Cooperative AND receptor for ion-pairs†
Michael D.
Lankshear
,
Andrew R.
Cowley
and
Paul D.
Beer
*
Department of Chemistry, Inorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QR. E-mail: paul.beer@chem.ox.ac.uk; Fax: +44 1865 272960; Tel: +44 1865 285142
First published on 11th January 2006
Abstract
A novel heteroditopic calix[4]diquinone receptor capable of binding an anion and cation simultaneously in a cooperative fashion is shown only to recognise halide anions in the presence of a suitable cobound cationic guest species, and displays affinity for certain ion-pairs where no affinity for either of the free ions is observed.
The design and application of new heteroditopic receptor systems capable of simultaneous coordination of both anionic and cationic guest species has recently attracted a great deal of interest, as these systems have the potential to act as salt solubilisation, extraction, and membrane transport agents.1 A number of receptors whereby an anion and cation are bound separately within the receptor have been reported previously.2 In these systems, the cation may be bound using a number of common motifs, while the anion is coordinated using Lewis acidic, electrostatic, or hydrogen bonding interactions.3 Interest in a contact ion-pair binding approach, wherein the anion and cation are bound essentially as one moiety, is particularly noteworthy as this avoids the energetically unfavourable separation of the two ions.4 Importantly, the geometry of the ditopic receptor must be optimised so that the anion and cation binding sites are located in proximity so as to enhance this interaction, as incorrect orientation could lead to the ion pair associating outside of the receptor, or solvent-separated ion binding. Herein we describe the design, synthesis and binding properties of one such rare system, which demonstrates a dramatic enhancement of anion binding by a cobound cation and, in some cases, strong binding with associated ion pairs where no affinity for the free ions is observed, which to our knowledge is without precedent.
The calix[4]diquinone unit has well-documented cation binding properties which may be readily probed by electrochemical and UV-visible spectroscopic methods.5 The isophthalamide cleft has been widely utilised as an anion binding unit in a variety of systems.6 By using a combination of these two moieties, it was hoped that a considerable enhancement of anion recognition would take place in the presence of a bound cationic guest species.
The design of this system was assisted by using the CAChe modelling program. MM3 and PM3 calculations indicated that the target receptor 1 (Fig. 1) provided an optimal spatial arrangement for the binding of a KCl ion pair within the macrocyclic cavity, with the calix[4]diquinone unit coordinating the cation, and the isophthalamide amide motif binding the anion. In addition, ether oxygens were included in the macrocycle design to provide extra stabilising units for the cation binding site. The resulting model of the host–guest complex is illustrated in Fig. 2.
![Ditopic calix[4]diquinone receptor 1.](/image/article/2006/CC/b515634c/b515634c-f1.gif) | ||
| Fig. 1 Ditopic calix[4]diquinone receptor 1. | ||
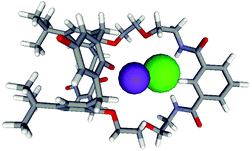 | ||
| Fig. 2 Optimised CAChe model of 1·KCl recognition. Potassium is in purple, chloride in green. | ||
The synthesis of the target receptor was accomplished by a four-step synthetic procedure (Scheme 1).7 This route provides a simple, high yielding pathway to the possible preparation of a number of calixarene species with macrocyclic function at the lower rim. The single crystal X-ray diffraction determined structure of 1 (Fig. 3) reveals the calixquinone species in a partial cone conformation, with one of the quinone oxygens hydrogen bonding to the isophthalamide amide protons.‡
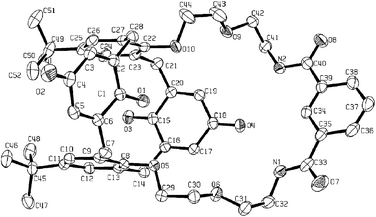 | ||
| Fig. 3 ORTEP thermal ellipsoid plot of 1 at 40% probability. Selected distances: N(1)⋯O(4) 3.171(3), N(2)⋯O(4) 3.237(3) Å. | ||
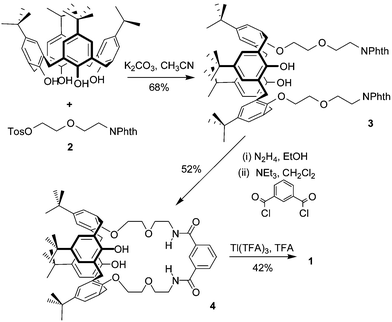 | ||
| Scheme 1 Formation of compound 1. Abbreviations used: Tos = tosylate, Phth = phthalimide. | ||
The anion binding properties of 1 were assessed by 1H NMR spectroscopic titration methods in deuterated acetonitrile solution. One equivalent of tetrabutylammonium (TBA) chloride was found to induce only very small downfield shifts in the signals arising from the amide (d, 0.057 ppm) and isophthalyl C-H (c, 0.012 ppm) protons of receptor 1 (Fig. 4). The change in chemical shift as a function of concentration revealed a linear relationship (Fig. 5), indicating that no anion binding was occurring; the change in chemical shift was attributed to an increase in the ionic strength of the solution.
