Formation of insoluble perylenetetracarboxylic diimide films by electro- or photo-crosslinking of pyrrole units†
Heung Cho
Ko
,
Suk-ho
Kim
,
Woonghyun
Choi
,
Bongjin
Moon
and
Hoosung
Lee
*
Department of Chemistry, Sogang University, Seoul, 121-742, Korea. E-mail: hlee@sogang.ac.kr; Fax: +82 2 701 0967; Tel: +82 2 705 8446
First published on 23rd November 2005
Abstract
Perylenetetracarboxylic diimide derivatives bearing 2- or 4-peripheral pyrrole pendants could be efficiently crosslinked to form an insoluble film either by electropolymerization or visible light induced oxidative photopolymerization of the pyrrole units.
Perylenetetracarboxylic acid diimide (PDI) derivatives might be one of the few examples in chemistry and materials science that have found numerous applications as a single species.1 Due to their unique optical and electrochemical properties, their applications now have rapidly expanded to many fields such as tunable laser dyes,2 light harvesting materials,3 light emitting diodes,4 electrogenerated chemiluminescence,5 electrochromes,6 solar cells,7 p–n junction diodes,8 and liquid crystal materials.9
However, owing to the intrinsic poor solubility of PDI, fabrication of a PDI film with desired morphology and properties has been a challenging issue especially in construction of a device based on PDI. When dealing with pristine PDI, one will be confronted with the difficulties in solution-to-film translation due to its extremely poor solubility arising from the π-stacking interaction between perylene moieties. The π-stacking effect can be reduced by introducing bulky groups into 1-, 6-, 7-, and 12-positions or into N-positions of PDI.10 Even though the improved solubility enables the solution-to-film translation, the high solubility also limits the choice of solvents for coating an upper-layer in fabrication of multi-layered film structures, for example. Therefore, a special strategy is required to satisfy the two contradictory requirements in solubility before and after PDI film preparation when a PDI film serves as an under-layer. Bearing this in mind, we have reported on the synthesis and electrochemical study of an analog of PDI-2Py (Fig. 1) containing two pyrrole units that can be electrochemically polymerized to form an insoluble PDI film.11 In this communication, we report on the synthesis and unique film formation characteristics of PDI-4Py either by electropolymerization or by photo-crosslinking induced by simple visible light irradiation (> 500 nm) to form a very robust insoluble PDI film. To the best of our knowledge, this is the first example of oxidative photopolymerization of pyrrole derivatives sensitized by a purely organic sensitizer such as PDI.
 | ||
| Fig. 1 The chemical structures of perylene derivatives used in this study. | ||
A series of PDI derivatives illustrated in Fig. 1 was prepared and fully characterized by 1H-NMR, 13C-NMR, EA and MALDI/TOF-HRMS. The detailed synthetic procedures are described in the Supplementary Information. When the three PDI-nPy (n = 0, 2, and 4) were subjected to cyclic voltammetry measurement (Fig. 2a), they exhibited almost identical electrochemical behavior in the range of −1.5 to 0 V (vs. Ag/Ag+) but showed quite different aspects in the range of 0.8 to 1.2 V: PDI showed reversible redox peaks but PDI-2Py and PDI-4Py displayed an irreversible oxidation peak which arises from the irreversible oxidation of pyrrole units. Especially, PDI-4Py showed more intense anodic current than PDI-2Py due to a higher amount of pyrrole units per PDI.
