Pharmaceutical quality control of acid and neutral drugs based on competitive self-assembly in amphiphilic systems
Ana Pedraza, María Dolores Sicilia, Soledad Rubio and Dolores Pérez-Bendito*
Department of Analytical Chemistry, Faculty of Sciences, University of Córdoba, Edificio Anexo Marie Curie. Campus de Rabanales, 14071- Córdoba, Spain. E-mail: qa1pebem@uco.es; Fax: +34-957-218644; Tel: +34-957-218644
First published on 17th November 2005
Abstract
An aggregation parameter-based methodology for determining acid and neutral drugs in pharmaceutical dosage forms is presented. The method is based on competitive self-assembly in ternary dye–surfactant–drug aqueous mixtures. Dyes bearing charge of opposite sign to that of surfactants bind to surfactant to form mixed dye–surfactant aggregates, which are monitored from changes in the spectra features of the dye. The drug competes with the dye to interact with the surfactant to form drug–surfactant aggregates, which results in a decrease in the surfactant to dye binding degree proportional to the drug concentration in the aqueous solution. Coomassie Brilliant Blue G (CBBG) and didodecyldimethylammonium bromide (DDABr) were the dye and surfactant reactant used, respectively. The suitability of the surfactant to dye binding degree (SDBD) method to determine drugs with very different molecular structure: propionic (flurbiprofen, ibuprofen, naproxen and ketoprofen) and acetic (diclofenac, felbinac and zomepirac) acids, indolines (indomethacin and sulindac), glycyrrhetinic acid derivatives (carbenoxolone and enoxolone), salicylates (diflunisal and phenyl salicylate), oxicams (meloxicam, piroxicam and tenoxicam), pyrazolones (phenylbutazone and sulfinpyrazone) and hydrocortisones (dexamethasone and prednisolone) has been proved. The proposed method was successfully applied to the determination of drugs in commercial formulates (effervescent granulates, tablets, suppositories, gels and blisters) with a minimum sample treatment (dilution of liquid samples and dissolution of solid samples).
Introduction
Amphiphiles, compounds that have both hydrophobic and hydrophilic groups, play an essential role in our world. Natural amphiphiles (e.g. phospholipids, glucolipids, llipopeptides, liposaccharides, etc.) are the principal component of biological membranes and guarantee the transport and exchange of materials.1 Both synthetic and natural amphiphilic substances are widely used in the industry, agriculture, medicine, pharmacology, etc. They are key ingredients of household products in every day use2 (e.g. laundry detergents, shampoos, textile, softeners, cosmetics, etc.) and are widely used as foodstuffs additives3,4 (antimicrobial preservatives, antioxidants, emulsifier, dough conditioners, etc.). Finally, amphiphiles acquire a special relevance in the pharmaceutical industry, since most of the therapeutic drugs are selected or designed to be amphiphilic in order to penetrate cells and tissues and to favour interaction of drug molecules with receptor sites.5 Thus, antibiotics, anti-cancer drugs, antihistamines, tricyclic antidepressant, phenothiazines and anti-inflammatory drugs, among others, are amphiphiles.The characteristic molecular structure of amphiphiles, known as amphipatic structure, is based on two phenomena which differentiate these compounds from other chemical substances: (a) adsorption at interfaces (gas–liquid, liquid–liquid and solid–liquid), which results in the formation of monolayers and multilayers, and (b) self-assembly association in bulk solution, which results in the formation of ordinary and reverse micelles, vesicles, bilayers, microemulsions, etc.
Based on the capability of amphiphiles to form self-assemblies in aqueous bulk solutions, our research group has developed two new analytical approaches,6,7 which have been demonstrated to be a powerful tool for the determination of amphiphilic compounds. The mixed aggregate (MA) method,6,8 based on measurements of the critical aggregate concentration of amphiphile mixtures, has been successfully used for the determination of global indexes (total concentration of non-ionic,6,9 anionic10 and cationic surfactants11 in aqueous environmental samples) and for quality control of household products,11,12 foodstuffs13 and pharmaceuticals.14–16 The surfactant to dye binding degree (SDBD) method,7 based on the effect of amphiphilic compounds on the degree of binding of a surfactant to dye molecules, has been also demonstrated to be suitable for environmental analysis7 and pharmaceutical quality control.17–19 The quantification of the total concentration of anionic surfactants in sewage samples7 and the determination of phenamic acids17 and fusidane antibiotics18 in pharmaceutical preparations have been reported.
The amphiphilic character of the analyte is not an essential requirement for MA and SDBD methods; the determination of aromatic hydrotropic drugs using the SDBD method has been recently described.19 However, the occurrence of both ionic/polar and hydrophobic moieties in the analyte molecules results in increased drug–surfactant bond strength, which permits one to develop more sensitive methods.
