 | — | — | 0.980 |
Table 2 also shows that the strength of the interactions between the calixarene and BSA is inversely proportional to the size of the macrocyclic ring: n = 4 > n = 6 ≫ n = 8. It has been previously reported that BSA has three sites for anionic molecules and in particular for long chain fatty acids, salicylate, and some sulfonamides.50 Binding of these anions involves, as expected, arginine or lysine residues with Lys-474 being implicated in the primary binding site, Lys-350 in the secondary site and Lys-116 in the weakest site. The three binding sites of BSA are present in domains I, II and III in order of increasing affinity.51 The results obtained for the para-sulfonato-calix[n]arenes and their derivatives in this study are hence in agreement with previous biochemical studies of anion binding to BSA (Fig. 18).50
![Molecular model of Human Serum Albumin showing one of the binding sites for para-sulfonato-calix[n]arenes. The cationic residues lysine and arginine within the binding pocket are shown in blue spheres, sulfonate groups of the calixarene molecule are shown in coloured spheres.](/image/article/2006/CC/b600720c/b600720c-f18.gif) | ||
| Fig. 18 Molecular model of Human Serum Albumin showing one of the binding sites for para-sulfonato-calix[n]arenes. The cationic residues lysine and arginine within the binding pocket are shown in blue spheres, sulfonate groups of the calixarene molecule are shown in coloured spheres. | ||
In conclusion, all these results show that the interaction between BSA and para-sulfonato-calix[n]arenes is different as a function of the macrocycle size. Nevertheless, we can expect to use these para-sulfonato-calix[n]arenes in physiological milieux due to their weak interactions with this protein.
5. Biological activities
In 1955, Cornforth et al.12 reported the first biological activity of calixarenes. Their studies concerned para-octyl-calix[8]arene having polyoxyethylene units on the lower rim and have shown that they were active as an anti-tuberculosis agent. The mechanism of action is completely different from the other drugs currently used against tuberculosis and, since the resistance toward conventional chemotherapeutic agents is increasing, these compounds are very promising.During more than 40 years, no more studies were carried out on the biological activities of calixarenes, until the patent of Hwang14 concerning the anti-viral activity of some calixarene derivatives.
5.1. Anti-viral activity
One of the interesting biological activities of the para-sulfonato-calix[n]arenes is their potential use in the treatment of viruses, such as HIV and Herpes.Hwang et al.14 patented a method for inhibiting cell infection by an enveloped virus, by administering to an infection site, a therapeutically effective amount of a calix[n]arene-derived compound, with polar substituents having terminal carboxylate, phosphate, or sulfonate (SC4) groups, including esters and amides which are cleavable in vivo. The mechanism of this action is proposed to be by interaction of the molecule with the viral envelope via electrostatic interactions, thus masking the recognition site for cells.
5.2. Anti-bacterial activity
Lamartine et al.52 have studied the anti-microbial activity of a series of water-soluble calixarenes.Preliminary screening of 57 calixarenes was conducted to assay their potential as anti-microbially active compounds against Corynebacterium. Of these compounds, seven calixarenes, amongst those SC4, SC6 and SC8, were found to exhibit suitable anti-microbial activity. These seven samples were then further tested to elucidate any anti-microbial activity they might have versus additional species. After examining the growth and inhibition values of these selected compounds, calixarenes SC4, SC6 and SC8 were shown to also display anti-microbial activity against Fusarium solani f. sp. Mori [F.s.-26] with an inhibition range of approximately 60–70%. Additionally, SC4, SC6, SC8 and others exhibited excellent and selective anti-microbial activity against the fungal strains, Rosellinia necatrix [R-8], and Colletotrichum dematium [C.d.8901].