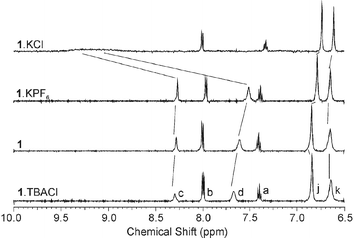 | ||
| Fig. 4 Changes in the aromatic region of the 1H NMR spectrum (CD3CN, 298 K) of 1 ∶ 1 mixtures of 1·TBACl, 1, 1·KPF6 and 1·KPF6·TBACl (1·KCl). | ||
![Plot of chemical shift of the NH protons of 1·M+ as a function of added chloride concentration (CD3CN, 298 K).All titrations conducted in CD3CN solution at 298 K. Halide added as TBAX. Metal cations added as MPF6 salt except for Li+ and Na+, where the ClO4− salts were used. At >1 equivalent of added Cl− in the cases of K, Li and NH4vs. TBACl titrations peak broadness led to significant errors in the chemical shift plotted. For Na the broadening was so severe only an extrapolation of δNH was possible (see Fig. 5).[1·M]+ = 0.0015 mol dm−3.](/image/article/2006/CC/b515634c/b515634c-f5.gif) | ||
| Fig. 5 Plot of chemical shift of the NH protons of 1·M+ as a function of added chloride concentration (CD3CN, 298 K)§.[1·M]+ = 0.0015 mol dm−3. | ||
Analogous 1H NMR titration experiments were then carried out in the presence of one equivalent of various cationic guest species. Remarkably, the signals arising from the amide, d, and isophthalyl, c, protons were found to broaden and shift downfield considerably on the addition of one equivalent of TBA chloride (∼1.51 and ∼1.135 ppm, respectively, in the case of potassium, see Fig. 4). Job Plot analysis indicated that the host–guest interaction demonstrated 1 ∶ 1 complex stoichiometry, but the interaction was too strong to allow accurate stability constant determination through WinEQNMR8 analysis of the titration binding curves (Fig. 5). This was found to be the case for a number of cationic guest species (Table 1), although importantly no chloride recognition was observed in the presence of one equivalent of a non-coordinating cationic species such as TBA or tetramethylammonium. These results illustrate a ‘switching on’ of chloride recognition in receptor 1, which appears to display absolutely no affinity for chloride in the absence of a suitable cationic guest, but which binds very strongly when this species is present. This is a very dramatic enhancement of anion binding effected by a cobound cation, one of the largest yet reported.
Furthermore, it was demonstrated that this effect is not confined to chloride recognition, as a similar enhancement in binding is induced by one equivalent of KPF6 in the cases of bromide and iodide anions in CD3CN solution. Neither anion binds to the free receptor, but, as with chloride, the stability constant for bromide in the presence of potassium is >104 M−1. With iodide, as may be expected, the enhancement is weaker, with KI = 380 M−1.§
The cation binding properties of this system were probed in CH3CN solution by UV-visible spectroscopic analysis of the quinone n–π* absorption band. The free receptor was found to bind only with potassium cations, as could be demonstrated by SPECFIT9 analysis of the titration spectra obtained, but in the presence of one equivalent of a coordinating anion (chloride) the binding constants obtained from all the cations studied were enhanced, suggesting a dramatic positive cooperativity effect whereby chloride binding can enhance the cation binding in these systems (Table 2). This effect is most noteworthy for ammonium and sodium binding, as in these cases cation binding can only be detected in the presence of chloride anion. No significant changes in the absorption spectra of 1 were induced on addition of TBA chloride. Thus, chloride can be said to ‘switch on’ sodium and ammonium cation recognition by the quinone groups of 1; indeed it seems that 1 will, in some cases, bind the ion-pair strongly where no affinity for the discrete ions is observed (e.g. NH4Cl, NaCl). To the best of our knowledge, this is the first such example of a system where the receptor displays no discernible affinity for either one of the free ions, but which binds the associated ion pair species strongly (for summary of potential solution equilibria, see Scheme 210). This behaviour can be treated through Boolean logic to show that this receptor behaves in a manner consistent with an AND gate; that is, it only demonstrates binding in the presence of both a suitable anion and cation.11 A possible reason for this phenomenon could be the self-inhibition of the cation and anion binding sites of the molecule by intramolecular hydrogen bonding of the quinone unit with the isophthalamide, as illustrated in the solid state structure of 1 (Fig. 3), which is disrupted only by a suitable ion-pair.