This work deals with the evaluation of the SDBD method to be used as a general methodological approach for the quality control of drugs in pharmaceutical preparations. For this study, a wide number of acid, neutral and basic amphiphilic drugs with very different molecular structure: propionic and acetic acids, indolines, glycyrrhetinic acid derivatives, salicylates, oxicams, pyrazolones, hydrocortisones, phenothiazines, ethanolamines and dibenzazepines were selected. The anionic dye Coomassie Brilliant Blue G (CBBG) was used as an inductor of aggregates of the cationic surfactant didodecyldimethylammonium bromide (DDABr), which were monitored from changes in the spectral features of the dye. In ternary CBBG–DDABr–drug mixtures, the drug competed with the dye to interact with the surfactant, which resulted in a decrease in the degree of binding of surfactant to dye molecules. Ibuprofen was selected as a model for the optimization of experimental conditions and the analytical features of the proposed method for the determination of the different drugs studied were evaluated. The feasibility of the SDBD method for the direct quantitation of drugs in pharmaceutical preparations was investigated by analysing effervescent granulates, tablets, suppositories, gels and blisters.
Experimental
Apparatus
A Metrohm 794 Basic Titrino titrator (Herisau, Switzerland) equipped with a 20 ml autoburet, a fan stirrer and a titration vessel was used for titrations. The detection unit was a Metrohm 662 spectrophotometer furnished with an immersion probe (1 cm path length). The instrument control and data processing were performed using a computer made up of a Pentium 4 processor, a Microsoft Windows XP operating system and a Metrohm TiNet 2.5 Light software. Light scattering measurements for drug–surfactant interactions studies were performed using a Hitachi U-2000 spectrophotometer (Tokyo, Japan).Reagents and solutions
Commercially available highest-grade reagents were used throughout, without further purification. Aqueous solutions (10 mM) of the surfactants: didodecyldimethylammonium bromide (DDABr), didecyldimethylammonium bromide (DDeABr), ditetradecyldimethylammonium bromide (DTeABr) (Fluka Chemie GmgH, Buchs, Germany) and cetylpyridinium chloride (Serva Feinbiochemica GmbH, Heidelberg, Germany) were prepared in distilled water. A 100 mM dodecyltrimethylammonium bromide (DTABr, Sigma Aldrich Chemie GmbH, Steinheim, Germany) was also prepared in distilled water. Aqueous solutions of the dyes: Coomassie Brilliant Blue G (CBBG, 0.14 mM), Acid Red 97 (AR97, 0.4 mM), Acid Red 88 (AR88, 1.2 mM) and Acid Orange 6 (AO6, 0.4 mM), were made by dissolving the reagent in 1 l of distilled water with sonication for 15 min. These solutions were prepared at least 24 h prior to use. CBBG was purchased from Sigma Chemical Co. (St. Louis, MO, USA) and AR97, AR88 and AO6 were supplied by Sigma Aldrich Chemie GmbH (Steinheim, Germany). The buffer solution used consisted of 0.1 M KH2PO4 with the pH adjusted to 5.9 with 2 M NaOH. Stock solutions (2 g l−1) of therapeutic drugs, ibuprofen, enoxolone (Fluka Chemie GmbH), flurbiprofen, ketoprofen, diclofenac, zomepirac, indomethacin, sulindac, phenyl salicylate, diflunisal, phenylbutazone, suphinpyrazone, meloxicam, carbenoxolone, dexamethasone, prednisolone (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), naproxen and felbinac ( Aldrich Chemie GmbH, Steinheim, Germany), were prepared in ethanol. Piroxicam (0.5 g l−1) and tenoxicam (0.1 g l−1) stock solutions were also made by dissolving each drug (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) in ethanol. Promethazine (2 g l−1), diphenhydramine (1 g l−1) and northiptyline (1 g l−1) stock solutions were prepared by dissolving the corresponding chlorhydrate (Sigma Chemical Co., St. Louis, MO, USA) in distilled water.Recommended procedure for the determination of acid and neutral drugs
Volumes of 4.0 ml of 0.14 mM CBBG solution (stable for one month), 12.5 ml of 0.1 M phosphate buffer (pH = 5.9) and an aliquot of standard or treated sample solution to give a final drug concentration between about 0.2 and 50 mg l−1 were placed in a 25 ml volumetric flask. Then, distilled water was added to the mark. This solution was placed in a 50 ml titration vessel and titrated with 1.0 mM DDABr delivered from the buret at a rate of 10 ml min−1. The stirring rate was set at 700 rpm. Titration curves were obtained by recording the absorbance at 590 nm as a function of the titrant volume.The concentration of therapeutic drug was determined from the following equation:7
