Two regioisomeric and exclusively selective Hg(II) sensor molecules composed of a naphthalimide fluorophore and an o-phenylenediamine derived triamide receptor†
Jiaobing
Wang
a and
Xuhong
Qian
*ab
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, PO Box 89, Zhongshan Road 158, Dalian, 116012, China. Fax: 86-411-83673488; Tel: 86-411-83687466.
bShanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237, China. E-mail: xhqian@ecust.edu.cn; Fax: 86-21-64252603
First published on 25th November 2005
Abstract
This communication presents two regioisomeric fluorescent PET Hg(II) sensors RS, which contain a novel o-phenylenediamine based triamide receptor; RS could be used to detect Hg(II) with exclusive selectivity, high fluorescence enhancement, and fast and reversible response in a neutral buffered aqueous solution.
Mercury is considered a highly toxic and hazardous pollutant with recognized accumulative and persistent character in the environment and biota. Excessive exposure of the body to mercury will lead to DNA damage, mitosis impairment, and nervous system defects.1 The development of fluorescent mercury chemosensors which could be used to reveal the detrimental cellular role of mercury in vivo as well as to monitor mercury concentration temporally in mercury-contaminated sources, has received much attention. We previously reported a Hg(II)-selective fluorescent sensor which was based on two 4-aminonaphthalimide (ANI) fluorophores,2 and a derivative of which had been used for the real-time detection of Hg(II) inside a single living cell.3 Ono and Togashi developed an oligonucleotide-based fluorescent Hg(II) sensor employing the strategy of fluorescence resonance energy transfer (FRET).4 Nolan and Lippard reported a fluorescein-based turn-on Hg(II) sensor MS1.5 In addition, some fluorophore-appended macrocycles (e.g., thia aza or aza crown ether) were shown to respond to Hg(II) via photoinduced charge transfer or inhibition of photoinduced electron transfer (PET).6 Nonetheless, despite impressive advances, most of these systems suffer from limitations, which include interference from other chemically closely related metal ions (e.g., Cd(II), Pb(II), Cu(II)), comparatively low fluorescence enhancement, lack of water solubility, or coordination-induced fluorescence quenching. Thus, to enhance practical utility and further widen the scope of molecular sensing chemistry, there is a great need for the development of new sensors capable of detecting Hg(II) with better properties.
In this communication, we present two regioisomeric sensors (RS), which are based on the ANI fluorophore. A novel mercury receptor is attached to the imide nitrogen (RS1) or the 4-amino group (RS2) through an ethylene spacer. This receptor contains three rigid amide groups, which cooperate with the two o-phenylenediamine nitrogens to achieve selective binding of the Hg(II) cation. In addition, the three hydroxyl-groups incorporated in the receptor moiety combined with the 2-(2-hydroxyethoxy)ethyl group on the fluorophore endow RS with excellent water solubility (Scheme 1). RS exhibit distinct advantages such as exclusive selectivity, significant fluorescence enhancement, and quick and reversible Hg(II) binding in a neutral buffered aqueous solution. Details on the synthesis and characterization of RS are provided in supporting information.
 | ||
| Scheme 1 Structures of the two regioisomeric Hg(II) sensors. | ||
Fig. 1 shows the emission spectra of the two sensors at various Hg(II) concentrations. The fluorescence of the unbound RS2 (Φf = 0.007) is obviously weaker than that of RS1 (Φf = 0.032).7 This different fluorescence behavior is thought to be caused by a photogenerated electric field reported by de Silva and coworkers:8 briefly, in the “push–pull” π electron system of the ANI fluorophore, a considerable dipole (positive pole at the 4-amino terminus) is generated upon excitation, which in turn gives rise to a strong photogenerated electric field. As a consequence, the PET fluorescence quenching process in RS2 is more efficient because the electron leaving the o-phenylenediamine moiety can be easily transferred to the excited ANI fluorophore across the 4-amino position with its attractive electric field. While in RS1, a higher kinetic barrier for the PET fluorescence quenching process is posed by the repulsive electric field in the imide moiety of the excited ANI fluorophore. Accordingly, unbound RS1 displays a stronger fluorescence. Titration with increasing concentrations of Hg(II) retards PET gradually, and switches on the fluorescence of both sensors. In the presence of 1 equiv. of Hg(II), high fluorescence enhancement factors (EF, I/I0) of 5.8 and 34.7 are achieved by the two sensors RS1 (Φf = 0.19) and RS2 (Φf = 0.24), respectively.
