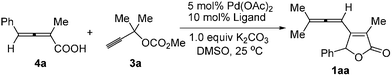
From allene to allene: a palladium-catalyzed approach to β-allenyl butenolides and their application to the synthesis of polysubstituted benzene derivatives†
Shengming
Ma
*ab,
Zhenhua
Gu
a and
Youqian
Deng
b
aState Key Laboratory of Organometallic Chemistry Shanghai Institute of Organic Chemistry Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, P. R. China. E-mail: masm@mail.sioc.ac.cn; Fax: +86 21-6416-7510
bLaboratory of Molecular Synthesis and Recognition, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang 310027, P. R. China
First published on 14th November 2005
Abstract
An allene to allene protocol for the synthesis of β-allenyl butenolides in moderate to high yields from 2,3-allenoic acids and propargylic carbonates catalyzed by Pd(OAc)2–TFP has been developed; the products were applied successfully to the Diels–Alder reaction with electron-deficient alkynes to afford polysubstituted benzene derivatives with an excellent regioselectivity.
Allenes are a class of compounds of current interest with unique reactivity.1–3 Recently, our group and others have established two- or three-component monoallene cyclization approaches to carbo- or heterocycles.2f,4 Hashmi et al. reported the Au(III)- and Pd(II)-catalyzed homodimerization reaction of 1,2-allenyl ketones forming corresponding 2- or 3-alkenyl furans.5 We also noticed the bisallene approaches of two 2,3-allenoic acids and the heterodimerization reaction of 2,3-allenoic acids with 1,2-allenyl ketones to afford bisbutenolides and 4-(furan-3′-yl)butenolides, respectively.6 Although these functionalized allenes afforded cyclic products efficiently, no allene structure was present in the products. In this communication we wish to report a Pd-catalyzed two-component reaction of 2,3-allenoic acid with propargylic carbonates, in which 2,3-allenoic acids cyclized to form the butenolide’s skeleton while a new allenyl moiety was formed from the propargylic carbonates (Scheme 1). Due to the high reactivity of the allene-ene functionality,7 the products have been successfully applied to the Diels–Alder reaction to afford polysubstituted benzene derivatives.
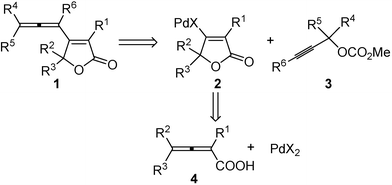 | ||
| Scheme 1 The proposed synthetic route. | ||
On the basis of the previous work, we reasoned that the β-allenyl butenolides 1 may be generally constructed from the reaction of propargylic carbonates 38 with intermediate 2, which may be generated easily via cyclic oxypalladation of the 2,3-allenoic acid 4 with Pd(II) species (Scheme 1).2f The challenge here is the formation of the allene moiety via β-heteroatom elimination9 and avoiding further reactions of the in situ formed allene moiety.
Our first approach was based on the reaction of 2,3-allenoic acid 4a and 3a. A survey of some of bidentate phosphine ligands indicates that (R)-BINAP can only afford a trace amount of product 1aa (Entry 1, Table 1). However, it is quite fortunate to see that dppb [1,4-bis(diphenylphosphino)butane] and dppp [1,3-bis(diphenylphosphino)propane] indeed afforded 1aa in 10% and 14% yields, respectively (Entries 2, and 3, Table 1). Furthermore, using dppe [1,2-bis(diphenylphosphino)ethane] as the ligand the reaction can afford a moderate yield of 1aa (Entry 4, Table 1). Further screening indicates that using tri-(2-furyl)phosphine (TFP) as the ligand can afford 1aa in 59% yield (Entry 7, Table 1).10 In terms of solvent effect, DMSO is better than other solvents, such as NMP, DMF, DME etc. (see supporting information for the results in different solvents†). The reduction of K2CO3 to 5 mol% led to a lower yield of 1aa (Entry 8, Table 1). Thus, we have established the proposed protocol for the cross-coupling cyclization of 2,3-allenoic acids 4 with 3 affording β-allenyl butenolides 1 (Entry 7, Table 1). The X-ray diffraction study of 1aa clearly proved the presence of the allenyl group in the β-position of the butenolide skeletons, which has not been further transformed under the current reaction conditions (Fig. 1).‡