| mS* − mS = βAmA | (1) |
![(A) Spectra for CBBG (22 µM) in (1) the absence and (2) the presence of 120 µM DDABr. (B) Variation of the absorbance of CBBG (22 µM) at 590 nm as a function of the volume of titrant (1 mM DDABr) added to a titration vessel containing (1) non-drug or ibuprofen at a concentration of (2) 14.5 µM, (3) 24.0 µM and (4) 44.0 µM. [phosphate buffer] = 50 mM; pH = 5.9. Spectra 2 in (A) recorded against 120 µM DDABr 50 mM buffer phosphate blank solutions.](/image/article/2006/AN/b509978a/b509978a-f1.gif) | ||
| Fig. 1 (A) Spectra for CBBG (22 µM) in (1) the absence and (2) the presence of 120 µM DDABr. (B) Variation of the absorbance of CBBG (22 µM) at 590 nm as a function of the volume of titrant (1 mM DDABr) added to a titration vessel containing (1) non-drug or ibuprofen at a concentration of (2) 14.5 µM, (3) 24.0 µM and (4) 44.0 µM. [phosphate buffer] = 50 mM; pH = 5.9. Spectra 2 in (A) recorded against 120 µM DDABr 50 mM buffer phosphate blank solutions. | ||
Light scattering studies on the formation of drug–surfactant aggregates
In a 25 ml volumetric flask were placed, in sequence, 12.5 ml of 0.1 M phosphate buffer (pH = 5.9), 75 µl of 2 g l−1 ibuprofen solution, appropriate volumes of 1.0 mM DDABr to give a final surfactant concentration between 0 and 75 µM, and distilled water to the mark. These ingredients were mixed by shaking and, then, the stopclock was started. An aliquot of the mixture was transferred to a 1 cm cell and its absorbance spectra between 200 and 500 nm were recorded exactly 5 min after the stopclock was started.Determination of anti-inflammatory drugs in pharmaceutical preparations
Several pharmaceutical samples including blisters, tablets, gels, suppositories and effervescent granulates were analysed. Their compositions are shown in Table 1. Blisters were diluted with ethanol without further treatment; these diluted solutions contained about 0.5 g l−1 of drug. For the analysis of solid formulations (tablets, gels, suppositories and effervescent granulates), an amount of sample containing about 200 mg of drug was accurately weighed and dissolved in about 80 ml of ethanol with sonication for 15 min (tablets and effervescent granulates) or with stirring for 30 min (gels and suppositories). Extraction of the drug from suppositories was performed under slight heating (about 30 °C) to aid dissolution of samples. If any insoluble material was present in the solution after this treatment, it was removed by filtration and washed several times with ethanol. Dissolved samples were diluted to 100 ml with ethanol and aliquots of them were analysed as described above.| Drugs | Commercial formulationa | Drugs nominal value | Drugs found (s)b |
|---|---|---|---|
| a Composition of commercial formulations: Neobrufen (Abbott Laboratories, Spain): saccharose, 51.45% (w/w) and others excipients (sodium carbonate, cellulose, sodium croscarmellose, malic acid, povidone, sodium hydrogen carbonate, orange essence, sodium dodecyl sulfate and sodium saccharin), 39.28% (w/w). Voltarén Retard (Novartis Pharmacéutica, Spain): saccharose, 40.16% (w/w) and others excipients (colloidal anhydrous silica, cetylic alcohol, magnesium stearate, povidone, hypromellose, red iron oxide, polysorbate 80, talc, titanium IV oxide and polyethylene glycol 8000), 26.08% (w/w). Inacid (Merck Sharp & Dohme, Spain): excipients (butylhidroxyanisol and butylhidroxytoluene), 93.9% (w/w). Sanodin (Altana Pharma, Spain): excipients (karaya gum, vaseline oil and polyethylene), 98% (w/w). Dolobid (Frosst Laboratories, Spain): excipients (sodium croscarmellose, hydroxypropyl methylcellulose, starch, propylene glycol, sodium stearyl fumarate, and titanium dioxide), 41% (w/w). Feldene (Nefox Pharma, Spain): ethanol, 12.8% (v/v) and others excipients (benzilic alcohol, sodium phosphate, nicotinamide, 1,2 propanediol, sodium hydroxyde, hydrochloric acid and water), 85.2% (w/v). Butazolidina (Padró Laboratory, Spain): excipients (triglycerides), 87.45% (w/w). Fortecortín (Merck Pharma and Chemistry, Spain): excipients (lactose, starch, talc and magnesium stearate), 98.99% (w/w).b s denotes standard deviation (n = 6). | |||
| Ibuprofen | Neobrufen (effervescent granulates) | 92.7 mg g−1 | 93.1 (0.4) mg g−1 |
| Diclofenac | Voltarén Retard (tablets) | 314 mg g−1 | 314 (1) mg g−1 |
| Indomethacin | Inacid (suppositories) | 60 mg g−1 | 60 (1) mg g−1 |
| Carbenoxolone | Sanodin (gel) | 20 mg g−1 | 20.1 (0.4) mg g−1 |
| Diflunisal | Dolobid (tablets) | 590 mg g−1 | 590 (7) mg g−1 |