5.3. Anti-thrombotic activity
As noted in Section 2, we have performed many experiments to study the complexation of para-sulfonato-calix[n]arenes with basic amino-acids lysine and arginine and di- and tripeptides composed by these amino-acids. These experiments were undertaken in view of the previously observed increase in blood coagulation time in the presence of these macrocycles. In view of their chemical structure, their conformational flexibility and the number of SO3 groups, these para-sulfonato-calix[n]arenes present analogies with glycosaminoglycans such as heparin.15,53 Furthermore, arginine and lysine are the major amino-acids present in the heparin recognition peptide sequences. This has lead us to study more deeply their anti-thrombotic activity.54The parent para-sulfonato-calix[n]arenes and six O-monosubstituted derivatives have been investigated in vitro for anti-coagulant activity. Different concentrations of calixarenes have been tested, showing that the compound SC8-b has a significantly strong prolongation on the activated partial thromboplastin time (APTT) and on the thrombin time (TT) than the other calixarenes. Secondly, investigation of whether the anti-coagulant behaviour was via interaction with antithrombin or heparin cofactor II was carried out. Thrombin inhibition mediated by antithrombin (AT) and heparin cofactor II (HCII) activation has been investigated in comparison to the biological activators, Heparin (Hep) and Dermatan sulfate (DS). The results are given in Fig. 19.
 | ||
| Fig. 19 HCII activation by calixarene derivatives at 100 µM (□) and 500 µM (■) compared to DS (100 µM). | ||
![Modelling of the interaction between HCII binding site and para-sulfonato-calix[8]arene SC8 (Alchemy).](/image/article/2006/CC/b600720c/b600720c-f20.gif) | ||
| Fig. 20 Modelling of the interaction between HCII binding site and para-sulfonato-calix[8]arene SC8 (Alchemy). | ||
The results showed that SC8-b and SC6 produce activation of HCII at 500 µM comparable to that induced by DS at 100 µM. However, activation of AT by all of the investigated calixarenes is between 10 and 50 times lower than that observed in the presence of heparin. Mono-substitution of para-sulfonato-calix[n]arenes can lead to an increase in their anti-coagulant properties with the highest activity observed for the carboxylic pendant arm functionalised para-sulfonato-calix[8]arene SC8-b. The mechanism of the anti-coagulant properties proceeds via interaction with the serine proteases inhibitors HCII and AT (Fig. 20). Chromogenic tests have indicated that interaction between the para-sulfonato-calix[n]arenes and HCII is the major factor in the activity of these molecules.
5.4. Enzyme inhibition
para-Sulfonato-calix[n]arenes have been used for the study of fibrolitic diseases of kidney, lung, liver and skin. L-Lysyl oxidase is an enzyme that assures the formation of covalent interactions between macromolecules of extra-cellular matrix and the initiated reticulation by this enzyme is an essential factor in fibrolitic diseases in organs. Inhibition of L-lysyl oxidase by the para-sulfonato-calix[6]arene has been reported by our group in a patent on the treatment and the cicatrisation of the skin.53 The inhibition has been explained by the electrostatic interaction between lateral chains of the basic amino-acid of the active site of the enzyme (RADVRDYDHRVLLRFPQRVK) and the sulfonate group of the calixarene.5.5. Ion channels
Numerous calixarenes have been used, either as inhibitors of ionic channels or as biomimetic systems of ion channels.For para-sulfonato-calix[n]arenes, Droogmans et al.55 first carried out the study of the inhibition of volume-regulated ionic channel (VRAC) present on endothelial cells which allows the passage of ions depending on the membrane potential, using para-sulfonato-calix[4]arene SC4 and its tetra-O-methyl derivative SC4-TM.SC4 and SC4-TM induced a fast inhibition at positive potentials but were ineffective at negative potentials. Results have shown that binding occludes VRAC at moderately positive potentials, but calix[4]arenes permeate the channel at more positive potentials. Their data suggest an open-channel blocking of VRAC by calix[4]arenes that also depends on the protonation of the binding site within the pore.
In a complementary experiment,56 they completed this work by studying the effect of para-sulfonato-calix[6]arene SC6 and of para-sulfonato-calix[8]arene SC8 on VRAC. At small positive potentials, SC4 was a more effective inhibitor than SC6 and SC8, which became more effective at more positive potentials. SC4, suramin and basile blue bind and occlude VRAC at moderate potentials, but permeate the channel at more positive potentials. SC6 and SC8 however do not permeate the channel.