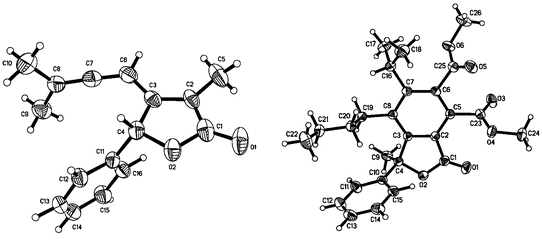 | ||
| Fig. 1 ORTEP structures of 1aa and 6fda. | ||
|
|
|||
|---|---|---|---|
| Entry | Ligand | Time/h | Yield of 1aa (%)b |
| a Under an argon atmosphere, the mixture of 0.25 mmol of 4a, 0.50 mmol of 3a, 5 mol% Pd(OAc)2, and 10 mol% ligand in 3 mL DMSO was stirred at 25 °C. b Isolated yield. c 5 mol% ligand was used. d 5 mol% of K2CO3 was used. | |||
| 1 | (R)-BINAPc | 19.5 | Trace |
| 2 | Dppbc | 17.5 | 10 |
| 3 | Dpppc | 21 | 14 |
| 4 | Dppec | 17 | 51 |
| 5 | PCy3 | 19.5 | 14 |
| 6 | P(o-tolyl)3 | 21 | 17 |
| 7 | TFP | 14 | 59 |
| 8d | TFP | 23 | 50 |
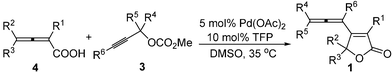
The optimized reaction conditions proved to be generally applicable, allowing for an efficient coupling of 2,3-allenoic acids 4 with 3. Some typical results are listed in Table 2. Various differently substituted 2,3-allenoic acids with R1 being alkyl (Entries 1–9, Table 2) or hydrogen (Entries 10–12, Table 2), R2 = aryl (Entries 1–8, and 10–12, Table 2) or alkyl (Entry 9, Table 2), and R3 being hydrogen (Entries 1–9, Table 2) or alkyl (Entries 10–12, Table 2) can smoothly afford the products 1 in moderate to high yields.
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Substrate 4 | Substrate 3 | Time/h | Yield of 1 (%)b | ||||
| R1 | R2 | R3 | R4 | R5 | R6 | |||
| a Under an argon atmosphere, the mixture of 0.25 mmol of 4, 0.50 mmol of 3, 5 mol% Pd(OAc)2, and 10 mol% TFP in 1 mL of DMSO was stirred at 35 °C for the time indicated in the table. b Isolated yield. c The reaction was carried out at 40 °C. | ||||||||
| 1 | Me | Ph | H (4a) | –(CH2)5– | H (3b) | 5 | 68 (1ab) | |
| 2 | Me | Ph | H (4a) | Et | Et | H (3c) | 10 | 60 (1ac) |
| 3 | Pr | Ph | H (4b) | Me | Me | H (3a) | 11 | 54 (1ba) |
| 4c | Pr | Ph | H (4b) | Et | Et | H (3c) | 9 | 64 (1bc) |
| 5 | Me | 1′-Nap | H (4c) | Me | Me | H (3a) | 10 | 63 (1ca) |
| 6c | Me | 1′-Nap | H (4c) | –(CH2)5– | H (3b) | 10 | 52 (1cb) | |
| 7c | Me | 1′-Nap | H (4c) | Et | Et | H (3c) | 10 | 68 (1cc) |
| 8 | Pr | 1′-Nap | H (4d) | Et | Et | H (3c) | 10 | 71 (1dc) |
| 9 | Me | Me | H (4e) | Me | Me | H (3a) | 6 | 54 (1ea) |
| 10 | H | Ph | Me (4f) | Et | Et | H (3c) | 12 | 82 (1fc) |
| 11 | H | Ph | Me (4f) | Me | Me | Bu (3d) | 16 | 93 (1fd) |
| 12 | H | Ph | Et (4g) | Me | Me | Ph (3e) | 17 | 91 (1ge) |
Synthesis of differently polysubstituted benzenes is a synthetic challenge.11 The Diels–Alder reaction of 1 with the electron-deficient alkynes 5a or 5b afforded polysubstituted benzo-γ-lactones 6 highly regioselectively in the presence of 7–14 mol% of hydroquninone in xylene (Scheme 2).12 The structure of these benzo-γ-lactones was further established by the X-ray studies of 6fda (Fig. 1).§
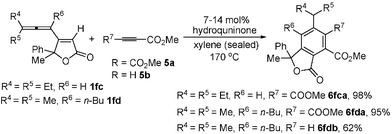 | ||
| Scheme 2 The Diels–Alder reaction of 1 with alkynes 5. | ||
In conclusion, we have established a protocol for palladium-catalyzed coupling reaction of 2,3-allenoic acids with propargylic carbonates forming β-allenyl butenolides, in which the newly formed allene moiety does not undergo further transformation. These compounds may be applied to the highly selective synthesis of polysubstituted benzene derivatives.
