Soft matter and art conservation. Rheoreversible gels and beyond
Emiliano
Carretti
a,
Luigi
Dei
a and
Richard G.
Weiss
*b
aDepartment of Chemistry and Consortium CSGI, University of Florence, via della Lastruccia, 3 I-50019 Sesto Fiorentino, Florence, Italy
bDepartment of Chemistry, Georgetown University, Washington, DC, 20057-1227, USA. E-mail: weissr@georgetown.edu
First published on 29th March 2005
Abstract
A form of soft matter, physical gels, has been exploited for many practical purposes for several centuries. During the last decade, several types of physical gels have been developed to clean surfaces for art conservation and restoration. A short history and an explanation of the structures and properties of these gels and recent modifications of them by the authors to allow “isothermal rheoreversibility” are described. Speculation about future developments and the factors that should be considered when formulating gels for art conservation and restoration is presented.
 Emiliano Carretti | Emiliano Carretti received his undergraduate degree in Chemistry in 1999 and his PhD in Science for Cultural Heritage Conservation in 2003 from the University of Florence. He is currently Research Fellow at the CSGI Consortium, University of Florence. His specialization is the physical chemistry of colloidal systems. |
 Luigi Dei | Luigi Dei is Associate Professor of Physical Chemistry at the Faculty of Sciences and president of the University Course in Technology for Cultural Heritage Conservation (2001–2007) at the University of Florence. He carries out research at the CSGI Consortium (National Center for Colloids and Surface Science), University of Florence, lead Center for the Cultural Heritage Conservation Applied Science. He has authored about 100 publications in the field of colloids and interfaces and physical chemistry applied to art conservation. |
 Richard G. Weiss | Richard G. Weiss is Professor of Chemistry at Georgetown University in Washington, DC, USA. He is a Senior Editor of Langmuir and has published more than 200 scientific articles on topics dealing with photochemistry, reaction mechanisms, reactions in confining environments, and the structure and dynamics of gels, liquid crystals and polymers. |
Gels, a soft matter
During his Nobel Lecture in 1991,1 Pierre-Gilles de Gennes mentioned a wide variety of materials that are considered “soft matter.” Although he recognized the place of physical gels within this class of material, they were not among those included. Yet, physical gels are the embodiment of “soft matter” because they combine the structural complexity and rheological flexibility that were the traits that de Gennes emphasized. Those gels that must undergo a self-assembly step as part of the formation of the 3-dimensional networks that entrap the liquid components have been especially interesting to others and us as media for cleaning surfaces of art objects. Here, we present a subjective overview of the status of gels in art conservation and perspectives for the future.As noted by Dorothy Jordon Lloyd,2 gels truly are more easily recognized than defined. Nearly 80 years ago, she stated: “Only one rule seems to hold for all gels, and that is that they must be built up from two components, one of which is a liquid at the temperature under consideration, and the other of which, the gelling substance proper,3 is a solid. The gel itself has the mechanical properties of a solid, i.e., it can maintain its form under the stress of its own weight, and under any mechanical stress, it shows the phenomenon of strain.” That definition holds in large part even today although not all networks need be solid, more than two components may constitute a gel, and the rheological criteria for a gel are more quantitatively defined now. Jordon Lloyd and we refer to ‘wet gels’ in our discussions; they are soft matter. Although the sol–gel process has led to many interesting new ceramic materials, and other ‘dry gels’, such as xerogels and aerogels, are also useful, they are not “soft matter” as defined by de Gennes. Flory suggested four different types of wet gels4 based principally on the natures of their 3-dimensional networks. We will concentrate on those with particulate disordered structures that are non-aqueous (i.e., organogels), although hydrogels are also used widely in art conservation.