| Piroxicam | Feldene (blisters) | 20 g l−1 | 20 (0.2) g l−1 |
| Phenylbutazone | Butazolidina (suppositories) | 125.4 mg g−1 | 125.3 (0.7) mg g−1 |
| Dexamethasone | Fortecortín (tablets) | 10.05 mg g−1 | 10.1 (0.1) mg g−1 |
Results and discussion
Competitive self-assembly in ternary aqueous drug–CBBG–DDABr mixtures
In aqueous solutions containing the surfactant didodecyldimethylammonium bromide (DDABr), the dye Coomassie Brilliant Blue G (CBBG) and an amphiphilic drug, two types of mixed aggregates, dye–surfactant and drug–surfactant aggregates, are simultaneously formed. The formation of CBBG–DDABr aggregates can be monitored from changes in the features of the dye spectrum (compare curves 1 and 2 in Fig. 1A). Thus, at DDABr concentrations higher than a threshold value (37 ± 4 µM under the experimental conditions given in the Experimental Section), the absorbance measured for CBBG at 590 nm decreases as a function of the surfactant concentration (Fig. 1B). These absorbance changes have been previously demonstrated to be related to the formation of CBBG–DDABr aggregates with well-defined stoichiometries.7The amount of DDABr required to form dye–surfactant aggregates of a given stoichiometry increases as a function of the drug concentration in the aqueous mixture (compare curves 1 with curves 2–4 in Fig. 1B) as a result of the competition established between drug and dye molecules to interact with the cationic surfactant to form mixed aggregates. The capability of amphiphilic drugs to form mixed aggregates with DDABr have been previously demonstrated.17,18Fig. 2 shows the results obtained from light scattering studies for the formation of ibuprofen–DDABr aggregates. The addition of DDABr to ibuprofen aqueous solutions (pH = 5.9) results in an increase in the absorbance measured for the drug in the UV region (compare spectra 1 with spectra 2–3 in Fig. 2A), which is related to the formation of drug–surfactant aggregates. The broken line obtained by plotting the absorbance measured for ibuprofen at 220 nm as a function of the [DDABr]/[ibuprofen] molar ratio indicates the formation of DDABr ∶ ibuprofen aggregates of different stoichiometries (between 1 ∶ 2 and 5 ∶ 2) as the cationic surfactant concentration increases.
![(A) Spectra for ibuprofen (29 µM) in (1) the absence and (2,3) the presence of DDABr: (2) 29 µM and (3) 58 µM. (B) Variation of the absorbance of ibuprofen (29 µM) at 220 nm as a function of the [DDABr]/[ibuprofen] molar ratio. [phosphate buffer] = 0.05 M; pH = 5.9.](/image/article/2006/AN/b509978a/b509978a-f2.gif) | ||
| Fig. 2 (A) Spectra for ibuprofen (29 µM) in (1) the absence and (2,3) the presence of DDABr: (2) 29 µM and (3) 58 µM. (B) Variation of the absorbance of ibuprofen (29 µM) at 220 nm as a function of the [DDABr]/[ibuprofen] molar ratio. [phosphate buffer] = 0.05 M; pH = 5.9. | ||
Experimental variables affecting the formation of mixed aggregates and sensitivity
Analytical response obtained for drugs using the SDBD method depends on both the molecular structure of the analyte and the experimental conditions used for its determination. The effect of the nature of the analyte on the analytical signal obtained will be discussed later. In this section, a systematic study of the effect of different variables (molecular structure of the dye and the surfactant reactant, pH, dye and buffer concentration, organic additives and temperature) on mS , mS* and the measurement parameter (mS − mS*) is presented. Ibuprofen was the drug selected as a model to perform this study.The surfactant reactants tested were dodecyltrimethylammonium bromide (DTABr), cetylpyridinium chloride (CPC) and dialkyldimethylammonium bromides (DDeABr, DDABr and DTeABr). No response was obtained for ibuprofen at concentrations up to 30 mg l−1 by using cationic surfactants containing a unique long alkyl chain in their molecular structure (i.e. DTABr or CPC). However, all double long alkyl chain cationic surfactants assayed (alkyl chain length between 10 and 14 carbon atoms) provided mS* values significantly higher than that of mS at ibuprofen concentrations at the low mg l−1 level. Increased drug–surfactant hydrophobic interactions due to the occurrence of a second hydrocarbon chain in the molecular structure of the cationic surfactant could explain the different behaviour observed for both types of ammonium salts. The highest reproducibility in the determination of the titration end-point was obtained using DDABr as titrant, so it was the surfactant reactant selected for further studies.