![Emission spectra of RS1 (left, λex. = 451 nm, λmax.em. = 545 nm) and RS2 (right, λex. = 443 nm, λmax.em. = 537 nm) in phosphate (0.1 M) solution (pH = 7.5, 23 °C) in the presence of different concentrations of Hg(ii). The up-arrow indicates the increase of [Hg(ii)] from 0 to 10 μM. The sensor concentration is 10 μM.](/image/article/2006/CC/b511319a/b511319a-f1.gif) | ||
| Fig. 1 Emission spectra of RS1 (left, λex. = 451 nm, λmax.em. = 545 nm) and RS2 (right, λex. = 443 nm, λmax.em. = 537 nm) in phosphate (0.1 M) solution (pH = 7.5, 23 °C) in the presence of different concentrations of Hg(II). The up-arrow indicates the increase of [Hg(II)] from 0 to 10 μM. The sensor concentration is 10 μM. | ||
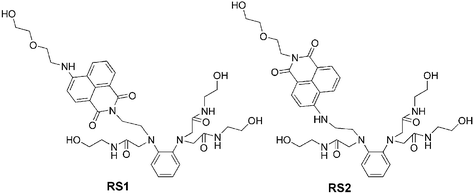
Fig. 2 displays the absorption spectra of RS2 taken in the course of the titration. The presence of two isosbestic points at 331 nm and 443 nm clearly reveals that only two species coexist in the aqueous solution corresponding to free RS2 and RS2–Hg(II) complex. The band centered at 456 nm progressively shifts to 433 nm with the addition of Hg(II), indicating that the electron donating 4-amino nitrogen of the fluorophore, in the ground state, participates in RS2–Hg(II) complexation. While in the internal charge transfer (ICT) excited state, Hg(II) is ejected from the 4-amino nitrogen due to the partial positive charge photogenerated on it,8,9 and is only grasped by the triamide receptor. This is reflected by the absence of an emission spectral shift. In contrast, no spectral shift is observed in either the absorption (λabs = 449 nm, log ε = 4.1) or emission spectra of RS1, indicating that none of the imido oxygens in the imide moiety acts as a Hg(II) chelating site in either the ground state or the ICT excited state.10 This is reasonable considering that “soft” Hg(II) ion prefers a “soft” coordination atmosphere. The above results suggest that the new o-phenylenediamine-based triamide receptor could suitably accommodate a Hg(II) ion by itself.
 | ||
| Fig. 2 Absorption spectra of RS2 (10 μM) in the presence of different concentrations of Hg(II). | ||
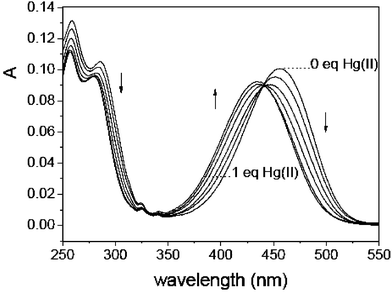
1H-NMR studies of the Hg(II) complexes of RS1 and RS2 present some useful information consistent with the above mentioned photophysical results. The peaks (around 6.50 in Fig. 3b or around 6.80 in Fig. 3d) assigned to the four aromatic protons in the benzene ring of the triamide receptor shift significantly to the downfield and get broadened around 7.35 ppm. Obviously, N–Hg(II) (N, o-phenylenediamine nitrogen) complexation lowers the electron density of the benzene ring and a deshielding effect is responsible for these NMR spectral changes. In contrast, only moderate signal changes are observed for the aromatic protons in the ANI fluorophore of RS1, indicating that Hg(II)–fluorophore interactions are very weak and no direct O–Hg(II) coordinate bond is formed between the imido oxygen and the grasped Hg(II) ion. The proton in the ortho-position of the electron donating 4-amino group of the ANI fluorophore of RS2 undergoes a clear downfield shift from 5.65 to 6.01 ppm, suggesting that the 4-amino nitrogen does participate in Hg(II)–RS2 complexation11 in the ground state as revealed by Fig. 2.
