Synthesis and disruption of a tetrametallic zinc hydrazide
Carl
Redshaw
*a and
Mark R. J.
Elsegood
b
aSchool of Chemical Sciences and Pharmacy, The University of East Anglia, Norwich, UK. E-mail: carl.redshaw@uea.ac.uk; Fax: +44 1603 592003; Tel: +44 1603 593137
bChemistry Department, Loughborough University, Loughborough, Leicestershire, UK LE11 3TU. E-mail: m.r.j.elsegood@lboro.ac.uk; Fax: +44 01509 223925; Tel: +44 01509 228751
First published on 3rd January 2006
Abstract
Treatment of 1,1′-dimethylhydrazine, Me2NNH2 with excess diethylzinc affords a novel tetrametallic Zn4N8 cage complex, which on further reaction with tBuLi forms a lithium-bridged chain of Zn4N7 cages.
In recent years, group 13 hydrazides have received attention principally for their potential as single source precursors to electronic materials, typically MN (M = Ga, Al).1 These studies have spawned a number of unusual structural motifs, for example, we have found that 1,1′-dimethylhydrazine upon treatment with trimethylaluminium affords, following work-up, a novel octanuclear ring system – best viewed as a structural analogue of the calix[4]pyrrole.2 Others have identified ladder-type structures,3 and a Ga4N8 cage structure was isolated from the reaction of trimethylgallium with phenylhydrazine.4 However, despite this research activity, there is a paucity of information regarding other main group element hydrazides.5
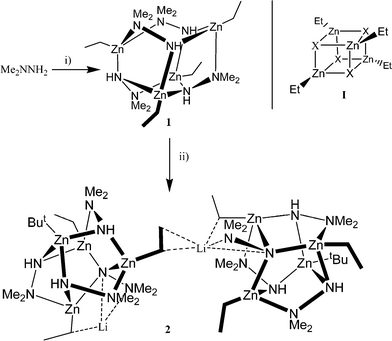
With this in mind, we have started to explore the chemistry of zinc alkyls in the presence of hydrazine ligands. Herein, we report our findings on the reaction of 1,1′-dimethylhydrazine with diethylzinc (Scheme 1), and find that the resulting novel tetrametallic zinc ‘cube’ can serve as a suitable starting material for further derivatization.
Reaction of Me2NNH2 with Et2Zn (1.1 equivalents) in refluxing toluene affords, following work-up and recrystallization from acetonitrile, the colourless complex [EtZn(NHNMe2)]4 (1) in good yield (ca. 65%).† Complex 1 is doubtless formed via the loss of ethane, and can readily be prepared on a multigram scale. Unlike the analogous preparation involving trimethylaluminium, there is no insertion of nitrile here.2 The 1H NMR spectrum is rather complex, with numerous overlapping resonances for the ethyl, δ −0.14 to −0.32 (Zn–CH2), 1.10 to 1.17 (Zn–CH2CH3) and dimethyl groups 2.36 and 2.72 ppm. In the IR spectrum, the v(NH) peak is clearly observed at 3150 cm−1. As expected, NMR studies indicate that 1 is not stable to prolonged exposure to air. Single crystals suitable for an X-ray diffraction study were obtained from a saturated acetonitrile solution on prolonged standing at ambient temperature. The solid-state structure (Fig. 1) reveals a novel tetrametallic cage structure in which tetrahedral zinc centres, each bearing one ethyl group, are linked via –N(Me)2NH– bridging groups. Indeed, 1 can be viewed as a variation of the tetrameric structure I proposed for both ethylzinc chloride and bromide, where X = –N(Me)2NH–.6 There are two 6-membered rings: that containing Zn(1)/Zn(2) adopts a chair conformation, whilst the Zn(2)/Zn(3) ring is a twisted chair. These two 6-membered rings are linked via four 5-membered rings. Both Zn(1) and Zn(3) possess the same N3C coordination environment, whereas Zn(2) and Zn(4) are different to one another, viz. NH + 2× NMe2 + ethyl and 3× NH + ethyl, respectively. The Zn–NH bonds all fall in the range 2.048(3)–2.098(3) Å, whereas the Zn–NMe2 bonds are somewhat longer [2.150(3)–2.192(3) Å] and are best considered dative. Although there are N–H groups present, the structure lacks any H-bonding, doubtless due to the paucity of acceptors. Overall, the structure adopts a layer motif, with the centroids of different molecules ca. 9 Å apart.