Previously, in 1996, Atwood had patented a study concerning the inhibition of chloride dependent channels in cells by calixarenes sulfonates and other calixarenes with a para-acid functionality.13 This study showed that the inhibition of the ionic channel increases with the size of the macrocycle (SC8 ≫ SC6 ≫ SC4).
6. Prion diagnostics57
The normal or native prion protein, designated as PrPC for the cellular prion protein, is a widely occurring protein, whose sequence is well conserved in mammals. Conformational changes in PrPC lead to propagation of the pathogenic protein PrPSC, which is resistant to diseases; including scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, chronic wasting disease in deer and variant Creutzfeldt-Jakob disease in humans. The slow development and appearance of symptoms in cattle infected with BSE, with average incubation periods from 4–6 years has greatly hindered the development of epidemic models. BSE is capable of being transmitted to humans and has led to the designation of a new variant of Creutzfeldt-Jakob disease (vCJD). The full extent of the spread of vCJD and the nature of the infectious process are at this point in time unclear. However, the detection of the first case of vCJD arising from infection by blood transfusion in 2004, and the subsequent determination of other cases infected in the same manner or by the iatrogenic route, requires the development of highly sensitive methods for the detection of PrPSC at very low concentration. Various antibody-based immunodetection methodologies have been developed for the detection of PrPSC and the removal of infected animals from the food chain. Similarly, methods for complexing peptides, small molecules or inhibitors to PrPSC to allow isolation or treatment of vCJD have been developed.The structure of the prion proteins presents a multi-histidine peptide sequence capable of binding a wide range of metallic cations, but which is always proteolytically cleared and a spherical core sequence with a highly negatively charged surface, and for which at least one positively charged pocket, rich in lysine or arginine residues, is always present. The presence of this positively charged binding pocket is related to the capacity of PrP to interact with the heparin sulfate glycofragment present at the cell surface and the 37 kDa/67 kDa laminin receptor (LRP). There are two types of interactions between LRP and PrP. The first involves the heparin sulfate chains of a heparin sulfate proteoglycan as intermediary. The second mechanism proceeds via a direct interaction between PrP and LRP involving the PrPLrpbd1 domain of PrP. This interaction allows the anchorage of the PrP at the surface via a GPI anchor and stabilizes it.
Given the known similitude between the para-sulfonato-calix[n]arenes and the glycosylaminoglycanes, the binding of these molecules to PrPSC was investigated, using Western blotting with the SAF84 antibody. The initial results, using monocarboxymethoxy para-sulfonato-calix[6]arene (SC6b) showed that, not only was there a clear interaction with PrPSC from bovine brain, but there was also a considerable amplification in the detection, allowing an order of magnitude lowering of PrPSC detection.
Further tests with a range of para-sulfonato-calix[n]arene derivatives (Fig. 21), showed a number of notable points: firstly that there is considerable selectivity between non-substituted molecules and those carrying mono-substitution at the lower rim, there is little difference between substitution with cationic or anionic groups at the lower rim and finally the sensitivity is a function of ring size.
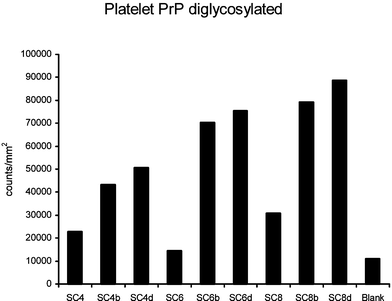 | ||
| Fig. 21 Effect of calixarene derivatives on the Western Blot detection of PrPSC. | ||
In a further development, the test was transferred to ELISA technology, for detection of PrP extracted from platlets using technology developed by bioMérieux SA., Immunorevelation is carried out with an anti-PrP specific Monoclonal antibody from bioMérieux, with coupling of mono-aminoethoxy para-sulfonato-calix[6]arene (SC6d) to the surface of NHS-activated 96 well titre plates (Fig. 22). Here the calixarene replaces the standard capture antibody used in ELISA tests. Use of a capture antibody is precluded in prion diagnostic tests which use Proteinase K to degrade all other proteins other than the resistant PrPSC. Such a transfer should in the near future allow automatisation of a diagnostic test for the prion in a wide range of biological fluids. The need for an ante-mortem blood based test in humans has become acute with the transfection of several persons via blood transfusion.
