Synthesis and crystal structure of Li4BH4(NH2)3†
Philip A.
Chater
a,
William I. F.
David
*bc,
Simon R.
Johnson
c,
Peter P.
Edwards
c and
Paul A.
Anderson
*a
aSchool of Chemistry, The University of Birmingham, Edgbaston, Birmingham, UK B15 2TT. E-mail: p.a.anderson@bham.ac.uk
bISIS Facility, Rutherford Appleton Laboratory, Chilton, Didcot, Oxon., UK OX11 0QX. E-mail: W.I.F.David@rl.ac.uk
cInorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX3 3QR. E-mail: peter.edwards@chemistry.oxford.ac.uk
First published on 16th February 2006
Abstract
The solid solution, (LiNH2)x(LiBH4)(1−x), formed through the reaction of the two potential hydrogen storage materials, LiNH2 and LiBH4, is dominated by a compound that has an ideal stoichiometry of Li4BN3H10 and forms a body-centred cubic structure with a lattice constant of ca. 10.66 Å.
The issue of storage is one of the major technical obstacles to the implementation of hydrogen as a fuel for mobile transport and much research effort has been devoted to developing new hydrogen storage methods and materials.1 It has been shown that vast improvements in the hydrogen storage properties of known materials can be achieved by catalytic or chemical modification, such as in NaAlH4 and MgH2.2,3 Although these developments are encouraging, it has also been recognized that an answer to the hydrogen storage challenge is likely to involve new, more complex materials systems.
LiNH2 and LiBH4 have both been extensively studied with regard to their potential hydrogen storage properties.4,5 Recently, it was shown that, in the presence of lithium hydride, lithium amide reversibly desorbs hydrogen.4,6,7 It has also been reported8–10 that lithium amide and lithium borohydride, heated or ball milled in a 2 ∶ 1 mole ratio, form a new body centred cubic phase of composition Li3BN2H8,8 but no further structural information was provided. This new quaternary hydride was shown to have much improved hydrogen desorption properties compared to the separate reactants. It is clear that the mixing of hydrides can provide alternative decomposition pathways promoting the release of hydrogen,10,11 which may result in new, improved hydrogen storage systems. Here we report the synthesis and structure of the new quaternary hydride, whose stoichiometric composition is in fact Li4BN3H10, but which tolerates a wide variation in stoichiometry.
LiNH2 and LiBH4 (Sigma-Aldrich) were ground together by hand in the desired mole ratio in an argon atmosphere glovebox (O2 content < 10 ppm) and placed in a quartz tube. The tube was sealed with a Young's tap via an Ultra-Torr fitting and removed from the glovebox, and the reactants were then heated in a tube furnace under a mixture of H2 and N2 gas (1 ∶ 9) at 1 bar.
In a series of experiments, we heated different mixtures of LiNH2 and LiBH4 over the entire composition range and identified that a body centred cubic phase with a unit cell of around 10.66 Å was always present across the full compositional range. Synchrotron X-ray powder diffraction experiments showed that the lattice parameters varied between 10.6664 and 10.7080 Å, and that the 80 ∶ 20 composition contained an amide impurity while LiBH4 was detected in the 70 ∶ 30 composition. We concluded that the most likely stoichiometric composition for this structure was 75 ∶ 25, giving the empirical formula Li4BN3H10. Subsequently we observed that a mixture in a 3 ∶ 1 mole ratio heated at around 100 °C (near the orthorhombic–tetragonal structural transition temperature of LiBH4)5 gave a pure12 phase after annealing at 180 °C.13
The FTIR spectrum14 of Li4BN3H10 suggests that NH2− and BH4− anions remain intact within the structure and that the compound should be regarded as Li4BH4(NH2)3. There were no absorptions observed in positions expected for bridging B–H–B vibrations, again consistent with the BH4− anion remaining intact.15 B–H stretching modes were shifted relative to LiBH4 by around 8 cm−1 to the higher frequencies of 2387, 2293, 2237 (shoulder) and 2225 cm−1. BH2 deformation bands at 1120 and 1092 cm−1 in LiBH4 were observed at 1126 and 1082 cm−1 in Li4BH4(NH2)3. In contrast, the observed symmetric and asymmetric stretches of the amide anion16 were reduced from 3312 and 3259 cm−1 in LiNH2 to 3303 and 3243 cm−1 in Li4BH4(NH2)3.