![(a) Cyclic voltammograms of 2 mM PDI-nPy monomers in acetonitrile (AN)/methylene chloride (MC) (1/2 by volume) containing 0.1 M Bu4NPF6 at 50 mV s−1 scan rate. (b): CVs of the corresponding electropolymerized films on GC electrodes in monomer-free electrolyte solutions [0.1 M Bu4NPF6 in AN/MC (1/2 by volume)] at 50 mV s−1.](/image/article/2006/CC/b513847g/b513847g-f2.gif) | ||
| Fig. 2 (a) Cyclic voltammograms of 2 mM PDI-nPy monomers in acetonitrile (AN)/methylene chloride (MC) (1/2 by volume) containing 0.1 M Bu4NPF6 at 50 mV s−1 scan rate. (b): CVs of the corresponding electropolymerized films on GC electrodes in monomer-free electrolyte solutions [0.1 M Bu4NPF6 in AN/MC (1/2 by volume)] at 50 mV s−1. | ||
After electropolymerization of the monomers by cycling the applied potential between 0 and 1.2 V for three cycles in a potentiodynamic mode, the resulting glassy carbon (GC) electrodes were rinsed thoroughly with methylene chloride and acetonitrile. The CVs of the resulting films in a monomer-free electrolyte solution are shown in Fig. 2b. While the PDI-treated electrode showed no recognizable PDI-redox peak in the potential range of −1.5 to −0.7 V, the other two electrodes treated with PDI-2Py and PDI-4Py respectively showed broad PDI redox peaks. The much more intensified PDI-redox peaks in the case of the PDI-4Py-modified electrode indicate that the electrodeposition efficiency of PDI-4Py is higher than that of PDI-2Py.
While the study was going on, we accidentally discovered that prolonged storage of a vial containing PDI-4Py solution under exposure to sunlight resulted in formation of an insoluble violet film inside the wall of the vial, while PDI-2Py solution did not show such behaviour. To investigate this interesting phenomenon in detail, we firstly spin-coated PDI-4Py solution on a glass substrate and irradiated the film with visible light. In order to choose visible light above ca. 500 nm that can excite only the PDI moiety without exciting the pyrrole unit, the light from a Xe-lamp was filtered with a filter (JB510, Zure Photonics) which cuts off the light below ca. 500 nm. A water chamber was also placed between the filter and the film to prevent IR-caused heating of the film. The temperature of the film was kept below 60 °C during the light-irradiation. After the photo-treatment, the film was rinsed thoroughly with chloroform to remove any soluble species. The change in film thickness before and after photo-treatment was investigated by comparing the maximum intensity of absorption spectra as shown in Fig. 3a and 3b. The absorption spectrum exhibits two distinct bands in the ranges 400–500 (S0–S2 transition) and 500–630 nm (S0–S1 transition).2 According to the absorption comparison data (Fig. 3a) before and after the photo-treatment and rinsing of the film, the minimum irradiation time for over 95% fixation of the film by cross-linking turned out to be ∼6 min. The kinetics with the non-linear increase of the film thickness points to the formation of crosslinked polymers (Fig. 3a). When we carried out the same experiments using PDI and PDI-2Py, we could not observe any intact film remaining after rinsing.
 | ||
| Fig. 3 (a) Electronic absorption ratios before and after exposure to light of a spin coated PDI-4Py film as a function of exposure time. All the photo-treated films were rinsed with chloroform before the measurement. (b) Electronic absorption spectra before and after exposure to light for 1 hour. (c) FT-IR spectra of a spin-coated film as a function of light exposure time. | ||
In order to figure out the structural change before and after photo-treatment, we spin-coated PDI-4Py onto a KBr pellet and monitored its FT-IR spectra as a function of exposure time. The peak intensity at 725 cm−1 (see α in Fig. 3c), which corresponds to the characteristic C–H bending vibration of pyrrole,12 decreased with the exposure time and eventually disappeared after 1 hour. These results indicate that almost all the pyrrole units reacted with each other upon light-exposure while conserving the PDI core structure. It is noteworthy that insoluble species are not formed when the exposure time is shorter than 5 min where approximately 60% of the C–H units have reacted. In the case of PDI-2Py, reduction of the peak intensity at 725 cm−1 was also observed but no residual film on the pellet after rinsing was observed, which means the formation of the soluble polymeric form instead of the insoluble crosslinked polymer.