 | ||
| Fig. 22 Effect of grafting SC6d solution concentration on platelet PrP ELISA detection. | ||
7. Toxicity of the para-sulfonato-calix[n]arenes
In order to proceed to full bio-medical applications, it has been necessary to obtain information on the interactions, first at the cellular level and then in vivo, between molecules and biological entities. For medical applications, the toxicity of molecules is evidently a key factor, and the measure of the haemolytic properties represents a useful starting point.A brief report in a review by Gutsche in 1985 stated that the calixarenes had shown no activity in the Ames test.1
In our group, the first studies on the cellular toxicity of a series of water soluble calix[n]arenes were performed in 2000 by Perret et al. and resumed in the abstracts of ISSC 2000. It is noted that certain calix[4]arene phosphonate derivatives showed no effect on the cell growth of human fibroblasts.58
More recently, a series of cell toxicity studies have been carried out by Da Silva et al. A paper on the haemolytic properties of a series of para-sulfonato-calix[n]arene derivatives59 has been reported. As indicated before in section 4.1, we have shown that the complexation of the para-sulfonato-calix[n]arenes by bovine serum albumin is strongly dependant on the size of the macrocycle. Furthermore, we have observed that both the strength of complexation and the selectivity of complexation are strongly modified by the presence of pendant groups (2-carboxy methoxy group, 2-amido methoxy group and 2-amino ethoxy group) functions at the lower rim of the para-sulfonato-calix[n]arenes. In particular both the carboxylic acid and amine functions induce strong complexation of aspartic acid. As a consequence, we have studied, both, the effects of macrocycle size and the effects due to the presence of pendant lower rim groups on the haemolytic properties of the para-sulfonato-calix[n]arenes.
In this study, it has been shown that para-sulfonato-calix[4]arene and three of its lower rim monosubstituted derivatives have effectively no haemolytic effects for concentrations up to 200 mM. For the para-sulfonato-calix[6]arene derivatives a maximum of 12% haemolysis occurs for the ones bearing an amine function at a concentration of 200 mM. Haemolysis of 29% is observed for para-sulfonato-calix[8]arene at 200 mM. However, for all derivatives, haemolysis of less than 5% is observed for concentrations below 50 mM. The haemolytic effect increases with macrocycle ring size. For the para-sulfonato-calix[4]arene and para-sulfonato-calix[6]arene derivatives, the presence of a single ethoxy-amine function on the lower rim increases the haemolytic effect at all concentrations. In all cases, the presence of a carboxy-ethoxy function leads to a significant reduction of the haemolytic effects at concentrations of 50 mM and above.
Recently,60 the effects of the para-sulfonato-calix[n]arene on neutrophils, a class of white blood cells involved in the inflammatory response, has been investigated. Activation of neutrophils would show a non-specific immune response. The results, in Fig. 23, show that there is both no activation of neutrophils and also that cell viability is fully retained.
 | ||
| Fig. 23 The effect of pre-treatment with 100 nM calixarenes on NADPH oxidase activity in PMA (phorbol myristate acetate)-stimulated neutrophils. | ||
From all these studies, it has been deduced that the para-sulfonato-calix[n]arenes and their derivatives will be innocuous with regard to haemolytic effects at doses of less than 50 mM.
