Photophysical properties of 2-amino-9,10-anthraquinone: evidence for structural changes in the molecule with solvent polarity†
Received
27th July 2004
, Accepted 4th October 2004
First published on 5th November 2004
Abstract
The photophysical properties of 2-amino-9,10-anthraquinone (2AAQ) have been investigated in different solvents and solvent mixtures and correlated with the Lippert–Mataga solvent polarity parameter, Δf. In the low solvent polarity region with Δf < ca. 0.1, the dye shows unusually high fluorescence quantum yields (Φf) and lifetimes (τf) in comparison to those in other solvents of medium to high polarities. Similarly, the radiative rate constants (kf) are relatively lower and the non-radiative rate constants (knr) are relatively higher in the low polarity solvents in comparison to those in the medium to high polarity solvents. The current results have been rationalized assuming that the dye adopts different structural forms below and above the Δf value of ≈0.1. It is inferred that in the low solvent polarity region the dye exists in a non-planar structure, with its 2-NH2 plane away from that of the 9,10-anthraquinone moiety in the ground state. In solvents of medium to high polarities, the dye exists in a polar intramolecular charge transfer (ICT) structure, where the amino lone pair of the 2-NH2 group is in strong resonance with the anthraquinone π-cloud in the ground state. In all the solvents, however the dye is inferred to exist in the ICT structure in its excited (S1) state. Supportive evidence for the above hypothesis has been obtained from the solvent polarity effect on the Stokes' shifts for the dye. Quantum chemical studies on the structures of 2AAQ dye in the gas phase also give qualitative support for the inferences drawn from the photophysical properties of the dye in different solvents.
1 Introduction
Amino and hydroxy substituted quinones are an important class of molecules, having immense importance in the dye industry, biology, and pharmaceutical chemistry.1–9 Due to the presence of the quinone moiety, these molecules are good electron acceptors, and have been used quite extensively to understand the dynamics and mechanism of electron transfer processes under different experimental conditions.10–14 Unlike simple quinones, the amino and hydroxy substituted anthraquinones show reasonable fluorescence yields, and thus the steady-state and time-resolved fluorescence measurements of these substituted anthraquinones have been used quite extensively to investigate the excited state behavior of the quinonoid molecules, especially to understand the role of intra- and inter-molecular hydrogen bonding on their internal conversion and intersystem crossing mechanisms.15–19 The effect of solvent polarity on the photophysical properties of the amino and hydroxy substituted anthraquinones have not, however, been studied that systematically.
In our recent studies with aminocoumarin dyes, e.g. coumarin-120 (C120; 7-NH2-4-CH3-1,2-benzopyrone) and coumarin-151 (C151; 7-NH2-4-CF3-1,2-benzopyrone) dyes, the molecules that are known to display intramolecular charge transfer (ICT) character in most of the solvents, for both the ground and excited singlet (S1) states, it has been observed that depending on the solvent polarity these dyes undergo conformational changes at their 7-NH2 substituent, and thus display photophysical characteristics in nonpolar solvents characteristically different than in other solvents of moderate to high polarities.20,21 It is understood that in nonpolar solvents, the C120 and C151 dyes adopt a nonplanar conformation with respect to the 7-NH2 substituent, and thus the lone pair of the amino nitrogen cannot participate in full resonance with the 1,2-benzopyrone π-cloud.20,21 Excluding nonpolar solvents, in all other solvents of moderate to high polarities, the C120 and C151 dyes exist in the usual ICT structure, where the NH2 group adopts a planar conformation and consequently the amino lone pair is in full resonance with the 1,2-benzopyrone π-cloud.20,21 Considering the presence of the same electron donating 2-NH2 substituent and an equivalent electron accepting 9,10-anthraquinone moiety, 2-amino-anthraquinone (2AAQ) was expected to show behavior similar to that of C120 and C151 dyes in solvents of different polarity. In the literature, there is no report on the solvent polarity dependent changes in the photophysical properties of 2AAQ dye. In the present work we thus investigate these properties of 2AAQ dye in different solvents and solvent mixtures with the aim of seeing if the 2AAQ dye also displays conformational changes at its amino substituent with a change in solvent polarity, as was observed for the C120 and C151 dyes in our earlier work.20,21 Theoretical studies on the structures of 2AAQ have also been carried out to support the experimental results and their interpretations. The chemical structures of 2AAQ, C120 and C151 dyes are shown in Chart 1 for comparison.