Recrystallization of the compound was attempted from a number of different solvents17 in order to produce a single crystal for structural determination, but unfortunately Li4BH4(NH2)3 proved to be similar to LiNH2 in its lack of solubility in common solvents. Instead, the crystal structure was solved using a combination of high resolution synchrotron X-ray and neutron powder diffraction measurements.18 Extremely sharp diffraction peaks were observed in the X-ray data, indicating a high degree of crystallinity (see Fig. 1(a)). The regular spacing of peaks is indicative of a cubic crystal structure, which was verified using the computer program DASH;19 the refined lattice constant and unit cell volume were 10.66445(1)
Å and 1212.875(3)
Å3, respectively. Consideration of the volumes of a single formula unit of LiNH2
(V = 32.6 Å3) and LiBH4
(V = 54.2 Å3) from diffraction data collected on ID31 leads to a 3 ∶ 1 ratio and 7.98 formula units in the unit cell. The closeness of this value to 8 is a strong indication that the ideal stoichiometry is indeed Li4BN3H10. The most probable space groups were also determined using DASH by means of a Bayesian algorithm20 that evaluates the relative probabilities of different space group extinction symbols; the most probable symbol was found to be I- - - which is associated with the space groups I23, I213, Im3, I432, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m and Im3m. Unless there is substantial disorder in the crystal structure, the only two space groups that are consistent with 8 formula units per unit cell are I23 and I213. Both were assessed in the structure solution process. The contributions of nitrogen and boron together correspond to over half the X-ray scattering in Li4BH4(NH2)3 and thus to simplify structure solution only these atoms were considered initially in the solution process which was undertaken using the computer program TOPAS.21 Solutions of similar quality were found for both space groups I23 and I213. Examination of the resulting structures indicated that the only difference between the two space groups was the permutation of N and B atoms over the same set of sites in the unit cell22 which explains the similar qualities of fit.
3m and Im3m. Unless there is substantial disorder in the crystal structure, the only two space groups that are consistent with 8 formula units per unit cell are I23 and I213. Both were assessed in the structure solution process. The contributions of nitrogen and boron together correspond to over half the X-ray scattering in Li4BH4(NH2)3 and thus to simplify structure solution only these atoms were considered initially in the solution process which was undertaken using the computer program TOPAS.21 Solutions of similar quality were found for both space groups I23 and I213. Examination of the resulting structures indicated that the only difference between the two space groups was the permutation of N and B atoms over the same set of sites in the unit cell22 which explains the similar qualities of fit.
