Selective production of aromatics from CO2†
Abstract
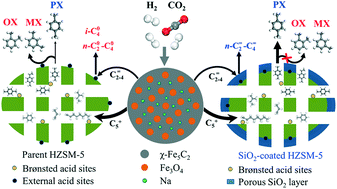
Direct catalytic hydrogenation of CO2 to value-added aromatics, particularly the desirable para-xylene, not only provides a complementary route to the aromatics market but also helps reduce emissions of the greenhouse gas CO2. Herein, we report a composite Na/Fe and HZSM-5 catalyst system used for the direct and selective production of aromatics from CO2. Over the composite catalyst, the selectivity to aromatics in liquid hydrocarbons could reach over 94% under industrially relevant conditions, and the selectivity to para-xylene in total xylenes remarkably increased from 24–26% to 70% as HZSM-5 zeolite was coated with SiO2. Experiments and DFT calculations confirmed that the Na-promoted Fe catalyst and HZSM-5 with high Brønsted acidity are key factors for enhancing the CO2 conversion and aromatics synthesis. Specifically, SiO2-coated HZSM-5 can suppress the isomerization of para-xylene and para-ethyltoluene, which are preferentially formed in the inner channels of HZSM-5, and over a 100 h test, the composite catalyst showed highly stable CO2 conversion with relatively stable aromatics synthesis. Interestingly, in the case of SiO2-coated HZSM-5, the light olefins (C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) –C4
–C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) remained largely in the gas phase rather than becoming isomerized and hydrogenated into light paraffins. This study opens a new way to reduce CO2 emissions via the efficient production of aromatics over a simple composite catalyst system.
) remained largely in the gas phase rather than becoming isomerized and hydrogenated into light paraffins. This study opens a new way to reduce CO2 emissions via the efficient production of aromatics over a simple composite catalyst system.


 Please wait while we load your content...
Please wait while we load your content...