 | ||
| Fig. 1 Selected bond lengths (Å) and angles (°) for 1: Zn(1)–N(1) 2.162(3), Zn(1)–N(4) 2.049(3), Zn(1)–N(6) 2.085(4), Zn(2)–N(3) 2.169(3), Zn(2)–N(5) 2.150(3), Zn(2)–N(8) 2.048(3), Zn(3)–N(2) 2.053(3), Zn(3)–N(6) 2.068(3), Zn(3)–N(7) 2.192(3), Zn(4)–N(2) 2.083(3), Zn(4)–N(4) 2.087(3), Zn(4)–N(8) 2.098(3); N(1)–Zn(1)–N(4) 93.96(12), N(4)–Zn(1)–N(6) 104.19(13), N(2)–Zn(3)–N(7) 93.23(11), N(2)–Zn(3)–N(6) 95.55(13), N(2)–Zn(4)–N(4) 97.77(12), N(2)–Zn(4)–N(8) 97.69(12). | ||
Given the presence of the NH functionality, it was of interest to establish the effect of deprotonation on the structural features of 1. With this in mind, 1 was treated with tBuLi (1 equivalent) in toluene at −78 °C, which resulted in a colourless solution, from which small crystals suitable for X-ray diffraction using synchrotron radiation7 could be isolated in moderate yield (ca. 25%).† The crystal structure (Figs. 2 and 3) reveals a remarkable chain-like structure in which the cages of 1 have been severely disrupted to afford new Zn4N7 cages that are linked via lithium bridges, viz. [(ZnEt)3(ZnBut)(NHNMe2)3(NNMe2)(μ-Li)]n2. Notable differences from the Zn4N8 cage of 1 include the now pendant N(8)Me2 group at N(7), and the presence of a tert-butyl group at Zn(1), the source of which can only be the lithium reagent. Furthermore, the cages of 2 are made up solely of 5-membered ring systems. In the cages of 2, three zinc centres (Zn(2)/(3) and (4)) possess the same N3C coordination, each being bonded to NMe2, NH, N(7) and an ethyl group, whereas Zn(1) is surrounded by three NH groups and the tert-butyl group. The pattern of Zn–N bond lengths is similar to 1, i.e. Zn–N(H) 2.0197(4)–2.1201(15) Å and Zn–N(Me)2 2.1913(14)–2.2000(4) Å. For the lithium cation, there are a number of close ‘agostic-type’ contacts. The Li–H bonds to the neighbouring anion at H(7A′) [2.06 Å], H(7B′) [2.06 Å] and H(8A′) [2.02 Å] are similar to those found in the interstitial hydrides {Li8(H)[N(2-pyridyl)Ph]}62+ [mean = 2.015 Å] and {Li7(H)[N(2-pyridyl)Ph]}6 [2.06 Å],8 whilst that to H(5B) [2.19 Å] is close to those observed in the interstitial hydride [Li8(H)(hpp)6]+ [2.10–2.17 Å].9 There is also a short Li–N(8) distance [1.934 Å], a somewhat longer bond to N(7) [2.388 Å], and long Li–C distances [2.258–2.467 Å]. Similar, long Li–C bonding [< 3.01 Å] has been implicated in a number of other systems.10 The Li(1)–Zn distances are larger than the sum of their covalent radii [ca. 2.45 Å].
 | ||
| Fig. 2 Selected bond lengths (Å) and angles (°) for 2: Zn(1)–N(1) 2.0751(13), Zn(1)–N(3) 2.1201(15), Zn(1)–N(5) 2.0809(13), Zn(2)–N(2) 2.1913(14), Zn(2)–N(3) 2.0197(14), Zn(2)–N(7) 2.0302(12), N(7)–N(8) 1.4918(17), Li(1)–N(7) 2.388(3), Li(1)–N(8) 1.934(3), Li(1)–H(5B) 2.19, Li(1)–C(5) 2.395, Li(1)–H(8A) 2.02, Li(1)–H(7A′) 2.06, Li(1)–H(7B′) 2.06, Li(1)–C(7′) 2.258(3), Li(1)–C(8′) 2.467(4); N(3)–Zn(1)–N(5) 97.80(5), N(1)–Zn(1)–C(1) 123.35(6), Zn(2)–N(7)–N(8) 113.57(9), N(6)–Zn(4)–N(7) 99.73(5). | ||
 | ||
| Fig. 3 Packing diagram of 2. | ||
In summary, 1,1′-dimethylhydrazine reacts with diethylzinc to afford a novel tetrametallic Zn4N8 cage. Further reaction with tBuLi involves deprotonation, alkyl exchange and disruption of the zinc cage to afford a new Zn4N7 system; the latter link up via lithium bridges to afford a novel chain-like structure. Further investigations to survey the scope of this reaction, including the use of other hydrazines and deprotonating agents, are in progress.
The EPSRC is thanked for beam-time at Daresbury Laboratory.