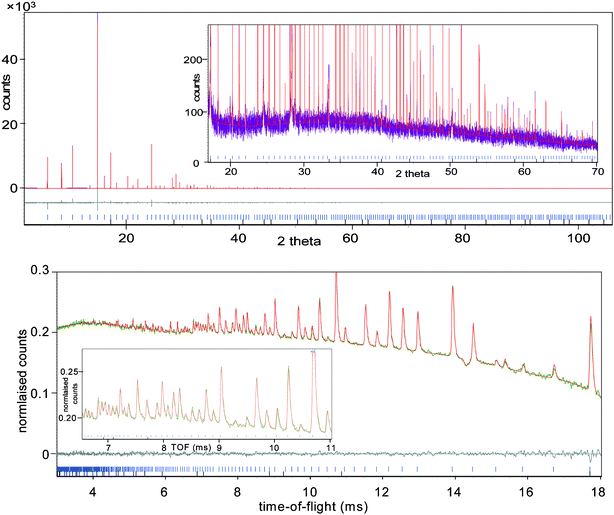 | ||
| Fig. 1 Final Rietveld plots for (a) X-ray and (b) neutron diffraction data showing observed (purple and green respectively), calculated (red) and difference (grey) plots. The inserts show the low d-spacing region at a magnified scale. Peak positions for Li4BH4(NH2)3 and Li2O are indicated. | ||
The B and N sites occupy half of the possible positions at approximately ([2ℓ + 1]/8, [2m + 1]/8, [2n + 1]/8) where ℓ,m,n = {0,1,2,3}; this arrangement is reminiscent of the nitrogen positions in LiNH2 (Fig. 2(b)). Building upon the apparent close similarity between Li4BH4(NH2)3 and LiNH2, all sites at (ℓ/4, m/4, n/4) (where ℓ,m,n = {0,1,2,3}) were considered to be possible lithium candidate positions. The next stage involved the refinement of the occupancies of each of these sites. At this point, I213 became a better option than I23. The final stage of the structure solution process involved neutron diffraction data.18 Usually deuteration and 11B enrichment are required for neutron powder diffraction experiments because of problems with high background and absorption. The high count-rate of GEM, however, means that natural Li4BH4(NH2)3 could be used without isotopic enrichment (see Fig. 1(b)). Four hydrogen atoms were placed in tetrahedral coordination around boron initially at 1.25 Å while two hydrogen atoms were located around each nitrogen atom at a distance of 1 Å in a configuration similar to LiNH2. Initial bond length and angle restraints were used for both BH4− and NH2− anions and all atomic positions were refined. In the final refinement using the neutron diffraction data (Table 1), all restraints were removed and an excellent fit was obtained over the full d-spacing range down to 0.44 Å. The resulting crystal structure is shown in Fig. 2(a). The corresponding fits to the X-ray and neutron diffraction data are shown in Figs. 1 (a) and (b) respectively.
| Atom | x/a | y/a | z/a | B iso/Å2 |
|---|---|---|---|---|
| a X-ray data18 – Rwp = 13.8, Rexp = 10.6, χ2 = 1.7. Neutron data23 – Rwp = 0.821 (11.9%), Rexp = 0.726 (10.5%), χ2 = 1.3. | ||||
| Li1 | 0.25 | −0.0428(14) | 0.0 | 1.5(1) |
| Li2 | −0.2622(16) | 0.0 | 0.25 | 1.5(1) |
| Li3 | 0.2722(12) | 0.2722(12) | 0.2722(12) | 1.5(1) |
| B | 0.1354(5) | 0.1354(5) | 0.1354(5) | 1.4(2) |
| H1 | 0.0721(15) | 0.0721(15) | 0.0721(15) | 4.1(2) |
| H2 | 0.0969(10) | 0.1361(9) | 0.2480(8) | 4.1(2) |
| N | −0.1559(3) | 0.1101(2) | 0.3668(3) | 1.8(1) |
| H3 | −0.1533(12) | 0.0533(15) | 0.4394(12) | 5.6(2) |
| H4 | −0.0773(12) | 0.1672(12) | 0.3768(14) | 5.6(2) |
 | ||
| Fig. 2 (a) Crystal structure of Li4BH4(NH2)3; (b) LiNH2 for comparison. Li atoms are depicted in red, N in blue, H in grey and BH4− as green tetrahedra. | ||
Interestingly, the B–H bonds in Li4BH4(NH2)3 are shorter than in LiBH4; however, as with LiBH4, one B–H bond is significantly (1.17 Å) shorter than the other three (1.27 Å).23 N–H bond lengths are close to the N–D bond lengths observed in LiND2.24 These observations agree with the FTIR data, which showed a strengthening and weakening of the B–H and N–H bonds relative to LiBH4 and LiNH2, respectively.