8. Determination of solution concentration
In view of these biological activities, it is now necessary to have a reliable method for assaying the concentration of the para-sulfonato-calix[n]arenes and their derivatives.Recently in our group, Rousseau et al.61 have constructed a spectrometric method for determination of para-sulfonato-calix[n]arenes concentration. The method is based on the use of DMMB, a spectrometric reagent previously used in assays for the glycosaminoglycans, for the determination of concentrations of para-sulfonato-calix[n]arenes and their derivatives in the concentration range 0–6 µg ml−1 (Fig. 24). The results show response curves dependant on both the macrocyclic ring size and the nature of substitution at the lower rim.
 | ||
| Fig. 24 Spectrometric titration curves for DMMB in the presence of increasing quantities of SC6. | ||
Conclusion
In this article, we have demonstrated how the para-sulfonato-calix[n]arenes are capable of complexing small biologically active molecules, amino-acids and proteins. This basic work has been extended by ourselves and other groups to demonstrate a strong potential of true bio-activity of these molecules with activity ranging from enzyme inhibition, through anti-thrombotic activity, anti-viral activity to anti-bacterial properties. In a major advance in the bio-applications of the para-sulfonato-calix[n]arenes, their incorporation into a working diagnostic kit for detection of prion based derivatives is being achieved.However, we are only at the beginning of the study of their toxicology and there are still several years before we can expect approval for these compounds in medicine. However, given the rapidity of the advances in the study of these compounds in biology, a promising future for work in the area seems assured.
Acknowledgements
The authors are deeply indebted to the past and present members of the research group, Assemblages d’Intérêt Biologiques, for their contribution to the work. One of us, F.P., acknowledeges financial support from a CNRS post-doctoral fellowship. We also wish to acknowledge the work of Maryline Dupin, Géraldine Ramage and Hervé Perron, Biomérieux, and Aly Moussa, AFSSA, concerning Prion diagnostics.References
- C. D. Gutsche, Host Guest Complex Chemistry Macrocycles; Synthesis, Structures, Applications, ed. E. Weber, Springer, Berlin, 1985 Search PubMed.
- C. D. Gutsche, Calixarenes Revisited: Monographs in Supramolecular Chemistry, The Royal Society of Chemistry, Cambridge, UK, 1998 Search PubMed.
- C. D. Gutsche and I. Alam, Tetrahedron, 1988, 44, 4689–4694 CrossRef CAS.
- C. D. Gutsche and J. A. Levine, J. Am. Chem. Soc., 1982, 104, 2652–2653 CrossRef CAS.
- C. D. Gutsche and K. C. Nam, J. Am. Chem. Soc., 1988, 110, 6153–6162 CrossRef CAS.
- Z. Asfari, V. Bohmer, J. M. Harrowfield and J. Vicens, Calixarenes 2002, Dordrecht, 2001 Search PubMed.
- A. von Baeyer, Dtsch. Chem. Ges., 1872, 5, 25 Search PubMed.
- A. Zinke, E. Ziegler, E. Martinowitz, H. Pichelmayer, M. Tomio, H. Wittmann-Zinke and S. Zwanziger, Chem. Ber., 1944, 77B, 264–272 CAS.
- A. Arduini, A. Pochini, S. Reverberi and R. Ungaro, J. Chem. Soc., Chem. Commun., 1984, 981–982 RSC.
- S. Shinkai, S. Mori, T. Tsubaki, T. Sone and O. Manabe, Tetrahedron Lett., 1984, 25, 5315–5318 CrossRef CAS.
- A. W. Coleman, S. G. Bott, S. D. Morley, C. M. Means, K. D. Robinson, H. Zhang and J. L. Atwood, Ang. Chem., Int. Ed., 1988, 27, 1361–1362 Search PubMed.
- J. W. Cornforth, P. D. Hart, G. A. Nicholls, R. J. Rees and J. A. Stock, Br. J. Pharmacol. Chemother., 1955, 10, 73–88 CAS.
- J. L. Atwood, R. J. Bridges, R. K. Juneja and A. K. Singh, US Patent, 5 489 612, 1996 Search PubMed.
- K. M. Hwang, Y. M. Qi, S. Y. Liu, T. C. Lee, W. Choy and J. Chen, US Patent, 5 409 959, 1995 Search PubMed.