On the macro scale, a large fraction of our bodily tissues, including key elements of our eyes,5 operates in a jellied state. Gels are nearly ubiquitous in our daily lives.6 They are a part of our food (jellies, puddings, Jell-O, and aspic are examples), our personal care products (toothpaste, shampoo, deodorants, etc.), electronic devices, agents for drug delivery (such as gel capsules for vitamin E), and tissue engineering.7 Perhaps the extremes are represented, on the one hand, by the delicate application of gels to the surfaces of oil paintings in art restoration (the subject of this Opinion) or the weaving of a spider's web8 and, on the other, by the massive pumping of gels into oil wells to increase the efficiency of crude oil recovery9 or to destroy lives with napalm.10 Were it not for the development of ‘dry’ gelatinous print film, modern photography would not have become available to the masses in the XIXth century.11 Of course, the advent of digital photography is rapidly supplanting the use of the film camera!
The history of gels in the scientific literature is very interesting because of the applications of gels and the scientists who investigated the properties of gels. Some of both have been mentioned already. The transformation of aqueous solutions containing lithium urate by Lipowitz in 1841 is one of the first formal scientific investigations of gelation.12 However, careful sleuthing has implicated the use of thixotropic hydrogels in a much earlier and more controversial application since the 14th century.13 Thomas Graham's account of the gel and jelly states of aqueous (and alcoholic) mixtures of silicic acid and several other metallo acids in 186414 is a classic precursor of sol–gel chemistry, and he reported experiments on gels even earlier15 while serving as Master of the Mint of Britain! Later, Meunier discovered gels of 1,3:2,4-di-O-benzylidene-D-sorbitol, a low molecular-mass organic gelator (LMOG16) used widely today,17 and Hardy reported the properties of thermally-reversible LMOG gels in 1912.18 Systematic treatments of the structures and properties of gels began to appear decades later, as the field of study matured. However, in most cases, the treatises were still a part of books devoted to colloids.19 Only later were gels discussed as a separate subject, although the focus was primarily on polymer gels.6 Interest in molecular gels (i.e., those in which the gelators are LMOGs) and their self-assembled fibrillar networks (SAFINs) has been much more recent.16
Due to the peculiar viscoelastic properties of physical gels, they remind one of liquid crystals, the class of substances thought of as “the fourth state of matter.”20 Above a well-defined temperature of transition, the gel point (Tg), a gel becomes a sol or solution and begins to behave rheologically as a normal (Newtonian) liquid. Frequently, a few percent or even less than one percent of the so-called gelator is sufficient to immobilize macroscopically the liquid component. Several reviews dealing with the rheology of physical gels are available,21 and many other techniques have been used to gain insights into their structures at different distance scales.16 As temperature is lowered from above Tg, the liquid becomes very viscous. This change is manifested quantitatively in the simultaneous increase of the storage modulus (G′) as a function of the angular frequency of imposed strain (ω). The simplest determinations of the gel point, the ‘tilt test tube’ and ‘falling ball’ methods,22 are based on the increase of viscosity and exploit the high viscosity of a gel to detect when the system stops or starts to flow.
In the case of LMOG gels, the transition from liquid-like to gel states is characterized structurally by formation of a SAFIN that entraps domains of the liquid, leading to increases of the storage modulus that can exceed 104 fold. Some gels form almost immediately upon cooling and others require protracted periods for their gelator networks to be formed. Although the interaction between gelator and solvent need not be strong at the molecular level, the influence of the interactions at the macroscopic level leads to the properties that make gels potentially interesting in art conservation: (i) their slow flow rate along even vertical surfaces and (ii) the very high tendency of the solvent molecules to remain within their SAFIN, attenuating their diffusion and migration, due to capillary forces. In the next paragraphs, we will explain how these two properties have been exploited to develop gel systems for cleaning painted surfaces and present perspectives for future developments of new generations of rheoreversible gels.