The Li4BH4(NH2)3 structure accommodates a wide range of stoichiometries. Direct substitution of NH2− ions by the larger BH4− ions (anion volumes of 0.43 and 0.66 nm3, respectively) results in an increase in observed lattice parameter.25 Annealing experiments are currently in progress to determine the exact limits of this non-stoichiometry; preliminary results indicate that the composition range for (LiNH2)x(LiBH4)(1−x) may extend from x = 0.5 to x = 0.8. It is worth noting that the replacement of an NH2− anion in the unit cell of LiBH4(NH2)3 by an additional BH4− necessarily results in shorter distances between BH4− ions than in the stoichiometric compound and that non-stoichiometric compositions may be less stable as a result. In samples containing less than 40 mol% LiNH2, we also observed a second body centred cubic phase with a larger unit cell size of ∼ 11.1 Å, which co-exists with the Li4BH4(NH2)3 structure and an excess of LiBH4. Early indications suggest that this phase may represent a second preferred composition within the (LiNH2)x(LiBH4)(1−x) solid solution range. Further studies to determine the stoichiometry of this second phase are ongoing, but this work is complicated by its lack of stability over time with respect to LiBH4(NH2)3 and LiBH4.
When Chen et al. observed that the intimate mixing of LiH with LiNH2 suppresses the usual release of ammonia in favour of hydrogen evolution at a much reduced decomposition temperature, it was suggested that this could be due to an interaction between partly positively charged hydrogen atoms in LiNH2 and the negatively charged hydride anions in LiH,26 and a mechanism for hydrogen desorption has recently been discussed.27 We hypothesize that a similar interaction may occur in Li4BH4(NH2)3, as H atoms in BH4− are also partially negative.28 Significantly, in Li4BH4(NH2)3 the BH4− ions are close to the NH2− on a molecular level, obviating the need for mechanical mixing. Initial findings confirm that the major gaseous decomposition product of Li4BH4(NH2)3 above 260 °C is indeed H2 with ammonia present only in trace amounts. Similar findings have been reported for the decomposition of the 2 ∶ 1 LiNH2/LiBH4 mixture.8,10 Remarkably, this new quaternary complex hydride, constituted mainly of—and with striking structural similarities to—LiNH2, has a completely different decomposition pathway from the pure amide, favouring H2 evolution over that of ammonia.
We wish to thank Andy Fitch for assistance in using beamline ID31 at the ESRF, Grenoble, and Ron Smith and Richard Ibberson for assistance at ISIS. SRJ acknowledges the EPSRC (SUPERGEN), and PAC the EPSRC for funding.
Notes and references
- See L. Schlapbach and A. Züttel, Nature, 2001, 414, 353 Search PubMed; R. Harris, D. Book, P. Anderson and P. Edwards, Fuel Cell Rev., 2004, 1, 17 CrossRef; A. Züttel, Naturwissenschaften, 2004, 91(4), 157 Search PubMed; W. Grochala and P. P. Edwards, Chem. Rev., 2004, 104, 1283 CrossRef; A. M. Seayad and D. M. Antonelli, Adv. Mater., 2004, 16, 765 CrossRef CAS; F. Schüth, B. Bogdanović and M. Felderhoff, Chem. Commun., 2004, 2249 CrossRef CAS and references therein.
- B. Bogdanović and M. Schwickardi, J. Alloys Compd., 1997, 253, 1 CrossRef.
- S. R. Johnson, P. A. Anderson, P. P. Edwards, I. Gameson, J. W. Prendergast, M. Al-Mamouri, D. Book, I. R. Harris, J. D. Speight and A. Walton, Chem. Commun., 2005, 22, 2823 RSC.
- P. Chen, X. Xiong, J. Luo, J. Lin and K. L. Tan, Nature, 2002, 420, 302 CrossRef CAS.
- A. Züttel, P. Wenger, S. Rentsch, P. Sudan, Ph. Mauron and Ch. Emmenegger, J. Power Sources, 2003, 118, 1 CrossRef CAS.