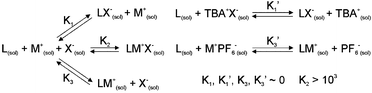 | ||
| Scheme 2 Summary of potential CH3CN solution equilibria for e.g., M = NH4, X = Cl. For M = potassium, K3′ and, by implication, K3, are >0. L = 1 in this case. N.B. equilibrium M+(sol) + X−(sol) = MX(sol) is also possible.10 | ||
| logKNa | logKK | logKNH4 | |
|---|---|---|---|
| a Measurements carried out at 298 K in dry CH3CN, spectra analysed using the SPECFIT computer program. Errors < 15%. In the case of lithium, SPECFIT analysis of the titration data failed to give an association constant value. b Insufficient changes in the absorption spectrum to infer binding. | |||
| 1 | —b | 3.08 | —b |
| 1·TBACl | 3.13 | 4.13 | 4.65 |
In order to gain more insight into the cooperative enhancement of cation recognition by the presence of an anion, 1H NMR spectroscopic analysis of the aliphatic region of 1·KPF6 was carried out. This suggests a conformational change of the calix[4]diquinone unit on binding chloride anion. Binding one equivalent of potassium ion leads to a widening of the splitting between the peaks arising from the bridging methylene protons i, an effect which is magnified when anions are added (Fig. 6). Changes are also seen in the signals arising from the alkyl protons e–h. These changes definitely imply a conformational change in the calixarene unit on cation-induced anion binding, and may indicate an enhancement of cation binding strength as the anion is added; certainly the complex 1·KCl is solely in the cone conformation with little in the way of conformational lability. Similar conformational changes are seen for LiCl, NaCl and NH4Cl recognition. These observations reinforce the suggestion of cooperative binding behaviour obtained from the UV-visible spectroscopic experiments.
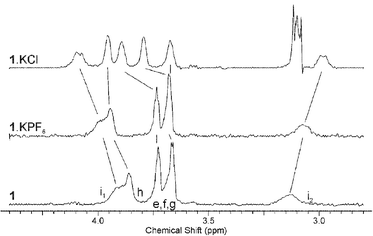 | ||
| Fig. 6 Changes in aliphatic region of 1H NMR spectrum (CD3CN, 298 K) of 1, 1·KPF6 and 1·KPF6·TBACl (1·KCl). | ||
These results lead us to propose that the new ditopic receptor 1 behaves in a manner consistent with the model calculations outlined above, that is it binds appropriate group 1 metal and ammonium chlorides as contact ion-pair species. This would account for the remarkable enhancement of both cation and anion recognition observed in the presence of an appropriate cobound guest. To the best of our knowledge, this system provides the first evidence of a neutral macrocyclic contact ion-pair binding system based on the calix[4]diquinone unit, which furthermore displays unprecedented properties consistent with the cooperative AND recognition of ion-pairs.
The authors wish to thank the EPSRC and GE Healthcare (Amersham) for a CASE supported studentship.
Notes and references
- N. Pelizzi, A. Casnati, A. Friggeri and R. Ungaro, J. Chem. Soc., Perkin Trans. 2, 1998, 1307 RSC; D. J. White, N. Laing, H. Miller, S. Parsons, S. Coles and P. A. Tasker, Chem. Commun., 1999, 2077 RSC; D. M. Rudkevich, J. D. Mercer-Chalmers, W. Verboom, R. Ungaro, F. de Jong and D. N. Reinhoudt, J. Am. Chem. Soc., 1995, 117, 612.