 | ||
| Fig. 3 Partial 1H-NMR spectra of RS (in D2O, 19 mM): a) RS1 in the presence of 2 equivalent of Hg(II); b) free RS1; c) RS2 in the presence of 2 equivalent of Hg(II); d) free RS2. | ||
pH titration profiles of RS in the presence or absence of Hg(II) exhibit a quite different feature and provide a deep insight into the interactions between Hg(II) and the receptor unit. The fluorescence intensity of the RS–Hg(II) solution steadily decreases on titration with HClO4, a process related to the dissociation of the RS–Hg(II) complex. And when the pH is decreased to 3.2, the fluorescence intensity comes to the same level as that of the unbound sensor, suggesting an entire dissociation; thus Hg(II) no longer has influence on the fluorescence of the sensor. The plot of fluorescence intensity vs. pH (from 3.2 to 8.3) displays a sigmoidal curve (Fig. 4). A pKa of 5.6, distinct from that of the unbound sensor (pKaRS1 = 2.4, pKaRS2 = 2.7) is derived. This result indicates a coordination-promoted amide deprotonation process, consistent with the amide–Hg(II) coordination chemistry reported by Goodgame et al.12 The observed pH titration profiles of RS resemble closely those of a Cu(II) sensor with a dioxotetraaza receptor reported by Fabbrizzi and co-workers, which chelates Cu(II) in the same way, through an amide deprotonation mechanism.13
![pH titration profiles of the unbound RS2 (triangles) and RS2–Hg(ii) complex (circles, [RS2] : [Hg(ii)] = 1 ∶ 1) in aqueous solution.](/image/article/2006/CC/b511319a/b511319a-f4.gif) | ||
| Fig. 4 pH titration profiles of the unbound RS2 (triangles) and RS2–Hg(II) complex (circles, [RS2] : [Hg(II)] = 1 ∶ 1) in aqueous solution. | ||
Job's plot indicates that RS form a 1 ∶ 1 complex with Hg(II) in aqueous solution (see supporting information). The association constants log Ka for RS1– and RS2–Hg(II) complex are determined to be 7.3 and 7.1, respectively. RS exhibit quick and reversible Hg(II) coordination in aqueous solution (see supporting information). Their fluorescence is switched on immediately after the addition of Hg(II). It was found that 1 equiv. of the heavy metal ion chelator N,N,N′,N′-tetra(2-picolyl)ethylenediamine (TPEN) effectively competes with RS for Hg(II) binding and switches off the fluorescence to 27.8% (RS1) or 5.6% (RS2) of the background value.
The selectivity of RS for Hg(II) over various detected cations was rather high. Even chemically closely related metal ions (e.g., Cu(II), Cd(II), Pb(II)) did not quench or generate fluorescence (Fig. 5). Also, the presence of these cations did not interfere with Hg(II) analysis (see supporting information).
![Fluorescence intensity change profiles of RS2 in the presence of 2 equiv. of the selected cations ([RS2] = 10 μM, 0.1 M phosphate, pH 7.5, 23 °C). λex. = 443 nm, the emission was integrated from 460 to 700 nm.](/image/article/2006/CC/b511319a/b511319a-f5.gif) | ||
| Fig. 5 Fluorescence intensity change profiles of RS2 in the presence of 2 equiv. of the selected cations ([RS2] = 10 μM, 0.1 M phosphate, pH 7.5, 23 °C). λex. = 443 nm, the emission was integrated from 460 to 700 nm. | ||
In summary, we have developed two regioisomeric turn-on Hg(II) sensors RS, which contain a novel o-phenylenediamine based triamide receptor. Both sensors display exclusive Hg(II) ion selectivity and could be used to detect Hg(II) with fast and reversible response in a neutral buffered aqueous solution. However, RS2 exhibits a higher sensitivity taking advantage of its larger EF. Comparison of the two regioisomers reveals some subtle but substantial differences in the fluorophore–receptor interactions, which suggests that much attention should be paid to the fluorophore–receptor-connecting fashion when a fluorescent molecular sensor is designed. Further work aimed at RS's biological utilization as well as the development of materials for mercury-contamination treatment is currently under way.