 |
| | Chart 1 | |
2 Materials and methods
2AAQ was obtained from TCI (Tokyo, Japan), and purified by repeated crystallization from ethanol. All spectroscopic grade solvents were used in the present work, and obtained either from Spectrochem (Mumbai, India), S.D. Fine Chemical (Mumbai, India), SISCO Research Laboratories (Mumbai, India), E. Merck (Mumbai, India) or Fluka (Buchs, Switzerland). Dielectric constants (ε) and refractive indices (n) of the pure solvents were taken from the literature,22 and those of the mixed solvents (εMS, nMS respectively) were estimated using eqns. (1) and (2),| |  | (1) |
| |  | (2) |
respectively,20,21,23–26 where, the subscripts A and B represent the co-solvents and f represents their volume fractions. Following Lippert and Mataga,27–30 the polarity parameters (f) for the different solvents were calculated using eqn. (3).| | 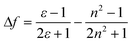 | (3) |
Absorption and fluorescence spectra were recorded using a JASCO (Tokyo, Japan, model V530) spectrophotometer and Hitachi (Tokyo, Japan, F-4010) spectrofluorometer, respectively. The Φf values of the dye were measured by a comparative method, using Φf of 1AAQ in benzene (ΦRf
= 0.058) as the reference.18 For all these measurements, optical density (O.D.) of the solutions was kept quite low (O.D. ≈0.2) at the excitation wavelength.
Fluorescence lifetimes of the dye in different solvents were measured using a time-correlated–single-photon-counting (TCSPC)31 spectrofluorometer, obtained from IBH (model IBH DataStation Hub, Glasgow, UK). In these measurements, a 455 nm LED (frequency 1 MHz) was used as the excitation source and a TBX4 detection module coupled with a special Hamamatsu PMT was used for the fluorescence detection. The instrument response function of the present setup was measured to be ≈1.2 ns at FWHM. For all cases, the observed fluorescence decays were analyzed as a single-exponential (eqn. (4)),
| | 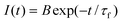 | (4) |
following a re-convolution procedure
31 where,
B is the pre-exponential factor and
τf is the fluorescence lifetime. A proper instrument response function, as obtained by replacing the sample cell with a light scatterer (suspended TiO
2 particles), was used for these re-convolution analyses. The goodness of the fits was judged from the reduced
χ2 values and the distribution of the weighted residuals among the data channels.
31 For all the fits, the
χ2 values were close to unity, and the weighted residuals were randomly distributed among the data channels used.
31
3 Results and discussion
3.1. Solvent polarity effect on the photophysical properties of the dye
Fluorescence quantum yields (Φf) of 2AAQ in different solvents and solvent mixtures were estimated at room temperature and are listed in Table 1 along with the solvent polarity parameter Δf. In the present study, the nonpolar solvents like hexane, cyclohexane, etc. could not be used, as the solubility of 2AAQ dye in these solvents is very small, and it was not possible to measure the photophysical properties in these solvents with confidence. Fig. 1A shows the variations in the Φf values of the dye with the solvent polarity function Δf. Two important points are to be noted from this figure. For medium to high polarity solvents with Δf > ca. 0.1, the plot is almost linear. For the lower polarity solvents with Δf < ca. 0.1, however, Φf is seen to be substantially deviated toward higher values. It is indicated from these results that the dye behaves differently in the lower solvent polarity region than in the medium to high solvent polarity region.