- Y. H. Hu and E. Ruckenstein, J. Phys. Chem. A, 2003, 107, 9737 CrossRef CAS.
- T. Ichikawa, S. Isobe, N. Hanada and H. Fujii, J. Alloys Compd., 2004, 356, 271 CrossRef.
- F. E. Pinkerton, G. P. Meisner, M. S. Meyer, M. P. Balogh and M. D. Kundrat, J. Phys. Chem. B, 2005, 109, 6 CrossRef CAS.
- M. Aoki, K. Miwa, T. Noritake, G. Kitahara, Y. Nakamori, S. Orimo and S. Towata, Appl. Phys. A, 2005, 80, 1409 CrossRef CAS.
- Y. Nakamori, A. Ninomiya, G. Kitahara, M. Aoki, T. Noritake, K. Miwa, Y. Kojima and S. Orimo, J. Power Sources, 2005 Search PubMed (in press).
- J. Lu and Z. Z. Fang, J. Phys. Chem. B, 2005, 109, 20830 CrossRef CAS.
- Li2O (Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a
= 4.609(5)
Å) present from the LiNH2 starting material was also observed at ca. 6%.
m, a
= 4.609(5)
Å) present from the LiNH2 starting material was also observed at ca. 6%. - Initially a small amount of lithium amide was present, which was incorporated into the structure by repeated grinding and annealing at 180 °C.
- FTIR spectra were acquired from samples contained in pressed KBr disks on a Nicolet Magna FTIR spectrometer equipped with a liquid nitrogen cooled MCTB detector, at a resolution of 2 cm−1.
- T. J. Marks and J. R. Klob, Chem. Rev., 1997, 77, 263.
- J.-P. O. Bohger, R. R. Eβmann and H. Jacobs, J. Mol. Struct., 1995, 348, 325 CrossRef CAS.
- Solvents tried were diethyl ether, THF, DMF and DMSO.
- X-ray diffraction data were collected on the ID31 diffractometer at the ESRF, Grenoble at a wavelength and stepsize of 0.80102 Å and 0.003° respectively. 354 reflections were used in the structural refinement. Neutron diffraction data were collected on GEM at the ISIS spallation neutron source at the Rutherford Appleton Laboratory. 1395 reflections were used in the refinement. CCDC 225200 & 294505. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b518243c.
- W. I. F. David, K. Shankland and N. Shankland, Chem. Commun., 1998, 8, 931 RSC.
- A. J. Markvardsen, W. I. F. David, J. C. Johnson and K. Shankland, Acta Crystallogr., Sect. A, 2001, 57, 47 CrossRef CAS.
- Bruker AXS (2004): TOPAS V3.0: General profile and structure analysis software for powder diffraction data. Bruker AXS, Karlsruhe, Germany.
- See Electronic Supplementary Information.
- J.-Ph. Soulié, G. Renaudin, R. Černý and K. Yvon, J. Alloys Compd., 2002, 346, 200 CrossRef CAS.
- M. Nagib and H. Jacobs, Atomkernenergie, 1973, 21(4), 275 Search PubMed.
- H. D. B. Jenkins, H. K. Roobottom, J. Passmore and L. Glasser, Inorg. Chem., 1999, 38, 3609 CrossRef.
- P. Chen, Z. Xiong, J. Luo, J. Lin and K. L. Tan, J. Phys. Chem. B, 2003, 107, 10967 CrossRef CAS.
- S. Isobe, T. Ichikawa, S. Hino and H. Fujii, J. Phys. Chem. B, 2005, 109, 14855 CrossRef CAS.
- Q. Ge, J. Phys. Chem. A, 2004, 108, 8682 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra, a comparison of symmetry groups and a virtual reality image of Fig. 2(a). See DOI: 10.1039/b518243c |
| This journal is © The Royal Society of Chemistry 2006 |