This work was supported by the National Basic Research Program of China (2003CB114400) and the National Natural Science Foundation of China. We thank the referees very much for their pertinent comments: some sentences in the text originate from their comments. We also thank Dr Y. Xiao and Dr J. Cui for valuable discussions.
Notes and references
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149 CrossRef CAS.
- X. Guo, X. Qian and L. Jia, J. Am. Chem. Soc., 2004, 126, 2272 CrossRef CAS.
- Z. Zhang, X. Guo, X. Qian, Z. Lv and F. Liu, Kidney Int., 2004, 66, 2279 CrossRef CAS.
- A. Ono and H. Togashi, Angew. Chem., Int. Ed., 2004, 43, 4300 CrossRef CAS.
- E. M. Nolan and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 14270 CrossRef CAS.
- (a) A. B. Descalzo, R. Martínez-máñez, R. Radeglia, K. Rurack and J. Soto, J. Am. Chem. Soc., 2003, 125, 3418 CrossRef CAS; (b) M. J. Choi, M. Y. Kim and S.-J. Chang, Chem. Commun., 2001, 1664 RSC; (c) F. Sancenón, R. Martínez-máñez and J. Soto, Chem. Commun., 2001, 2262 RSC; (d) K. Rurack, M. Kollmannsberger, U. Resch-Genger and J. Daub, J. Am. Chem. Soc., 2000, 122, 968 CrossRef CAS; (e) L. Prodi, C. Bargossi, M. Montalti, N. Zaccheroni, N. Su, J. S. Bradshaw, R. M. Izatt and P. B. Savage, J. Am. Chem. Soc., 2000, 122, 6769 CrossRef CAS.
- The fluorescence quantum yields were determined by using N-butyl-4-butylamino-1,8-naphthalimide in absolute ethanol (Φf = 0.81) as a reference. M. S. Alexiou, V. Tychopoulos, S. Ghorbanian, J. H. P. Tyman, R. G. Brown and P. I. Brittain, J. Chem. Soc., Perkin Trans. 2, 1990, 837 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne, J.-L. Habib-Jiwan, C. P. McCoy, T. E. Rice and J.-P. Soumillion, Angew. Chem., Int. Ed. Engl., 1995, 34, 1728 CrossRef.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef; L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59 CrossRef CAS.
- However, ion–dipole interactions between the chelated Hg(II) ion and the imide moiety of the excited ANI fluorophore in RS1–Hg(II) complex might occur and result in a heavy atom effect, which combined with the photogenerated electric field effect prevents RS1 from achieving higher fluorescence enhancement rates.
- Although 2D NMR techniques were also employed for the assignment of the aliphatic protons, efforts aimed at abstracting more detailed information about the actual RS–Hg(II) complexation structure failed, because the peaks corresponding to the aliphatic protons were congested in a short resonance segment (3.0–4.0 ppm) and got broadened after Hg(II) was added.
- D. M. L. Goodgame, A. M. Khaled, C. A. O'Mahoney and D. J. Williams, J. Chem. Soc., Chem. Commun., 1990, 850 Search PubMed.
- L. Fabbrizzi, M. Licchelli, P. Pallavicini, A. Perotti and D. Sacchi, Angew. Chem., Int. Ed. Engl., 1994, 33, 1975 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and spectroscopic data. See DOI: 10.1039/b511319a |
| This journal is © The Royal Society of Chemistry 2006 |