Gels in art conservation
Apart from removing dirt and grime, cleaning often causes undesired elimination of integral layers of varnish, gilding, and paint from the surface of an oil painting. An important challenge for conservators is to find highly selective agents that act only on the deleterious layers, do not damage chemically or mechanically the surface that is to be cleaned, and do not leave residues on the surface after removal of the cleaning agents.Application of pure organic solvents is still one of the main techniques used to remove selectively foreign and undesired components of an easel painting. Unfortunately, solvent penetration into the original paint layers, especially via capillary action, can lead to swelling and leaching of varnishes and binders (such as surfactants) from of the surface of the object. As observed by Stolow,22 after solvents have been removed and residual solvent has dried, treated surfaces (N.B., films) appear more compact; a second exposure of the surface of a film to the solvent results in swelling without additional shrinkage from leaching. An important conclusion from this research is that the swelling behavior by a pure solvent or a mixture of solvents on a painted surface (whose common binding medium is aged linseed oil or stand oil) can be predicted in terms of Hildebrand solubility parameters;23 empirically, the maximum swelling was caused by chlorinated solvents. Stolow also studied quantitatively the leaching of artificially aged lead white linseed oil films. The maximum amount of extracted dried oil was 15–30% by weight, depending on the swelling power of the solvent employed. The upper limits were achieved by halogenated and oxygenated solvents, and kinetic studies indicated that 50% of the extraction was complete within the first 100 seconds of surface contact.
Furthermore, it was found that diffusion rates of the solvent molecules are dependent on both molecular volume and liquid viscosity, and that swelling occurs more rapidly in thin films than in thick ones and more slowly in pigmented than in non-pigmented ones.23 Recently Michalski reinterpreted the swelling data from previous studies using a three-dimensional representation of solubility parameters that allows for a more accurate distinction between different solvent properties.24
Wolbers has suggested and implemented a methodology to decrease the deleterious effects of solvent contact with a painted surface that employs the solvents in their gelated states.25 For one, capillary penetration of the solvent (when immobilized within a gel network) into the binding matrix of a surface is reduced and can be controlled temporally to a much greater extent. Many of the ungelated solvents capable of dissolving coating varnishes penetrate the paint layer at ca. 10 µm s−1.24 By reducing the penetration rate and the concentration of the solvents in the painted layers, swelling of the binding medium is reduced as well. Both the very high retention of a solvent in its gel and the reduced rate of solvent evaporation are advantageous properties. The gels also make possible the use of volatile solvents that would otherwise be inappropriate because of their short contact time before evaporation and local cooling effects on the painted surface.
The gel-based cleaning technique is very versatile not only because of the attributes discussed above, but also because many types of organic solvents and other selective cleaning reagents (such as enzymes and chelating agents26) can be gelated. This broad spectrum of cleaning agents translates, in principle, to high selectivity in what and how much is removed from a painted surface! In addition, gels have been used successfully to clean painted glass and metal and objects as delicate as feathers.27
The Wolbers gel-based cleaning tools, introduced at the beginning of the last decade,28 are the most commonly employed currently in cultural heritage conservation. The gelator in these systems is ∼1% by weight of polyacrylic acid (molecular weights as high as 4 × 106 Dalton) that exists mostly in folded conformations. When the acidic functional groups of the polymer are converted to negatively charged carboxylates by treatment with base, repulsive forces alter intra- and inter-chain interactions, allowing the chains to unfold and the gel networks to form.29 Deprotonation is usually accomplished by addition of a non-ionic surfactant, Ethomeen C12 or C25, when organogels are sought. Ethomeens have a long alkyl chain and their amino functionalities form salts with the carboxylic groups of the polyacrylic acid. The gelating properties of the modified polymer are dependent on the alkyl chain lengths of the Ethomeen. Organo- and hydrogels of polyacrylic acid gels can be made and both have been employed for art conservation. The main difference between the modes of formation of the organo- and hydrogels is related to the nature of the unfolding process of the polyacrylic acid.
Other formulations employing cellulose derivatives26 or agar-agar as the gelator have been employed, but they are much less versatile gel systems. They are used especially as hydrogelators for delivery of lipases and EDTA at controlled pH and concentrations. In many cases, the reduced mobility of the active cleaning agent in these gels allows them to remain in contact with the painted surface for a longer period.30
Application of any of these gels has followed two procedures for the most part. In the first, a part of a pre-formed gel is placed gently on a surface for ≤1 minute. In the second, application is by a swab roll in order to initiate a mechanical force on the surface of the painting.