- K. M. Hwang, Y. M. Qi, S. Y. Liu, T. C. Lee, W. Choy and J. Chen, US Patent, 5 196 452, 1991 Search PubMed.
- R. Lamartine, J.-B. Regnouf-de-Vains, P. Choquar and A. Marcillac, World Patent, WO 97/49677, 1997 Search PubMed.
- S. Shinkai, T. Tsubaki, T. Sone and O. Manabe, J. Chem. Soc., Perkin Trans. 1, 1987, 11, 2297 RSC.
- Y. Morzheriny, D. M. Rudkevich, W. Verboom and D. N. Reinhoudt, J. Org. Chem., 1993, 58, 7602–7605 CrossRef CAS.
- M. Makha and C. L. Raston, Tetrahedron Lett., 2001, 42, 6215–6217 CrossRef CAS.
- G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049–1052 CrossRef.
- E. Da Silva, L. Memmi, A. W. Coleman, B. Rather and M. J. Zaworotko, J. Supramol. Chem., 2002, 1, 135–138 Search PubMed.
- E. Da Silva and A. W. Coleman, Tetrahedron, 2003, 59, 7357–7364 CrossRef.
- N. Douteau-Guevel, A. W. Coleman, J.-P. Morel and N. Morel-Desrosiers, J. Phys. Org. Chem., 1998, 11, 693–696 CrossRef CAS.
- N. Douteau-Guevel, A. W. Coleman, J.-P. Morel and N. Morel-Desrosiers, J. Chem. Soc., Perkin Trans. 2, 1999, 629–634 RSC.
- N. Douteau-Guevel, F. Perret, A. W. Coleman, J.-P. Morel and N. Morel-Desrosiers, J. Chem. Soc., Perkin Trans. 2, 2002, 524–532 RSC.
- O. I. Kalchenko, F. Perret, N. Morel-Desrosiers and A. W. Coleman, J. Chem. Soc., Perkin Trans. 2, 2001, 258–263 RSC.
- G. Arena, A. Contino, F. G. Gulino, A. Magri, F. Sansone, D. Sciotto and R. Ungaro, Tetrahedron Lett., 1999, 40, 1597–1600 CrossRef CAS.
- H. J. Buschmann, L. Mutihac and E. Schollmeyer, J. Incl. Phenom. Macro., 2003, 46, 133–137 Search PubMed.
- L. Mutihac, H.-J. Buschmann, R.-C. Mutihac and E. Schollmeyer, J. Incl. Phenom. Macro., 2005, 51, 1–10 Search PubMed.
- M. Selkti, A. Tomas, A. W. Coleman, N. Douteau-Guevel, I. Nicolis, F. Villain and C. de Rango, Chem. Commun., 2000, 161–162 RSC.
- J. L. Atwood, T. Ness, P. J. Nichols and C. L. Raston, Cryst. Growth Des., 2002, 2, 171–176 CrossRef CAS.
- P. J. Nichols and C. L. Raston, Dalton Trans., 2003, 2923–2927 RSC.
- A. Lazar, E. Da Silva, A. Navaza, C. Barbey and W. Coleman Anthony, Chem. Commun., 2004, 2162–2163 RSC.
- J. L. Atwood, S. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337–339 RSC.
- A. N. Lazar and W. Coleman Anthony, IBCP, 2005, unpublished results.
- K. Goto, Y. Yano, E. Okada, C.-W. Liu, K. Yamamoto and R. Ueoka, J. Org. Chem., 2003, 68, 865–870 CrossRef CAS.
- A. N. Lazar and W. Coleman Anthony, IBCP, 2005, unpublished results.
- J. S. Millership, J. Incl. Phenom. Macro., 2001, 39, 327–331 Search PubMed.
- E. Da Silva, C. Valmalle, M. Becchi, C.-Y. Cuilleron and A. W. Coleman, J. Incl. Phenom. Macro., 2003, 46, 65–69 Search PubMed.