 |
| | Fig. 1 Variations in (A) fluorescence quantum yields (Φf) and (B) fluorescence lifetimes (τf) of 2AAQ with the solvent polarity function Δf. The results indicate the differences in the behavior of the dye in the solvents of low polarities (Δf < ca. 0.1) compared with the solvents of medium to high polarities (Δf > ca. 0.1). | |
Table 1 Fluorescence quantum yields (Φf), fluorescence lifetimes (τf), radiative rate constants (kf) and nonradiative rate constants (knr) for 2AAQ in different solvents and solvent mixtures. The solvent polarity function (Δf) is also listed for the solvents used
| Solventa |
Δf |
Φ
f
|
τ
f/ns |
k
f/106 s−1 |
k
nr/108 s−1 |
|
Abbreviations for the solvents and solvent mixtures are used as follows: AN: acetonitrile; CHX: cyclohexane; EA: ethyl acetate; CHXxEAy
=
x% CHX and y% EA (v/v); EAxANy
=
x% EA and y% AN (v/v).
|
| CHX95EA5 |
0.025 |
0.036 |
6.00 |
5.95 |
1.61 |
| CHX90EA10 |
0.046 |
0.031 |
4.74 |
6.62 |
2.04 |
| CHX85EA15 |
0.058 |
0.028 |
3.75 |
7.51 |
2.59 |
| CHX80EA20 |
0.081 |
0.026 |
2.81 |
9.08 |
3.47 |
| CHX75EA25 |
0.088 |
0.024 |
2.52 |
9.59 |
3.87 |
| CHX70EA30 |
0.108 |
0.021 |
2.13 |
10.01 |
4.59 |
| CHX60EA40 |
0.128 |
0.019 |
1.94 |
9.97 |
5.05 |
| CHX40EA60 |
0.160 |
0.017 |
1.68 |
10.12 |
5.85 |
| CHX20EA80 |
0.183 |
0.015 |
1.51 |
9.93 |
6.52 |
| EA |
0.201 |
0.014 |
1.38 |
9.86 |
7.15 |
| EA90AN10 |
0.239 |
0.011 |
1.10 |
10.00 |
8.99 |
| EA80AN20 |
0.259 |
0.009 |
0.99 |
9.11 |
10.03 |
| EA70AN30 |
0.272 |
0.009 |
0.97 |
9.28 |
10.22 |
| EA60AN40 |
0.280 |
0.009 |
0.91 |
9.89 |
10.89 |
| EA40AN60 |
0.292 |
0.008 |
0.89 |
8.99 |
11.14 |
| EA20AN80 |
0.300 |
0.008 |
0.83 |
9.64 |
11.95 |
| AN |
0.305 |
0.007 |
0.80 |
9.34 |
12.41 |
To substantiate the above results, the fluorescence decays of 2AAQ were also measured at room temperature in different solvents. A single-exponential analysis was seen to give a reasonably good fit for the observed fluorescence decays in all the solvents studied. The τf values thus obtained for the dye in different solvents are listed in Table 1. Fig. 1B shows a plot of the τf values of the dye against the Δf values of the solvents. It can be seen from this figure that the variations in the τf values with Δf show a trend very similar to that observed in the Φfvs. Δf plot, showing almost linear τfvs. Δf correlation for the medium to high polarity solvents (Δf > ca. 0.1) but the data points deviate largely toward higher values in the lower solvent polarity region (Δf < ca. 0.1). The variations in the Φf and τf values with Δf thus clearly indicate that the dye 2AAQ behaves differently in the lower solvent polarity region than in the medium to high solvent polarity region, and it could be related to the kind of structural changes predicted for C120 and C151 dyes in nonpolar and other solvents.20,21
Since the Φf and τf values arise due to the combined effect of the radiative (kf) and nonradiative (knr) decay rate constants of the dye fluorescent state (S1 state), for a better understanding of the solvent polarity dependent changes in Φf and τf values it is very important to know the solvent polarity effect on the kf and knr values. The kf and knr values for the dye in different solvents were estimated using eqns. (5) and (6).27,28
| |  | (5) |
| |  | (6) |
Table 1 lists the kf and knr values for 2AAQ dye in different solvents. Figs. 2A and B show the plots of the kf and the knr values for the dye, respectively, vs. the solvent polarity function Δf. It is seen from Fig. 2A that the kfvs. Δf plot is almost linear for medium to high polarity solvents (Δf > ca. 0.1). Though there is not a clear theoretical basis for such a linear correlation for the kf values with Δf, a systematic and almost linear kfvs. Δf correlation suggests that there is no significant structural change involved for the dye (both in its exited and ground state) in these solvents of medium to high polarity. Unlike these solvents, in the lower polarity solvents with Δf < ca. 0.1, however, the kf values are seen to be substantially lower and naturally do not correlate with the trend observed for the rest of the solvents. These lower kf values in the lower polarity solvents suggest that the probability of radiative transition reduces substantially as the solvent polarity reduces below Δf
≈0.1. Such behavior is expected if the dye undergoes some kind of structural change in either of its electronic states that are involved in the radiative transition. From Fig. 2B it is seen that the knrvs. Δf plot is also almost linear for the medium to high polarity solvents. At the lower solvent polarity region, however, knr appears to deviate gradually toward higher values, though the deviations in this case are not as drastic as observed for the kf values. These results are also in support of the results obtained for the kf values, suggesting that the dye undergoes some kind of structural change in the lower solvent polarity region in comparison to what exists in other solvents of medium to high polarity.