‘Normal’ gels, isothermally rheoreversible gels, and beyond
Ensuring complete removal of both the gelator and the reagent and solvent of a polymer gel after its application to a painted surface has been one of the biggest challenges associated with the use of gels as cleaning agents. Burnstock and Kieslich31 demonstrated by gas chromatography/mass spectrometry and SEM analyses that residues of Ethomeen are present when a polyacrylic acid-based gel is removed either mechanically by a swab roll or by solubilization. The long-term effects of residues on surfaces are still being investigated and debated.32Unremoved residues and their long-term influence on works of art are not the only serious limitations to the expanded application of gels for art conservation. The mechanical force necessary to wipe away gels can scratch a surface or even remove additional layers from delicate objects. Of course, one can simply eliminate the gelator component and employ the liquid alone, but this method then opens other complications—control of spreading the liquid on the object surface and increased rates of diffusion of the liquid into sub-layers of the object (as noted above). We have devised gel systems that are intended to exploit the positive attributes of gels and free liquids while minimizing their negative ones.
The methodology involves isothermal rheoreversibility, the application of a gel to a surface, leaving it there as long as desired, and then removing it as a free-flowing liquid rapidly and ‘completely’. Initially, we employed thermal changes to cycle between the gel and sol/solution states (i.e., from below to above Tg). Many examples of gels that are thermally reversible are known.16 They are useful for other purposes, but they are impractical for art conservation because it is unwise to subject art objects to sudden temperature changes. Thus, warming or cooling a surface can result in serious damage, forming microfissures to surfaces and undesired adsorption of gel molecules on the surface or removing molecules in paints or other components of the surface.
Adding a co-gelator agent to a solution to effect gelation and then removing it isothermally is another approach. Thus, we have developed a class of low molecular mass and polymeric amine-based gelators whose solutions become gels when a simple gas, CO2, is added by bubbling (see eqn. (1)).33 The gels can be returned to their free-flowing solution states by bubbling a ‘displacing gas’, N2, at a slightly elevated temperature (to accelerate the displacement of CO2). The basis for gelation here is introduction of strong electrostatic forces between what were previously amine groups. Addition of one CO2 molecule transforms two primary or secondary amino groups into an ammonium (cationic) carbamate (anionic) pair. In organic liquids, especially those of low dielectric constant, the electrostatic attractions are very strong, and intimate ion-pairing is assured. Although this procedure does not entail the use of excessive heat to reverse gelation, so that the liquids can be removed easily from the surface of an object, it does require some physical perturbations, a mechanically difficult step of bubbling a gas through a thin film on the object surface, and time periods to effect liquification that may be too long for precise control of cleaning.
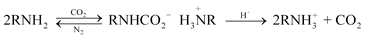 | (1) |
We have developed a rapid, completely isothermal method for return of a physical gel to its solution state that does not involve the drawbacks associated with N2 displacement,34 and it has been used to clean several art objects.35 It involves application of a polyamine–CO2 gel to a surface and addition of a drop of a weak acid, usually dilute acetic acid, at the moment the gel is to be removed (see eqn. (1) and Fig. 1). The influx of protons is sufficient to displace the CO2 from the anionic carbamate groups, transforming them within seconds to cationic ammonium groups. Thus, the pairs of attractive anions and cations in the gel become repulsive, due to the cationic nature of both entities, and the gel becomes a free-flowing liquid again within a few seconds. Current extensions of this method involve the use of different polyamines, additives that interact with a small fraction of the amino groups to make the polymer gels compatible with a wider variety of liquids, and acids that can diffuse rapidly through low polarity liquids.36 To date, most of our tests have been conducted using gels with 1-pentanol, 1-octanol, or 1-methyl-2-pyrrolidinone as the liquid. Our goal is to create a repertoire of gels (and acids to destroy them) in which the liquid components will span a wide spectrum of polarities. In this way, the conservator will be able to select the liquid of choice and then the gelator (and acid) that is compatible with it.