Fig. 3A shows the molecular structure of the dyes investigated in this study. Addition of DDABr to dye aqueous solutions caused spectral modifications for all dyes evaluated. Fig. 3B shows these modifications and the wavelength at which the titration curves were recorded. The results obtained for mS and mS* as a function of the dye concentration are depicted in Fig. 4. The lowest dye concentration tested was that providing a reproducible measurement of the titration end-point, whereas the highest dye concentration assayed was that not causing saturation of the detector response. Since both mS and mS* values linearly increased as a function of the dye concentration in a similar way, the measurement parameter was kept constant over the whole concentration range studied for each dye. The molecular structure of the dye greatly affected the sensitivity achieved for the determination of drugs using the SDBD method. The sensitivity decreased as the dye–surfactant bond strength increased and followed the order AR97 < AR88 < AO6 < CBBG. The low sensitivity obtained using AR97 (Fig. 4A) is a consequence of the two anionic groups and the great hydrophobic moiety in its molecular structure, which results in strong electrostatic and hydrophobic interactions with DDABr. For dyes bearing a unique anionic group (i.e. AR88 and AO6), the sensitivity is higher when the hydrophobic moiety is lower (see Fig. 4B and C). Repulsive electrostatic interactions between positively charged groups in CBBG and DDABr probably disfavour the formation of CBBG–DDABr aggregates, which permitted the drug to very effectively compete with the dye to interact with the surfactant (Fig. 4D).
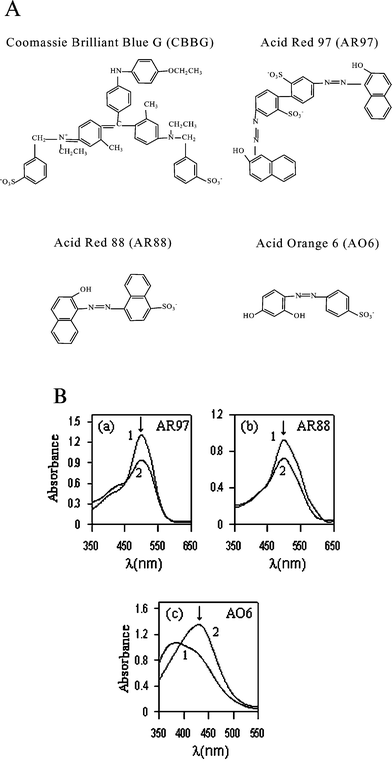 | ||
| Fig. 3 (A) Molecular structures of the tested dyes. (B) Spectra for Acid Red 97 (AR97, 25 µM), Acid Red 88 (AR88, 50 µM) and Acid Orange 6 (AO6, 65 µM) in (1) the absence and (2) the presence of DDABr at a concentration of: (a) 30 µM, (b) 20 µM and (c) 120 µM. pH = 5.9 adjusted with 1 M NaOH. Wavelengths selected to record titration curves for each dye are marked on their corresponding spectra with the symbol: ↓. | ||
![Influence of the concentration of the dyes: (A) AR97, (B) AR88, (C) AO6 and (D) CBBG on (○) mS and (◆) mS*. pH = 5.9 adjusted with 1 M NaOH; [ibuprofen] = (A) 50 mg l−1, (B) 30 mg l−1, (C) 12 mg l−1 and (D) 12 mg l−1.](/image/article/2006/AN/b509978a/b509978a-f4.gif) | ||
| Fig. 4 Influence of the concentration of the dyes: (A) AR97, (B) AR88, (C) AO6 and (D) CBBG on (○) mS and (◆) mS*. pH = 5.9 adjusted with 1 M NaOH; [ibuprofen] = (A) 50 mg l−1, (B) 30 mg l−1, (C) 12 mg l−1 and (D) 12 mg l−1. | ||
The pH of the titration medium should be adjusted between 5.5 and 7.0 in order to ensure reproducibility in the determination of the titration end-point. Within this interval, decreased mS and mS* values were obtained as the pH value increased. The measurement parameter, however, was kept constant. For adjustment of the pH, a phosphate buffer (pH = 5.9) was used. Electrolytes are known to decrease electrostatic interactions between oppositely charged organic molecules, so, phosphate buffer disfavoured the formation of both dye–surfactant and drug–surfactant aggregates. The effect of phosphate buffer on the interaction between dye and surfactant was more pronounced than that on the drug–surfactant interaction, which resulted in an increase in the measurement parameter (about two times) as the buffer concentration increased up to about 0.04 M. No influence of the buffer concentration in the interval 0.04–0.06 M on the sensitivity achieved for the determination of ibuprofen was observed.
The influence of the temperature on the formation of mixed dye–surfactant and drug–surfactant aggregates and on the measurement parameter was studied in the range 5–60 °C. The DDABr concentration required to form dye–surfactant aggregates slightly decreased as a function of the temperature. At temperatures higher than about 40 °C, this effect was more pronounced in the presence than in the absence of drug, which resulted in a decrease of the measurement parameter by a factor of about 2 times when the temperature increased from 40 to 60 °C. The measurement parameter was kept constant at temperatures comprised between 5 and 40 °C, therefore, measurements were performed at room temperature.