- J. M. Lehn, R. Meric, J.-P. Vigneron, M. Cesario, J. Guilhem, C. Pascard, Z. Asfari and J. Vicens, Supramol. Chem., 1995, 5, 97–103 CrossRef CAS.
- T. Jin, J. Incl. Phenom. Macro., 2003, 45, 195–201 Search PubMed.
- A. Specht, F. Ziarelli, P. Bernard, M. Goeldner and L. Peng, Helv. Chim. Acta, 2005, 88, 2641–2653 CrossRef CAS.
- A. Specht, M. Goeldner, J. Wirz and L. Peng, Synlett, 1999, 981–983 CAS.
- W. Yang and M. M. de Villiers, AAPS J., 2005, 7, E241–E248 Search PubMed.
- W. Yang and M. M. De Villiers, Eur. J. Pharm. Biopharm., 2004, 58, 629–636 CrossRef CAS.
- W. Yang and M. M. De Villiers, J. Pharm. Pharmacol., 2004, 56, 703–708 CrossRef CAS.
- T.-M. Yao, Z.-F. Ye, L. Wang, J.-Y. Gu, S.-D. Yao and X.-F. Shi, Spectrochim. Acta, Part A, 2002, 58A, 3033–3038 CAS.
- L. Memmi, A. Lazar, A. Brioude, V. Ball and A. W. Coleman, Chem. Commun., 2001, 2474–2475 RSC.
- E. Da Silva, C. F. Rousseau, I. Zanella-Cléon, M. Becchi and A. W. Coleman, J. Incl. Phenom. Macro., 2006, 54, 53–59 Search PubMed.
- T. J. Peters, All About Albumin. Biochemistry, Genetics and Medical Applications, Academic Press, New York, 1996 Search PubMed.
- R. G. Reed, J. Biol. Chem., 1986, 261, 15619–15624 CAS.
- R. Lamartine, M. Tsukadab, D. Wilson and A. Shirata, C. R. Chimie, 2002, 5, 163–169 Search PubMed.
- E. Aubert-Foucher, A. W. Coleman and D. J. S. Hulmes, French Patent, FR60252J, France, 1999 Search PubMed.
- E. Da Silva, D. Ficheux and A. W. Coleman, J. Incl. Phenom. Macro., 2005, 52, 201–206 Search PubMed.
- G. Droogmans, J. Prenen, J. Eggermont, T. Voets and B. Nilius, Am. J. Physiol., 1998, 275, C646–652 Search PubMed.
- G. Droogmans, C. Maertens, J. Prenen and B. Nilius, Br. J. Pharmacol., 1999, 128, 35–40 CrossRef CAS.
- A. Benscik-Reynier, A. W. Coleman, E. Da Silva, M. Dupin, E. Leclerc, A. Martin, A. Moussa, H. Perron and F. Ronzon, French Patent, 004/06538, 2005 Search PubMed.
- F. Perret, M. Mazzorana, P. Shahgaldian and A. W. Coleman, Abstracts of ISSC XI, 2000, Fukuoka, Japan Search PubMed.
- E. Da Silva, P. Shahgaldian and A. W. Coleman, Int. J. Pharm., 2004, 273, 57–62 CrossRef.
- M.-H. Paclet, C. F. Rousseau, Y. Campion, F. Morel and A. W. Coleman, J. Incl. Phenom. Macro., 2005 Search PubMed , in press.
- C. F. Rousseau, S. Cecillon, E. da Silva, A.-M. Freyria, D. Herbage, E. Leclere and A. W. Coleman, J. Incl. Phenom. Macro., 2005, 53, 9–13 Search PubMed.
Footnote |
| † We have chosen to use the term para-sulfonato-calix[n]arenes even with regard to the fact that for the parent sulfonic acids, the pKa value of the sulfonic acid functions are such that these functions are always present as fully ionized sulfonato groups at anything nearing normal biological conditions. |
| This journal is © The Royal Society of Chemistry 2006 |