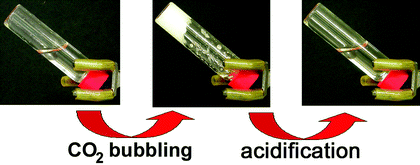 | ||
| Fig. 1 From left to right: a polyallylamine–1-pentanol solution, gel formation after CO2 addition, the gel after weak acidification (reprinted by permission of the American Chemical Society from ref. 35). | ||
Rheoreversible gels offer, in principle, the possibility to switch from the liquid-like to the gel state isothermally and rapidly. Thus, a liquid-like sample can be transformed to a gel immediately before its application to a painted surface, and then to the liquid-like state when removal from the surface is desired. Once in the liquid-like state, it can be removed by adsorption into tissue, cloth, cotton swabs, etc. and simple chemical action. This transformation simplifies the quantitative removal of the components of the gel (as well as the materials removed by them) from the surface of the painting.
The first tests with rheoreversible gels on both laboratory samples and small portions of old paintings have been promising (see Fig. 2). Results from some of those tests are summarized very briefly below. We emphasize, however, that these gels and the associated methods of treatment of painted surfaces with them are still in the experimental stages; the consequences of treating surfaces with them must be explored in much greater detail before they are presented as a cleaning alternative to art conservators.
 | ||
| Fig. 2 A gilded XIXth century frame overlaid with a surface layer of a degraded natural varnish before (A) and after (B) after application and removal of a 1-pentanol–polyallylamine rheoreversible gel.34 | ||
Thus far, infrared spectroscopy and X-ray energy dispersive spectroscopy have been the main tools that we have employed to evaluate the ability of the rheoreversible gels to remove targeted surface layers and to assess the presence of residues from the gels on the surfaces after cleaning. Although both techniques indicate that the desired cleaning actions are taking place, neither is sufficiently sensitive to assure the necessary degree of success. Future analyses will be expanded to include coupled gas chromatography/mass spectrometry (GC/MS) and pyrolysis-GC/MS. If the rheoreversible gels are found to be as efficient as traditional gels used for art conservation, they will be applied to cleaning of other art objects, such as frescoes, terracotta objects, wood and stone sculptures, etc.
It should be possible to design gels for conservation purposes whose rheoreversibility is based on concepts different from those mentioned above. For instance, gels that can be converted isothermally by exposing them to low doses of UV-vis radiation have been reported.37 The bases for the phase changes are isomerizations of chromophores that result in shape changes of the gelators and, consequently, destruction of the 3-dimensional networks. If the chromophores can be re-isomerized to their original forms by heat or radiation, the gels can be reformed.
Since it is not important that gels for art conservation be reusable, another photochemical approach is conceivable to effect their rheoreversibility. In it, a molecule or polymer as gelator will be designed to contain chromophores that expel fragments upon absorption of UV-vis radiation. These gelators will undergo irreversible chemical changes that lead to the rapid loss of the gel network. Several candidates can be envisioned based on what is known from classical photochemistry. Among them are ketones that undergo the Norrish–Yang reaction in which Cβ–Cγ bonds to the carbonyl are cleaved, expulsions of N2, CO, or CO2 from azo, keto, or carboxy containing gelators, irreversible isomerizations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds or di-π-methane rearrangements involving them, and cyclodimerizations of aromatic and other unsaturated groups.38
C bonds or di-π-methane rearrangements involving them, and cyclodimerizations of aromatic and other unsaturated groups.38
In all cases, the chromophores to be introduced and the radiation employed must meet stringent requirements to avoid damage to the surface that is to be cleaned. Thus, the excited electronic states of the chromophores should be unreactive toward the surface of the art object and the radiation wavelengths and flux must be adjusted to ensure that the pigments and paint binders of the object are not damaged in any way. In addition, it is imperative that free-radicals and other reactive intermediates, if formed, be unable to diffuse to the interface with the surface of the art object. These are not inconsequential requirements; significant efforts will be required to ensure that any system that appears a good candidate is safe. Furthermore, the quantum yield of the isomerization reaction should be high so that the transformation of the gelators to their non-gelling forms is rapid and requires the fewest number of photons possible.