Organic solvents are frequently used to extract active ingredients from solid pharmaceutical samples and to dilute liquid formulates. So, the effect of organic additives on mS, mS* and the measurement parameter was studied by adding ethanol to the titration medium at concentrations up to 10%. These parameters were found to remain constant at alcohol concentrations up to 3%. Higher ethanol concentrations disfavoured the formation of both dye–surfactant and drug–surfactant aggregates, which resulted in a decrease in the measurement parameter by a factor of 1.7 when the alcohol content was increased from 3 to 10%. Ethanol has been reported to break down the structured water around the hydrophobic parts of organic molecules hindering hydrophobic interactions between them.20
Analytical performance
Calibration curves were run for therapeutic drugs belonging in different structural groups, namely, propionic (flurbiprofen, ibuprofen, naproxen and ketoprofen) and acetic (diclofenac, felbinac and zomepirac) acids, indolines (indomethacin and sulindac), glycyrrhetinic acid derivatives (carbenoxolone and enoxolone), salicylates (diflunisal and phenyl salicylate), oxicams (meloxicam, piroxicam and tenoxicam), pyrazolones (phenylbutazone and sulfinpyrazone), hydrocortisones (dexamethasone and prednisolone), phenothiazines (promethazine), ethanolamines (diphenhydramine) and dibenzazepines (nortriptyline). Linear calibration curves were obtained for all drugs tested bearing no charge or opposite charge to that of the cationic surfactant DDABr at the working pH 5.9 (molecular structures are depicted in Table 2). The sensitivity, defined as the slope of the calibration graph, the detection limit and the linear concentration range of the proposed method for the determination of these pharmaceuticals are shown in Table 3. Standard errors of the estimate and correlation coefficients varied over the ranges 0.3–2.3 and 0.995–0.9998, respectively and the intercept values of the calibration graph were not significantly different from zero. Therefore, results obtained for neutral and acid drugs, fitted to eqn (1) and the drug–surfactant binding degree at the titration end-point, βA, remained constant over the linear concentration range. The precision, expressed as relative standard deviation, was estimated to be 0.1% (n = 11) for a concentration of ibuprofen of 6.0 mg l−1.| Structural group | General formula | Drug | X | RI | RII |
|---|---|---|---|---|---|
| Propionic acids | 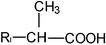 | Flurbiprofen | 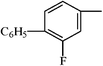 | ||
| Ibuprofen |  | ||||
| Naproxen | 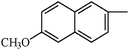 | ||||
| Ketoprofen |  | ||||
| Acetic acids | R1–CH2–COOH | Diclophenac | 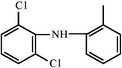 | ||
| Felbinac |  | ||||
| Zomepirac | 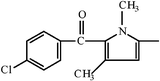 | ||||
| Indolines | 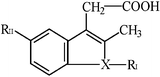 | Indomethacin | N |  | –OCH3 |
| Sulindac | CH | 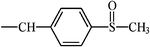 | –F | ||
| Glycyrrhetinic acid derivatives |  | Carbenoxolone | 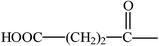 | ||
| Enoxolone | –H | ||||
| Salicylates | 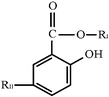 | Diflunisal | –H | 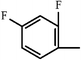 | |
| Phenyl salicylate | –C6H5 | H | |||
| Oxicams |  | Meloxicam | 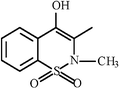 |  | |
| Piroxicam | 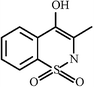 |  | |||