In Scheme 1 is provided a plausible mechanism of the pyrrole coupling based on PDI-sensitized photo-oxidation of pyrrole moieties. Once the PDI moiety is photoexcited by visible light (λmax of PDI = ∼600 nm, path a in Scheme 1), one electron oxidation of pyrrole may occur via electron transfer from the HOMO level of pyrrole to that of PDI (path b). Since the HOMO levels of PDI and Py are estimated to be 0.88 eV and 0.77 eV vs. Ag/Ag+, respectively, based on the CVs of PDI and N-methylpyrrole, the electron transfer (ET) from the slightly higher HOMO level of pyrrole to the lower HOMO level of PDI seems to be a reasonable process. Rapid oxidation of the resulting PDI anion to neutral PDI by oxygen will produce superoxide ion O2˙− (path c). The resulting pyrrole cation radicals then can have two possible fates, self-coupling followed by re-oxidation loosing two protons to superoxide ions (path d), or reaction with superoxide ion or peroxide radical to give oxygenated pyrroles (path e). It will also be possible for these two pathways to occur in combination, of course. A few similar examples of photo-sensitized polymerization of pyrrole using Ru(bpy)32+ as a sensitizer have been reported and they are proposed to proceed by a similar mechanism.13 Because figuring out the chemical identity of the photo-polymerized PDI-4Py film is limited by its insolubility, we attempted to monitor the photo-polymerization of PDI-2Py by 1H-NMR spectroscopy. We found that the resonances of the pyrrole moiety at δ 6.68 and δ 6.11 gradually disappeared and new broad resonances at δ 3.8–4.2 and δ 1.4–2.1 appeared. The absence of new resonances in the aromatic region indicates that the newly coupled species is not in a form of conjugated polypyrrole but probably in a dearomatized and oxygenated form of polypyrrole (see Supplementary Information for details). Elemental analysis of the resulting product also indicated that oxygen content has been significantly increased from 15.8% to 22.7% after the photopolymerization. From these observations, it seems that the pyrrole crosslinking reaction follows the oxygenation pathway (e) rather than conjugated polypyrrole pathway (d) in Scheme 1. Indeed, the CV of a photopolymerized PDI-4Py film on a glassy carbon electrode showed that the film is an insulating layer, which is different to the case of electropolymerization of PDI-4Py shown in Fig. 2b (see Supplementary Information for CV of a photopolymerized PDI-4Py film).
 | ||
| Scheme 1 A proposed mechanism of PDI-4Py photo-crosslinking. | ||
The morphologies of the PDI-4Py films generated by the two different methods were also different as shown in Fig. 4a. The electropolymerized PDI-networked film has a rough surface with lots of granules in its SEM image which are commonly observed in the case of electropolymerized conducting polymer. On the other hand, the surface of the photo-treated PDI-networked film was as smooth as spin-cast PDI-4Py film and no granules were observed as shown in Fig. 4b and 4c. XRD analysis of the films showed that they are amorphous (see Supplementary Information). In order to demonstrate photo-patterning with PDI-4Py, we prepared a negative mask of a Sogang University logo on a flexible transparent polyester film using a conventional laser printer (Fig. 4d left), spin-coated PDI-4Py on the back of the mask, and exposed the coated film to visible light (λ > 500 nm) for 10 min. After rinsing the film with chloroform to remove the printed mask, we were able to obtain a PDI-networked film with the logo pattern (Fig. 4d right).
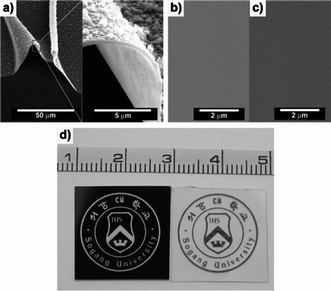 | ||
| Fig. 4 (a) SEM image of a PDI-networked film prepared by electro-treatment; (b) and (c) SEM images of spin-coated PDI-4Py film before and after light exposure. The exposure time was 1 hour. (d) Photographs of a transparent polyester film (left) with a negative mask printed on the back and a PDI-networked film (right) patterned on the front of the polyester film. | ||
In summary, we prepared PDI-nPy series with various numbers of pyrrole units (n = 0, 2, and 4) for coupling groups and found that PDI-networked films can be formed from PDI-4Pyvia pyrrole-coupling more efficiently than from PDI-2Py by electro-/photo-treatment. Especially, photo-crosslinking occurred by exposure to visible light (> 500 nm) that can excite the PDI moiety only. The chemical identity of the photo-crosslinked film seems to be different to that of electro-crosslinked film as judged by 1H-NMR spectroscopy and elemental analysis. The photo-treated film had a smooth granule-free surface while that of an electropolymerized PDI-networked film has significant roughness with lots of granules. We think our discovery will provide more flexibility in processing PDI containing films.