- A. J. Evans and P. D. Beer, Dalton Trans., 2003, 4451 RSC; M. T. Reetz, C. M. Niemeyer and K. Harms, Angew. Chem., Int. Ed. Engl., 1991, 30, 1472 CrossRef; J. M. Oh, E. J. Cho, B. J. Ryu, Y. J. Lee and K. C. Nam, Bull. Korean Chem. Soc., 2003, 24, 1538 CAS; T. Nabeshima, T. Saiki, J. Iwabuchi and S. Akine, J. Am. Chem. Soc., 2005, 127, 5507 CrossRef CAS.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef.
- J. M. Mahoney, A. M. Beatty and B. D. Smith, Inorg. Chem., 2004, 43, 7617 CrossRef CAS; M. Cametti, M. Nissinen, A. Dalla Cort, L. Mandolini and K. Rissanen, J. Am. Chem. Soc., 2005, 127, 3831 CrossRef CAS.
- P. D. Beer, P. A. Gale, Z. Chen, M. G. B. Drew, J. A. Heath, M. I. Ogden and H. R. Powell, Inorg. Chem., 1997, 36, 5880 CrossRef CAS.
- K. Kavallieratos, C. M. Bertao and R. H. Crabtree, J. Org. Chem., 1999, 64, 1675 CrossRef CAS; M. A. Hossain, J. M. Llinares, D. Powell and K. Bowman-James, Inorg. Chem., 2001, 40, 2936 CrossRef CAS; S. J. Brooks, L. S. Evans, P. A. Gale, M. B. Hursthouse and M. E. Light, Chem. Commun., 2005, 734 RSC.
- A. McKillop, J. S. Fowler, M. J. Zelsko, J. D. Hunt, E. C. Taylor and G. McGillivray, Tetrahedron Lett., 1969, 29, 2423 CrossRef; P. A. Reddy, R. P. Kashyap, W. H. Watson and C. D. Gutsche, Isr. J. Chem., 1992, 32, 89 CAS.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 2, 311 RSC.
- SPECFIT, v. 2.02, Spectrum Software Associates, Chapel Hill, NC, USA. Search PubMed.
- R. Shukla, T. Kida and B. D. Smith, Org. Lett., 2000, 2, 3099 CrossRef CAS.
- A. P. De Silva and N. D. McClenaghan, Chem. Eur. J., 2004, 10, 574 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation data for compounds 1–4, general procedures used for 1H NMR and UV-visible spectroscopic titration experiments, single X-ray crystal data for 1. See DOI: 10.1039/b515634c |
‡ Crystal data for 1: Chemical formula C54H59N3O10; M = 910.08; T = 150 K; triclinic; space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; a = 10.0261(3), b = 12.5372(4), c = 20.4416(6) Å; α = 102.137(3), β = 94.601(3), γ = 20.4416(6)°; V = 2442.24(14) Å3; Z = 2.00; μ = 0.085 mm−1; 22 ; a = 10.0261(3), b = 12.5372(4), c = 20.4416(6) Å; α = 102.137(3), β = 94.601(3), γ = 20.4416(6)°; V = 2442.24(14) Å3; Z = 2.00; μ = 0.085 mm−1; 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001 reflections measured; 11 001 reflections measured; 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 unique reflections; Rint = 0.030; R (wR) = 0.0447 (0.0514) for the 5735 unique data with I > 3σ(I) and 612 parameters. CCDC 289857. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b515634c 760 unique reflections; Rint = 0.030; R (wR) = 0.0447 (0.0514) for the 5735 unique data with I > 3σ(I) and 612 parameters. CCDC 289857. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b515634c |
| § All titrations conducted in CD3CN solution at 298 K. Halide added as TBAX. Metal cations added as MPF6 salt except for Li+ and Na+, where the ClO4− salts were used. At >1 equivalent of added Cl− in the cases of K, Li and NH4vs. TBACl titrations peak broadness led to significant errors in the chemical shift plotted. For Na the broadening was so severe only an extrapolation of δNH was possible (see Fig. 5). |
| This journal is © The Royal Society of Chemistry 2006 |