Another potential approach involves the addition of a reagent other than an acid to convert a gel to a sol or solution. We imagine that gelators that are very susceptible to nucleophilic or electrophilic attack can be made. Thus, when such a gel system is to be removed, a drop of solution containing the nucleophile or electrophile will be added and the free-flowing liquid of the gel will be removed. This approach involves fragmentation of the gelator and avoids radiation, but it introduces other potential problems.
Finally, it may be possible to exploit the intrinsic viscoelastic properties of the gel to achieve its rapid destruction. A gelator could be transformed momentarily into a sol with a short pulse of microwave radiation or ultrasonic waves. In fact, any mechanical force that exceeds the load limit of the gel, whether applied macroscopically or microscopically, will break down the gel into a free-flowing mass. Our approach to creating rheoreversibility by such means must take into account the depth of penetration of the radiation or waves, their influence on the surface of the art object through secondary transmission of effects, and the need to avoid the abrasive characteristics of removing cleaning gels by wiping.
Acknowledgements
The authors express their gratitude to Barbara Berrie (Senior Conservation Scientist, The National Gallery of Art in Washington), David Erhardt (Research Organic Chemist, Smithsonian Center for Materials Research and Education of the Smithsonian Institution), Mathew George and Christina Capacci (Georgetown University), Daniele Rossi, Paola De Santis, and Pamela Betts (conservators), and Anna Maria Guiducci (Soprintendenza ai Beni Artistici, Storici e Demoetnoantropologici per le Province di Siena e Grosseto) for stimulating discussions, suggestions and assistance during the tests. Financial support from Consorzio Interuniversitario per lo Sviluppo dei Sistemi a Grande Interfase, Florence to EC and LD and from the US National Science Foundation and Georgetown University to RGW is gratefully acknowledged.References
- P.-G. De Gennes, Nobel Lecture entitled ‘Soft Matter’, 9 December 1991.
- D. Jordon Lloyd, in Colloid Chemistry, ed. J. Alexander, The Chemical Catalog Co., New York, 1926, vol. 1, pp. 767–782 Search PubMed.
- The gelling substance is often spoken of as the ‘gelator’ or ‘gellant’ when a polymer. For the sake of simplicity, only the term ‘gelator’ will be used here.
- P. J. Flory, Discuss Faraday Soc., 1974, 57, 7–18 RSC.
- (a) M. J. Hogan, J. A. Alvarado and J. E. Weddell, Histology of the human eye; an atlas and textbook, Saunders, Philadelphia, 1971 Search PubMed; (b) M. F. Land and D.-E. Nilsson, Animal eyes, Oxford University Press, New York, 2002 Search PubMed.
- (a) Gels Handbook, ed. Y. Osada and K. Kajiwara, Academic Press, San Diego, 2001+, vol. 1–4 Search PubMed; (b) Polymer gels: fundamentals and applications, ed. H. B. Bohidar, P. Dubin and Y. Osada, ACS Symposium Series #833, American Chemical Society, Washington, DC, 2003 Search PubMed; (c) Reversible polymeric gels and related systems, ed. P. R. Russo, ACS Symposium Series #350, American Chemical Society, Washington, DC, 1987 Search PubMed; (d) Responsive gels: volume transitions (Adv. Polym. Sci. series), ed. K. Dusek, Springer-Verlag, New York, 1993, vol. 109, 110 Search PubMed; (e) Food polymers, gels and colloids, ed. E. Dickinson, Royal Society of Chemistry, Cambridge, England, 1991 Search PubMed; (f) Hydrogels in medicine and pharmacy, ed. N. A. Peppas, CRC Press, Boca Raton, FL, 1986–1987, vol. 1–3 Search PubMed.
