Phosphor and nitrogen co-doped rutile TiO2 covered on TiN for oxygen reduction reaction in acidic media†
Abstract
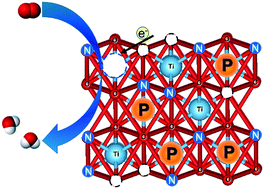
Phosphor and nitrogen atoms were simultaneously doped into rutile TiO2 covered on TiN for oxygen reduction reaction in acidic media, without using carbon supports. A recently reported facile combustion method was modified by simply adding H3PO4 to the precursor dispersion. Pentavalent phosphor (P5+) atoms were doped into both the bulk and surface to form new active sites without altering the number of oxygen vacancies on the rutile surface, which was formed by nitrogen doping. The P5+-doping successfully enhanced the ORR mass activity by more than double. However, the activity was not stable after 5000 potential cycles between 1.0 and 1.5 V versus reversible hydrogen electrode, due to the partial loss of both phosphor and nitrogen atoms. The stabilization of these foreign atoms at high potential was revealed to be necessary to use this catalyst without incurring costly potential protection systems.


 Please wait while we load your content...
Please wait while we load your content...