Notes and references
- (a) The Chemistry of the Allenes, ed. S. R. Landor, Academic, London, 1982, vol. 1 Search PubMed; (b) Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004, vol. 1–2 Search PubMed; (c) J. Tsuji, in Topics in Organometallic ChemistrySpringer-Verlag, Berlin Heidelberg, 2005 Search PubMed.
- (a) R. Zimmer, C. U. Dinesh, E. Nandanan and F. A. Khan, Chem. Rev., 2000, 100, 3067 CrossRef; (b) R. W. Bates and V. Satcharoen, Chem. Soc. Rev., 2002, 31, 12 RSC; (c) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS; (d) A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef; (e) H.-U. Reissig, W. Schade, M. O. Amombo, R. Pulz and A. Hausherr, Pure Appl. Chem., 2002, 74, 175 CrossRef CAS; (f) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef.
- For some of the most recent typical reactions of allenes, see: (a) B. M. Trost, C. Jakel and B. Plietker, J. Am. Chem. Soc., 2003, 125, 4438 CrossRef CAS; (b) J. Franzen and J.-E. Bäckvall, J. Am. Chem. Soc., 2003, 125, 6056 CrossRef CAS; (c) J. Huang and R. P. Hsung, J. Am. Chem. Soc., 2005, 127, 50 CrossRef CAS; (d) K.-J. Chang, D. K. Rayabarapu, F.-Y. Yang and C.-H. Cheng, J. Am. Chem. Soc., 2005, 127, 126 CrossRef CAS; (e) S.-S. Ng and T. F. Jamison, J. Am. Chem. Soc., 2005, 127, 7320 CrossRef CAS.
- (a) R. D. Walkup and G. Park, J. Am. Chem. Soc., 1990, 112, 1597 CrossRef CAS; (b) F. P. J. T. Rutjes, K. C. M. F. Tjen, L. B. Wolf, W. F. J. Karstens, H. E. Schoemaker and H. Hiemstra, Org. Lett., 1999, 1, 717 CrossRef CAS; (c) H. Ohno, M. Anzei, A. Toda, S. Ohshi, N. Fujii, T. Tanaka, Y. Takanato and T. Ibuka, J. Org. Chem., 2001, 66, 4904 CrossRef CAS; (d) Y.-H. Ha and S.-K. Kang, Org. Lett., 2000, 4, 1143; (e) S.-K. Kang, Y.-H. Ha, B.-S. Ko, Y. Lim and J. Jung, Angew. Chem., Int. Ed., 2002, 41, 343 CrossRef CAS; (f) H. Hamaguchi, S. Kosaka, H. Ohno and T. Tanaka, Angew. Chem., Int. Ed., 2005, 44, 1513 CrossRef CAS.
- (a) A. S. K. Hashmi, Angew. Chem., Int. Ed. Engl., 1995, 34, 1581 CrossRef; (b) A. S. K. Hashmi, T. L. Ruppert, T. Knöfel and J. W. Bats, J. Org. Chem., 1997, 62, 7295 CrossRef CAS; (c) A. S. K. Hashmi, L. Schwarz, J. –H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS.
- (a) S. Ma, Z. Yu and Z. Gu, Chem.–Eur. J., 2005, 11, 2351 CrossRef CAS; (b) S. Ma and Z. Yu, Angew. Chem., Int. Ed., 2002, 41, 1775 CrossRef CAS.