- D. G. Wallace and J. Rosenblatt, Adv. Drug Delivery Rev., 2003, 55, 1631–1649 CrossRef CAS.
- (a) H.-J. Jin and D. L. Kaplan, Nature, 2003, 424, 1057–1061 CrossRef CAS; (b) R. Valluzzi and H.-J. Jin, PCT Int. Appl. WO 2004 41,845 (Cl. C07K), 21 May 2004 Search PubMed.
- The first patent for this application: J. B. Clark, Treatment of Wells, US Pat. 2,596,844; May 13. 1952 Search PubMed.
- L. P. Fieser, G. C. Harris, E. B. Hershberg, M. Morgana, F. C. Novello and S. T. Putnam, Ind. Eng. Chem., 1946, 38, 768–773 CrossRef CAS.
- (a) R. L. Maddox, Br. J. Photogr., 1871, 18, 422–423 Search PubMed; (b) G. Eastman, US Pat., 388,850, 4 Sept. 1888 Search PubMed.
- A. Von Lipowitz, Liebigs Ann. Chem. Pharm., 1841, 38, 348–355 Search PubMed.
- L. Garlaschelli, F. Ramaccini and S. Della Sala, Nature, 1991, 353, 507 CrossRef CAS.
- T. Graham, J. Chem. Soc., 1864, 17, 318–327 RSC.
- T. Graham, Philos. Trans. R. Soc., 1861, 151, 183–224 CrossRef.
- (a) P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3159 CrossRef; (b) Molecular Gels. Structures of Self-Assembled Fibrillar Networks, ed. R. G. Weiss and P. Terech, Springer, Dordrecht, scheduled for 2005 Search PubMed.
- M. J. Meunier, Ann. Chim. Phys., 1891, 22, 412 Search PubMed.
- W. B. Hardy, Proc. R. Soc. London (A), 1912, 87, 29–37 Search PubMed.
- (a) Colloid Chemistry, ed. J. Alexander, The Chemical Catalog Co., New York, 1926, vol. 1, pp. 767–782 Search PubMed; (b) R. B. Dean, Modern Colloids, D. Van Nostrand Co., New York, 1948, p. 2 Search PubMed; (c) Colloid Science, ed. H. R. Kruyt, Elsevier, London, vol. I, 1952; vol. II, 1949 Search PubMed; (d) J. D. Ferry, Viscoelastic Properties of Polymers, Wiley, New York, 1961, p. 391 Search PubMed.
- Liquid crystals: the fourth state of matter, ed. F. D. Saeva, Marcel Dekker, New York, 1979 Search PubMed.
- (a) A. H. Clark and S. B. Ross-Murphy, Adv. Polym. Sci., 1987, 83, 57 CAS; (b) J. M. Guenet, Thermoreversible Gelation of Polymers and Biopolymers, Academic Press, London, 1993 Search PubMed; (c) K. te Nijenhuis, Thermoreversible Networks. Viscoelastic Properties and Structure of Gels, Springer Verlag, Berlin, 1997 Search PubMed.
- (a) N. Stolow, Nature, 1957, 16, 179–182; (b) N. Stolow, Studies in Conservation, 1957, 3, 40–44 Search PubMed.
- (a) N. Stolow, J. Oil Colour Chemists’ Assoc., 1957, 40, 488–499 Search PubMed; (b) N. Stolow, J. Oil Colour Chemists' Assoc., 1957, 40, 377–402 Search PubMed.
- S. Michalski, Cleaning, retouching and coatings. Preprints of the contributions to the Brussels Congress, September 1990, IIC, London, 1990, pp. 85–92 Search PubMed.
- R. C. Wolbers, Restoration '92: conservation, training, materials and techniques, latest developments, IIC, London, 1992, pp. 74–75 Search PubMed.
- R. C. Wolbers, Cleaning Painted Surface. Aqueous Method, Archetype Publications, London, 2000, pp. 109–156 Search PubMed.