| Tenoxicam | 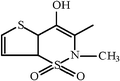 | 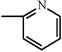 | |||
| Pyrazolones | 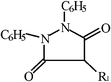 | Phenylbutazone | –CH2–(CH2)2–CH3 | ||
| Sulfinpyrazone | 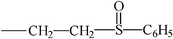 | ||||
| Hydrocortisones |  | Dexamethasone | –CH3 | ||
| Prednisolone | –H |
| Drug | Detection limita/mg l−1 | Linear concentration rangeb/mg l−1 | Intercept ± s/µM | Slope ± s/µM l mg−1 | rc | Syxd |
|---|---|---|---|---|---|---|
| a Calculated as 3-fold the standard deviation of mS.b Quantification limit calculated as 10-fold the standard deviation of mS.c Correlation coefficient.d Standard error of the estimate. | ||||||
| Flurbiprofen | 0.1 | 0.3 − 3.5 | 0.3 ± 0.3 | 14.2 ± 0.1 | 0.9998 | 0.4 |
| Ibuprofen | 0.1 | 0.4 − 10 | 1 ± 1 | 11.4 ± 0.2 | 0.9994 | 1.7 |
| Naproxen | 0.2 | 0.7 − 12 | 1 ± 1 | 6.0 ± 0.1 | 0.9990 | 1.2 |
| Ketoprofen | 0.3 | 1 − 20 | −2 ± 2 | 4.4 ± 0.1 | 0.997 | 2.3 |
| Diclofenac | 0.1 | 0.4 − 10 | 1 ± 1 | 9.7 ± 0.2 | 0.9990 | 1.8 |
| Felbinac | 0.1 | 0.5 − 7 | 1 ± 1 | 9.0 ± 0.1 | 0.9996 | 0.8 |
| Zomepirac | 0.3 | 1 − 15 | −0.3 ± 0.5 | 3.90 ± 0.05 | 0.9997 | 0.6 |
| Indomethacin | 0.1 | 0.4 − 3 | 1 ± 1 | 12.0 ± 0.4 | 0.997 | 1.1 |
| Sulindac | 0.15 | 0.5 − 7 | 1 ± 1 | 8.2 ± 0.2 | 0.998 | 1.5 |
| Carbenoxolone | 0.1 | 0.4 − 7 | 1 ± 1 | 12.1 ± 0.3 | 0.9990 | 1.7 |
| Enoxolone | 0.2 | 0.6 − 9 | −1 ± 1 | 7.0 ± 0.1 | 0.9994 | 0.9 |
| Diflunisal | 0.1 | 0.4 − 12 | 1 ± 1 | 9.9 ± 0.2 | 0.9991 | 1.9 |
| Phenyl salicylate | 0.8 | 3 − 30 | 0.7 ± 0.9 | 2.50 ± 0.05 | 0.9997 | 1.2 |
| Meloxicam | 0.1 | 0.4 − 12 | −1 ± 1 | 11.6 ± 0.2 | 0.9994 | 2.1 |
| Piroxicam | 0.06 | 0.2 − 2 | 1 ± 1 | 21 ± 1 | 0.995 | 1.8 |
| Tenoxicam | 0.06 | 0.2 − 2 | 0.4 ± 0.4 | 22.1 ± 0.4 | 0.9997 | 0.5 |
| Phenylbutazone | 0.1 | 0.5 − 4 | −0.1 ± 0.6 | 9.0 ± 0.2 | 0.9996 | 0.9 |
| Sulfinpyrazone | 0.1 | 0.3 − 3 | 2 ± 2 | 8.4 ± 0.8 | 0.995 | 2.2 |
| Dexamethasone | 2.5 | 8 − 50 | 0.2 ± 0.2 | 0.52 ± 0.01 | 0.9992 | 0.4 |
| Prednisolone | 2.3 | 8 − 25 | −0.2 ± 0.2 | 0.55 ± 0.01 | 0.998 | 0.3 |
Basic pharmaceuticals (e.g. promethazine, diphenhydramine and nortriptyline) provided no analytical response at least up to drug concentrations of 200 mg l−1, using DDABr as titrant. Repulsive electrostatic forces between drug and surfactant molecules bearing the same charge disfavoured the formation of drug–DDABr aggregates, which could not effectively compete with the formation of CBBG–DDABr aggregates in ternary drug–surfactant–dye aqueous mixtures.
The parameter βA, which can be easily calculated from the slopes obtained for each of the drug tested taking into account their molecular weight, is a measure of the sensitivity of the SDB method. Table 4 shows the βA values obtained for the neutral and acid drugs tested. The value of this parameter, and, therefore, the sensitivity of the proposed method, was highly dependent on the molecular structure of the drug. The drug–DDABr binding degree reached at the titration end-point was found to depend on the occurrence and number of anionic groups in the drug; it was higher for anionic than for neutral drugs and it increased with the number of anionic groups (e.g. compare the βA values for carbenoxolone and enoxolone). Generally, the βA value was higher for drug molecules with large hydrophobic moieties (e.g. compare the βA values for diclofenac and zomepirac), although some exceptions were found (e.g. drugs belonging the oxicam structural group).