- (a) M. Murakami, K. Itami and Y. Ito, Angew. Chem., Int. Ed. Engl., 1995, 34, 2691 CrossRef CAS; (b) M. S. Sigman and B. E. Eaton, J. Am. Chem. Soc., 1996, 118, 11783 CrossRef CAS; (c) M. Murakami, K. Itami and Y. Ito, J. Am. Chem. Soc., 1997, 119, 7163 CrossRef CAS; (d) M. V. Chevliakov and J. Montgomery, J. Am. Chem. Soc., 1999, 121, 11139 CrossRef CAS; (e) K. M. Brummond, H. Chen, B. Mitasev and A. D. Casarez, Org. Lett., 2004, 6, 2161 CrossRef CAS; (f) T. Makino and K. Itoh, J. Org. Chem., 2004, 69, 395 CrossRef CAS.
- (a) J. Tsuji and T. Mandai, Angew. Chem., Int. Ed. Engl., 1995, 34, 2589 CrossRef CAS; (b) J. Tsuji and Y. Mandai, in Metal-Catalyzed Cross-Coupling Reaction, ed. F. Diedrich and P. J. Stang, Wiley-VCH, New York, 1998, p. 455 Search PubMed.
- The formation of allenes from β-H or β-heteroatom elimination of transition-metals is not easy, for β-H elimination to afford allenes, see: (a) S. Pivsa-Art, T. Satoh, M. Miura and M. Nomura, Chem. Lett., 1997, 823 CrossRef CAS; (b) C. Fu and S. Ma, Org. Lett., 2005, 7, 1605 CrossRef CAS. For β-heteroatom elimination to afford allenes, see: (c) S. Ogoshi, S. Nishiguchi, K. Tsutsumi and H. Kurosawa, J. Org. Chem., 1995, 60, 4650 CrossRef CAS.
- D. Bouyssi, J. Gore, G. Balme, D. Louis and J. Wallach, Tetrahedron Lett., 1993, 34, 3129 CrossRef CAS.
- (a) N. E. Schore, Chem. Rev., 1988, 88, 1081 CrossRef CAS; (b) M. Murakami, M. Ubukata, K. Itami and Y. Ito, Angew. Chem., Int. Ed., 1998, 37, 2248 CrossRef CAS; (c) T. Takahashi, M. Ishikawa and S. Huo, J. Am. Chem. Soc., 2002, 124, 388 CrossRef CAS; (d) T. Takhashi, Y. Li, P. Stepnicka, M. Kitamura, Y. Liu, K. Nakajima and M. Kotora, J. Am. Chem. Soc., 2002, 124, 576 CrossRef; (e) Z. Xi, K. Sato, Y. Gao, J. Lu and T. Takahashi, J. Am. Chem. Soc., 2003, 125, 9568 CrossRef CAS.
- (a) T. Tokoroyama, Y. Fukuyama, T. Kubota and K. Yokotani, J. Chem. Soc., Perkin Trans. 1, 1981, 1557 RSC; (b) P. Wu, M. Chu and F. W. Fowler, J. Org. Chem., 1988, 53, 963 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data of all new compounds. See DOI: 10.1039/b513371h |
| ‡ Crystal data for 1aa: C16H16O2, MW = 240.29, Orthorhombic, space group Pbca, Final R indices [I > 2σ(I)], R1 = 0.0409, wR2 = 0.0625, R indices (all data) R1 = 0.1877, wR2 = 0.0882, a = 11.592(2) Å, b = 14.568(3) Å, c = 16.342(3) Å, α = 90°, β = 90°, γ = 90°, V = 2759.7(8) Å3, T = 293(2) K, Z = 8, reflections collected/unique: 15724/3266 (Rint = 0.1496), number of observations [>2σ(I)] 917, parameters: 227. CCDC 270605. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513371h |
| § Crystal data for 6fda: C26H30O6, MW = 438.50, Monoclinic, space group P2(1)/n, Final R indices [I > 2σ(I)], R1 = 0.0511, wR2 = 0.0965, R indices (all data) R1 = 0.0992, wR2 = 0.1120, a = 10.0344(17) Å, b = 13.210(2) Å, c = 18.172(3) Å, α = 90°, β = 99.356(4)°, γ = 90°, V = 2376.7(7) Å3, T = 293(2) K, Z = 4, reflections collected/unique: 14147/5404 (Rint = 0.0670), number of observations [>2σ(I)] 2955, parameters: 328. CCDC 273892. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513371h |
| This journal is © The Royal Society of Chemistry 2006 |