Notes and references
- (a) A. H. Cowley and R. A. Jones, Angew. Chem., Int. Ed. Engl., 1989, 28, 1208 CrossRef; (b) F. C. Sauls and L. V. Interrante, Coord. Chem. Rev., 1993, 128, 193 CrossRef CAS.
- V. C. Gibson, C. Redshaw, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 1999, 38, 961 CrossRef CAS.
- J. S. Silverman, C. D. Abernethy, R. A. Jones and A. H. Cowley, Chem. Commun., 1999, 1645 RSC.
- D. W. Peters, M. P. Power, E. D. Bourret and J. Arnold, Chem. Commun., 1998, 753 RSC.
- (a) J. Goubeau and U. Kull, Z. Anorg. Allg. Chem., 1962, 316, 182 CrossRef CAS; (b) B. Fröhling, G. Kreiner and H. Jacobs, Z. Anorg. Allg. Chem., 1999, 625, 211 CrossRef.
- J. Boersman, in Comprehensive Organometallic Chemistry I, Eds. G. Wilkinson, F. G. A. Stone, E. W. Abel; Pergamon, Oxford, 1982, Vol. 2, Chapter 16 Search PubMed.
- (a) W. Clegg, M. R. J. Elsegood, S. J. Teat, C. Redshaw and V. C. Gibson, J. Chem. Soc., Dalton Trans., 1998, 3037 RSC; (b) W. Clegg, J. Chem. Soc., Dalton Trans., 2000, 3223 RSC.
- D. R. Armstrong, W. Clegg, R. P. Davies, S. T. Liddle, D. J. Linton, P. R. Raithby, R. Snaith and A. E. H. Wheatley, Angew. Chem., Int. Ed., 1999, 38, 3367 CrossRef.
- S. R. Boss, M. P. Coles, R. Haigh, P. B. Hitchcock, R. Snaith and A. E. H. Wheatley, Angew. Chem., Int. Ed., 2003, 42, 5593 CrossRef CAS.
- W. Clegg, K. W. Henderson, R. E. Mulvey and P. A. O'Neil, J. Chem. Soc., Chem. Commun., 1994, 769 RSC and references therein.
Footnote |
| † Satisfactory microanalyses have been obtained for 1 and 2. Selected spectroscopic data: For 1: 1H NMR (CDCl3, 298 K, 400 MHz) δ: 2.77 (m, 12H, N(CH3)2), 2.36 (m, 12H, N(CH3)2), 2.31 (s 1H, N–H), 2.20 (s 1H, N–H), 2.08 (s 1H, N–H), 2.03 (s 1H, N–H), 1.14 (overlapping m, 12H, Zn–CH2CH3), −0.17 (q, 2H, 2JHH 8.2 Hz, Zn–CH2), −0.22 (overlapping m, 4H, 2× Zn–CH2), −0.29 (q, 2H, 2JHH 8.1 Hz, Zn–CH2). IR: v(NH): 3149 cm−1. Mass Spec (FAB+): 585 (M+ − Et). For 2: 1H NMR (CDCl3, 298 K, 400 MHz) δ: 2.82–2.37 (overlapping m, 27H, 3x ‘internal’ N(CH3)2 + ‘external’ N(CH3)2 + 3NH), 1.25–0.91 (m, 9H, 3× Zn–CH2CH3), 0.83 (s, 9H, + (CH3)3), 0.22 to −0.10 (m, 6H, Zn–CH2CH3). IR: v(NH): 3172 cm−1. Mass Spec (FAB+): 553 (M+ − 3Et–Li). Crystal data for 1: C16H48N8Zn4, M = 614.10, monoclinic, space group P21/n, a = 11.5497(4), b = 14.6647(5), c = 16.2586(6) Å, β = 101.473(2)°, U = 2698.74(16) Å3, T = 150(2) K, Z = 4, μ(Mo-Kα) = 3.529 mm−1, λ = 0.71073 Å, 20882 reflections measured, 5280 unique (Rint = 0.019) which were used in all calculations. The final wR2 = 0.106 (all data) and R1 = 0.037 (for 4641 data with F2 > 2σ(F2)). Crystal data for 2: C18H51LiN8Zn4, M = 648.09, monoclinic, space group P21/c, a = 12.9610(6), b = 14.6764(7), c = 15.5138(7) Å, β = 98.069(2)°, U = 2921.8(2) Å3, T = 151(2) K, Z = 4, μ = 3.263 mm−1, synchrotron radiation at Daresbury Laboratory, Station 9.8, silicon 111 monochromator, λ = 0.6765 Å, 34776 reflections measured, 9877 unique (Rint = 0.032) which were used in all calculations. The final wR2 = 0.079 (all data) and R1 = 0.029 (for 8625 data with F2 > 2σ(F2)). CCDC 290422 & 290423. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b516431a |
| This journal is © The Royal Society of Chemistry 2006 |