| Drug | βA ± sa | Log P | Drug | βA ± sa | Log P |
|---|---|---|---|---|---|
| a s denotes standard deviation (n = 5).b Octanol/water partition coefficient values were obtained from the Virtual Computational Chemistry Laboratory (http://www.vcclab.org).c Octanol/water partition coefficient values were obtained from Scifinder Scholar. | |||||
| Propionic acids | Salicylates | ||||
| Flurbiprofen | 2.4 ± 0.2 | 4.2b | Diflunisal | 2.47 ± 0.05 | 4.3c |
| Ibuprofen | 2.35 ± 0.04 | 4.0b | Phenyl salicylate | 0.53 ± 0.01 | 3.6c |
| Naproxen | 1.38 ± 0.02 | 3.2b | Oxicams | ||
| Ketoprofen | 1.12 ± 0.02 | 3.1b | Meloxicam | 4.32 ± 0.07 | 3.4b |
| Acetic acids | Piroxicam | 6.9 ± 0.3 | 3.1b | ||
| Diclofenac | 3.08 ± 0.06 | 4.5b | Tenoxicam | 7.4 ± 0.1 | 1.4c |
| Felbinac | 1.91 ± 0.02 | 3.3c | Pyrazolones | ||
| Zomepirac | 1.13 ± 0.01 | 2.3c | Phenylbutazone | 2.77 ± 0.03 | 3.2b |
| Indolines | Sulfinpyrazone | 3.4 ± 0.3 | 2.3b | ||
| Indomethacin | 4.3 ± 0.1 | 4.3b | Hydrocortisones | ||
| Sulindac | 2.92 ± 0.07 | 3.4b | Dexamethasone | 0.204 ± 0.004 | 1.8b |
| Glycyrrhetinic acid derivatives | Prednisolone | 0.198 ± 0.004 | 1.6b | ||
| Carbenoxolone | 6.9 ± 0.2 | 7.3c | |||
| Enoxolone | 3.29 ± 0.05 | 6.6c | |||
Determination of drugs in pharmaceutical preparations
The proposed method (see Procedure) was applied to the determination of neutral (dexamethasone) and acid (ibuprofen, diclofenac, indomethacin, carbenoxolone, piroxicam and phenylbutazone) drugs in commercial formulations. Results obtained for different pharmaceutical dosage forms: effervescent granulates, tablets, suppositories, gels and blisters, are shown in Table 1. As it can be seen, all of them were consistent with the nominal contents, which prove the suitability of the SDBD method to analyse a high number of drugs in pharmaceutical preparations with a minimum sample treatment (only dilution of liquid samples and dissolution of solid samples).Conclusions
The use of the surfactant DDABr and the dye CBBG has permitted the accurate, precise and selective determination of a variety of acid and neutral drugs, based on surfactant to dye binding degree measurements. Because of the advantageous features of the SDBD method (versatility, rapidity, simplicity and low cost) and the great number of drugs that can be determined, this methodology can be considered a useful tool for pharmaceutical quality control. Nowadays, our investigations on the use of the SDBD method to determine drugs in formulates continue with the aim of extending this new aggregation parameter-based method to the determination of basic drugs. Promising results have been already obtained using the anionic surfactant sodium dodecylsulfate (SDS) and the dye Cresyl Violet.Acknowledgements
The authors gratefully acknowledge financial support from MCyT (Proyect no. BQU2002-01017)References
- S. Lang, Curr. Opin. Colloid Interface Sci., 2002, 7, 12–20 Search PubMed.
- G. J. Tiddy and A. Khan, Curr. Opin. Colloid Interface Sci., 2000, 4, 379–380 Search PubMed.
- J. M. Rodríguez, M. I. Martínez, N. Horn and H. M. Dodd, Int. J. Food Microbiol., 2003, 80, 101–116 CrossRef CAS.
- A. M. Fernández, U. Held, A. Willing and W. H. Breuer, Prog. Org. Coat., 2005, 53, 246–255 CrossRef CAS.
- http://www.facm.ucl.ac.be/conferences/2000/Geneve-antibiotic-efflux-pumps-05-12-00/sld018.htm.
- D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chem., 1995, 67, 1872–1880 CrossRef CAS.
- R. Fabios, D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chem., 2003, 75, 6011–6016 CrossRef CAS.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, Trends Anal. Chem., 2001, 20, 241–254 CrossRef CAS.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, J. AOAC Int., 2002, 85, 173–181 CAS.
- I. Casero, D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chim. Acta, 1997, 345, 75–86 CrossRef CAS.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, Int. J. Environ. Anal. Chem., 1999, 75, 181–200 CrossRef CAS.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, Analyst, 2000, 125, 1507–1512 RSC.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chim. Acta, 1999, 384, 175–183 CrossRef CAS.
- E. Borrego, D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chim. Acta, 1998, 362, 285–297 CrossRef CAS.
- M. P. San Andrés, D. Sicilia, S. Rubio and D. Pérez-Bendito, J. Pharm. Sci., 1998, 87, 821–826 CrossRef.
- M. P. San Andrés, D. Sicilia, S. Rubio and D. Pérez-Bendito, Analyst, 1998, 123, 1079–1084 RSC.
- A. Pedraza, M. D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chim. Acta, 2004, 522, 89–97 CrossRef CAS.
- E. M. Costi, M. D. Sicilia, S. Rubio and D. Pérez-Bendito, Anal. Chim. Acta, 2005, 549, 159–165 CrossRef CAS.
- A. Pedraza, M. D. Sicilia, S. Rubio and D. Pérez-Bendito, Analyst, 2005, 130, 1102–1107 RSC.
- S. Bračko and J. Špan, Dyes Pigm., 2001, 50, 77–84 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