This work was supported by a Korea Research Foundation Grant (KRF-2004-005-C00010). We are grateful to Mr Jin Seok Lee for SEM imaging.
Notes and references
- (a) H. Langhals, Heterocycles, 1995, 40, 477 CrossRef CAS; (b) H. Langhals, Helv. Chim. Acta, 2005, 88, 1309 CrossRef CAS; (c) F. Würthner, Chem. Commun., 2004, 1564 RSC.
- R. Gvishi, R. Reisfeld and Z. Burshtein, Chem. Phys. Lett., 1993, 213, 338 CrossRef CAS.
- R. S. Loewe, K. Tomizaki, W. J. Youngblood, Z. Bo and J. S. Lindsey, J. Mater. Chem., 2002, 12, 3438 RSC.
- C. Ego, D. Marsitzky, S. Becker, J. Zhang, A. C. Grimsdale, K. Müllen, J. D. MacKenzie, C. Silva and R. H. Friend, J. Am. Chem. Soc., 2003, 125, 437 CrossRef CAS.
- S. K. Lee, Y. Zu, A. Herrmann, Y. Geerts, K. Müllen and A. J. Bard, J. Am. Chem. Soc., 1999, 121, 3513 CrossRef CAS.
- W. Lu, J. P. Gao, Z. Y. Wang, Y. Qi, G. G. Sacripante, J. D. Duff and P. R. Sundararajan, Macromolecules, 1999, 32, 8880 CrossRef CAS.
- K. Takahashi, I. Nakajima, K. Imoto, T. Yamaguchi, T. Komura and K. Murata, Sol. Energy Mater. Sol. Cells, 2003, 76, 115 CrossRef CAS.
- T. Kudo, M. Kimura, K. Hanabusa and H. Shirai, J. Porphyrins Phthalocyanines, 1998, 2, 231 CrossRef CAS.
- (a) R. A. Cormier and B. A. Gregg, Chem. Mater., 1998, 10, 1309 CrossRef CAS; (b) B. A. Gregg and R. A. Cormier, J. Phys. Chem. B, 1998, 102, 9952 CrossRef CAS; (c) C. W. Struijk, A. B. Sieval, J. E. J. Dakhorst, M. van Dijk, P. Kimkes, R. B. M. Koehorst, H. Donker, T. J. Schaafsma, S. J. Picken, A. M. van de Craats, J. M. Warman, H. Zuilhof and E. J. R. Sudhölter, J. Am. Chem. Soc., 2000, 122, 11057 CrossRef.
- (a) G. Seybold and G. Wagenblast, Dyes Pigm., 1989, 11, 303 CrossRef; (b) D. Dotcheva, M. Klapper and K. Müllen, Macromol. Chem. Phys., 1994, 195, 1905 CrossRef CAS; (c) W. E. Ford and P. V. Kamat, J. Phys. Chem., 1987, 91, 6373 CrossRef CAS.
- W. Choi, H. C. Ko, B. Moon and H. Lee, J. Electrochem. Soc., 2004, 151, E80 CrossRef CAS.
- D. Stanke, M. L. Hallensleben and L. Toppare, Synth. Met., 1995, 72, 95 CrossRef CAS.
- (a) H. Segawa, T. Shimidzu and K. Honda, J. Chem. Soc., Chem. Commun., 1989, 132 RSC; (b) A. Deronzier, P. Jardon, A. Martre, J. Moutet, C. Santato, V. Balzani, A. Credi, F. Paolucci and S. Roffia, New J. Chem., 1989, 1, 33 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: synthetic procedures and characterization data of PDI-nPy (n = 0, 2, 4). See DOI: 10.1039/b513847g |
| This journal is © The Royal Society of Chemistry 2006 |