- (a) M. Tomozei and Z. Balta, Metal 98: Proceedings of the International Conference on Metals Conservation. Draguignan-Figanières, France, 27–29 May 1998, James & James, London, 1998, pp. 188–191 Search PubMed; (b) N. Valentin, A. Sánchez and I. Herraez, ICOM Committee for Conservation, 11th Triennal Meeting in Edinburgh, Scotland, 1–6 September 1996: Preprints, ed. J. Bridgland, James & James, London, 1996, pp. 851–856 Search PubMed.
- R. C. Wolbers, Restoration '92: conservation, training, materials and techniques, latest developments, IIC, London, 1992, pp. 74–75 Search PubMed.
- P. Cremonesi, A. Curti, L. Fallarini and S. Raio, Progetto Restauro, 2000, 15, 25–33 Search PubMed.
- A. Burnstock and R. White, Cleaning, Retouching and Coatings, Preprints of the contributions to the Brussels Congress, September 1990, IIC, London, 1990, pp. 111–118 Search PubMed.
- A. Burnstock and T. Kieslich, ICOM committee for conservation, 11th triennial meeting in Edinburgh, James & James, London, 1996, pp. 253–262 Search PubMed.
- See for instance: (a) D. Stulik, D. Miller, N. Khandekar, R. Wolbers, J. Carlson and W. C. Petersen, Solvent Gels for the Cleaning of Works of Art. The Residue Question, ed. V. Dorge, The Getty Research Institute, Los Angeles, 2004 Search PubMed; (b) D. Erhardt and J. J. Bischoff, Studies in Conservation, 1994, 39, 3–27 Search PubMed; (c) C. S. Tumosa, J. Millard, J. Erhardt and M. F. Mecklenburg, Preprints of the 12th Annual Meeting of the ICOM Committee for Conservation, vol. I, James and James, London, 1999, pp. 347–352 Search PubMed.
- (a) M. George and R. G. Weiss, J. Am. Chem. Soc., 2001, 123, 10393–10394 CrossRef CAS; (b) M. George and R. G. Weiss, Langmuir, 2002, 18, 7124–7135 CrossRef CAS; (c) M. George and R. G. Weiss, Langmuir, 2003, 19, 1017–1025 CrossRef CAS; (d) M. George and R. G. Weiss, Langmuir, 2003, 19, 8168–8176 CrossRef CAS.
- E. Carretti, L. Dei, P. Baglioni and R. G. Weiss, J. Am. Chem. Soc., 2003, 125, 5121–5129 CrossRef CAS.
- E. Carretti, A. Macherelli, L. Dei and R. G. Weiss, Langmuir, 2004, 20, 8414–8418 CrossRef CAS and unpublished data.
- C. Capacci and R. G. Weiss, unpublished results.
- See for instance: (a) K. Murata, M. Aoki, T. Nishi, A. Ikeda and S. Shinkai, J. Chem. Soc., Chem. Commun., 1991, 1715–1718 RSC; (b) K. Murata, M. Aoki and S. Shinkai, Chem. Lett., 1992, 739–742 CAS; (c) K. Murata, M. Aoki, T. Suzuki, T. Harada, H. Kawabata, T. Komori, F. Ohseto, F. Ueda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 6664–6676 CrossRef CAS; (d) S. A. Ahmed, X. Sallenave, F. Fages, G. Mieden-Gundert, W. M. Muller, U. Muller, F. Vogtle and J.-L. Pozzo, Langmuir, 2002, 18, 19, 7096–7101 CrossRef CAS; (e) T. Yi, K. Sada, K. Sugiyasu, T. Hatano and S. Shinkai, Chem. Commun., 2003, 344–345 RSC; (f) J. J. D. de Jong, L. N. Lucas, R. M. Kellogg, J. H. van Esch and B. L. Feringa, Science, 2004, 304, 278–281 CrossRef CAS.
- See for instance: N. J. Turro, Modern Molecular Photochemistry, University Publishers, Sausalito, CA, 1991 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |