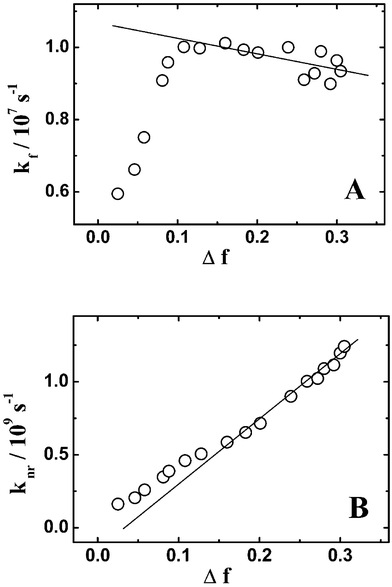 |
| | Fig. 2 Plot of (A) radiative (kf) and (B) nonradiative (knr) decay rate constants for 2AAQ dye against Δf. The kf values are abnormally lower and the knr values are unusually higher in the solvents of low polarity (Δf < ca. 0.1). | |
At this point it is interesting to have a comparison of the results obtained with the 2AAQ dye with those observed in our earlier work with C120 dyes.20 For the latter dye, the kfvs. Δf plot was seen to be reasonably linear for almost all of the solvents studied excluding the nonpolar solvents (Δf
≈0), for which kf largely deviated toward lower values.20 Similarly, the knrvs. Δf plot was also reasonably linear for almost all the solvents studied excluding the nonpolar solvents (Δf
≈0), for which the knr largely deviated toward higher values.20 These results are thus qualitatively very similar to those of 2AAQ, except that the critical solvent polarity to observe deviations in the kf and knr values for 2AAQ is extend up to a somewhat higher Δf value of ≈0.1, which for C120 dye was very close to Δf
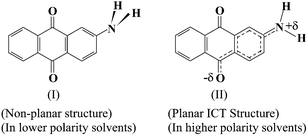
≈0.20 For C120 dye, the observed changes in the kf and knr values with Δf was also substantiated by the solvent polarity dependent changes in the absorption and fluorescence spectral characteristics and also in the Stokes' shifts. It was inferred that the dye C120 adopts a non-planar structure in nonpolar solvents, where the 7-NH2 group of the dye is in a pyramidal conformation and the amino lone pair is quite out of resonance with the π-cloud of the 1,2-benzopyrone moiety. In all other solvents, the dye was inferred to exist in a planar intramolecular charge transfer (ICT) structure, where the amino lone pair is in full resonance with the 1,2-benzopyrone π-cloud. Drawing an analogy between the results of 2AAQ and C120 dyes, we infer that the dye 2AAQ also adopts two different structural forms similar to those of C120 dye, one in the lower solvent polarity region (Δf < ca. 0.1) and the other in the medium to high solvent polarity region (Δf > ca. 0.1). Schematically these structures can be shown as in Chart 2, where the non-planar structure, as predicted to exist in lower solvent polarity region, is presented by structure I, and the polar ICT structure, as predicted to exist in medium to high solvent polarity region, is presented by structure II.
 |
| | Chart 2 | |
If the dye 2AAQ adopts a different structural form in the lower solvent polarity region in comparison to that in the medium to higher solvent polarity region, the absorption and fluorescence spectral characteristics of the dye are also expected to show some differences in the two solvent polarity regions. Thus, the absorption and the fluorescence spectra of 2AAQ were recorded in different solvents and solvent mixtures. Table 2 lists the longer wavelength absorption maxima (λmaxabs) and the fluorescence maxima (λmaxfl) of the dye in different solvents and solvent mixtures. Interesting to note from these results is that for the first three low-polarity solvents (cf. Table 2) the λmaxabs values show a sharp blue shift whereas for all other solvents the λmaxabs values do not show that drastic a change with solvent polarity. To understand more about these unusual blue shifts in the λmaxabs values in very low polarity solvents, we compared the shapes of the absorption spectra of the dye in different solvents very critically. Such a comparison revealed that the absorption maxima for 2AAQ dye in the first three low polarity solvents (cf. Table 2) correspond to a vibrational band that is next highest in energy compared to the vibrational band that corresponds to the λmaxabs positions in the rest of the solvents. Thus, to have a one to one correspondence, for the first three low polarity solvents, the wavelength of the vibrational band that corresponds to the λmaxabs values in the rest of the solvents were also noted and are listed in Table 2 within the parenthesis under the same λmaxabs column. Unlike absorption spectra, the fluorescence spectra of the dye are seen to be quite featureless for all the solvents studied, and there are no such changes in the relative intensities of the vibrational bands corresponding to the λmaxfl values in the different solvents studied.
Table 2 Absorption maxima (λabs), fluorescence maxima (λfl) and Stokes' shift (Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) ) for 2AAQ dye in different solvents and solvent mixtures
) for 2AAQ dye in different solvents and solvent mixtures
| Solventa |
Δf |
λ
abs/nmb |
λ
fl/nm |
Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) /cm−1 /cm−1 |
|
Abbreviations for the solvents and solvent mixtures are as given in the footnote of Table 1.
Values in the parenthesis for the first three low polarity solvents are the wavelength of the vibrational band that corresponds to the λmaxabs values in rest of the solvents.
|
| CHX95EA5 |
0.025 |
408(421.8) |
532 |
4912 |
| CHX90EA10 |
0.046 |
411(422.6) |
543 |
5247 |
| CHX85EA15 |
0.058 |
415(423.4) |
549 |
5400 |
| CHX80EA20 |
0.081 |
423.6 |
556 |
5621 |
| CHX75EA25 |
0.088 |
424.4 |
558 |
5642 |
| CHX70EA30 |
0.108 |
425 |
560 |
5673 |
| CHX60EA40 |
0.128 |
425 |
566 |
5862 |
| CHX40EA60 |
0.160 |
426 |
574 |
6053 |
| CHX20EA80 |
0.183 |
427 |
577 |
6088 |
| EA |
0.201 |
427.5 |
580 |
6150 |
| EA90AN10 |
0.239 |
428 |
588 |
6358 |
| EA80AN20 |
0.259 |
428.5 |
594 |
6502 |
| EA70AN30 |
0.272 |
429 |
599 |
6615 |
| EA60AN40 |
0.280 |
429.4 |
601 |
6655 |
| EA40AN60 |
0.292 |
429.5 |
603 |
6699 |
| EA20AN80 |
0.300 |
430 |
604 |
6700 |
| AN |
0.305 |
430 |
607 |
6781 |
Fig. 3A shows the Δf dependent changes in the energies corresponding to the λmaxabs values in different solvents excluding the first three low polarity solvents that showed unusual blue shifts in the λmaxabs values. For the latter three solvents, energies corresponding to the vibrational band that is related to the λmaxabs values in the rest of the solvents are in fact plotted in Fig. 3A for a one to one correspondence. It is seen from this figure that the plot is almost linear for all the solvents studied, without showing any visible difference between the solvents of low polarity (Δf > ca. 0.1) and those of medium to high polarities (Δf > ca. 0.1). Unlike in Fig. 3A, the plot of the energies corresponding to the λmaxfl values in different solvents vs. theΔf values, as shown in Fig. 3B, clearly indicate the deviations in the data points at the lower solvent polarity region (Δf > ca. 0.1) in comparison to the almost linear correlation observed for the solvents of medium to high polarity. Thus the plot in Fig. 3B supports the results obtained in the Φf and τf measurements, indicating that the dye behaves differently in the two regions of solvent polarity. If the nature of the ground and the excited electronic states, that are involved in the absorption and fluorescence processes, remain unchanged with solvent polarity, the Stokes' shift between the absorption and fluorescence spectra (Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) ) is expected to follow a linear relation with Δf, as suggested in the Lippert–Mataga relationship,27–30
) is expected to follow a linear relation with Δf, as suggested in the Lippert–Mataga relationship,27–30
| | 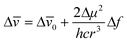 | (7) |
where Δ
μ
(Δ
μ
=
μe
−
μg) is the difference between the excited and ground state dipole moments,
μe and
μg respectively,
h is the Planck's constant,
c is the velocity of light and
r is the Onsager radius of the dipole–
solvent interaction sphere. For the present system, Δ
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif)
values were estimated as the difference in energy between the absorption and fluorescence maxima for most of the
solvents except the first three low polarity
solvents mentioned earlier. For the latter three
solvents instead of the observed absorption maxima, the vibrational band that corresponds to the absorption maxima in all other
solvents were considered in the calculation of the Δ
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif)
values. The Δ
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif)
values thus estimated for the
dye in different
solvents are listed in
Table 2.
Fig. 3C shows the plot of the Δ
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif)
values of the
dye against the
solvent polarity function Δ
f. It is seen from this figure that the plot is linear within experimental error for the
solvents of medium to high polarities (Δ
f >
ca. 0.1) but the data points are deviated towards lower values at Δ
f <
ca. 0.1. Similar results were also obtained in our earlier studies with C120
dye,
20 and these results indicate that the fluorophore is relatively less polar in structure in the lower polarity
solvents than in the
solvents of medium and high polarity.
 |
| | Fig. 3 Plot of (A) absorption maxima (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) abs), (B) fluorescence maxima ( abs), (B) fluorescence maxima (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) fl) and (C) Stokes' shift of 2AAQ dye against Δf. The results substantiate the observations made in the Φf and τf measurements of the dye in different solvents. fl) and (C) Stokes' shift of 2AAQ dye against Δf. The results substantiate the observations made in the Φf and τf measurements of the dye in different solvents. | |
For the medium to higher solvent polarity region, in which the Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) vs. Δf plot is linear, the data were analyzed according to eqn. (7). In this analysis, the Onsager radius r was considered to be 3.32 Å, as estimated on the basis of Edward's volume addition method.32 The Δμ value for 2AAQ is thus estimated to be ≈4.4 D, which is a substantially high value, suggesting the possible ICT character for the fluorescent state of the dye (cf. Chart 2). As the Stokes’ shifts are also higher for this solvent polarity region compared to those in the lower solvent polarity region, we suppose that the ground state of the dye also has reasonable ICT character in the former solvents. In the lower solvent polarity region, the lower than expected Δ
vs. Δf plot is linear, the data were analyzed according to eqn. (7). In this analysis, the Onsager radius r was considered to be 3.32 Å, as estimated on the basis of Edward's volume addition method.32 The Δμ value for 2AAQ is thus estimated to be ≈4.4 D, which is a substantially high value, suggesting the possible ICT character for the fluorescent state of the dye (cf. Chart 2). As the Stokes’ shifts are also higher for this solvent polarity region compared to those in the lower solvent polarity region, we suppose that the ground state of the dye also has reasonable ICT character in the former solvents. In the lower solvent polarity region, the lower than expected Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values, on the basis of the linear Δ
values, on the basis of the linear Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) vs. Δf correlation observed for the rest of the solvents, suggest that the dye structure could be relatively less polar in the former solvents than in the latter. These results are thus is accordance with our inference that the dye adopts a non-planar structure in the low solvent polarity region (cf. Chart 2).
vs. Δf correlation observed for the rest of the solvents, suggest that the dye structure could be relatively less polar in the former solvents than in the latter. These results are thus is accordance with our inference that the dye adopts a non-planar structure in the low solvent polarity region (cf. Chart 2).
3.2. Quantum chemical calculations on the structures of 2AAQ
To have theoretical support for the inferences made in the earlier sections, we also carried out quantum chemical calculations on the structures of 2AAQ dye in the gas phase. Full geometry optimization of 2AAQ was performed to predict the ground state structure under isolated gas phase conditions based on the DFT level of theory with B3LYP functional adopting a large basis function, 6-311++G(d,p). Ground state calculations were performed adopting the GAMESS suite of program on a PC-based LINUX cluster.33 The most relaxed ground state structure of the dye in the gas phase is shown in Fig. 4A. In this structure, the three rings of the molecule are in the same plane. However, the two amino hydrogen atoms are out of the above molecular plane. The calculated dihedral angles of the two amino hydrogen atoms are found to be ≈19 ° as shown in Fig. 4A. In the lower solvent polarity region, as the dielectric interaction of the solvent with the dye molecule is not that large, the dye structure is expected to be similar to that obtained in the quantum chemical calculation in the gas phase and thus supports our hypothesis depicted in Chart 2. Excited singlet (S1) state geometry optimization for the dye under isolated gas phase condition was carried out following time dependent DFT level of theory (TDDFT) using the 6-311++G(d,p) basis set. The most relaxed first singlet state structure of the dye in the gas phase is shown in Fig. 4B. In this structure, the three rings of the molecule and the two amino hydrogen atoms are almost in the same plane. Based on these theoretical results in conjunction with the observed photophysical properties in different solvents we infer that in the lower solvent polarity region (Δf < ca. 0.1) the dye 2AAQ exists in a non-planar structure in its ground state but adopts a planar ICT structure in its fluorescent (S1) state. In the medium to high polarity solvents, it is inferred that not only the fluorescent state of the dye but also its ground state adopts an ICT character.
 |
| | Fig. 4 The most relaxed ground state (A) and excited S1 state (B) structures of 2AAQ in the gas phase as obtained from quantum chemical calculations are shown. The calculated dihedral angles for the amino hydrogen atoms are also indicated in the figure. | |
4 Conclusions
The photophysical properties of 2AAQ dye show different behavior in the lower polarity solvents (Δf < ca. 0.1) than in other solvents of medium to high polarity (Δf > ca. 0.1). Substantially higher Φf, τf and knr values, and unusually lower kf values for the dye in the lower solvent polarity region in comparison to those in the higher solvent polarity region indicate that the dye adopts different structural forms in the two regions of solvent polarity. This is further supported by the Stokes' shift studies for the dye in different solvents. Based on the experimental results and the quantum chemical calculations it is inferred that in the lower solvent polarity region the dye adopts a non-planar structure in its ground state whereas in the medium to high solvent polarity region the dye adopts a polar ICT type of structure in its ground state. In all the solvents, however, the results indicate that in the excited (S1) state the dye adopts a polar ICT structure.
Acknowledgements
Authors are thankful to the Director cum Chief Forensic Scientist, DFS, MHA, New Delhi, Director, CFSL, Hyderabad, and Head, Analytical Chemistry Division, BARC, for their encouragement and support during the course of this work. The authors are also thankful to Prof. T. Kundu of IIT Bombay for the excited state calculation. Parmila Dahiya is also thankful to DFS for the Junior Research Fellowship.
References
- M. W. Rembold and H. E. A. Kramer, The role of anthraquinonoid dyes in the catalytic fading of the dye mixtures: substituent dependent triplet yield of diaminoanthraquinone, J. Soc. Dyers Colour., 1980, 96, 122–126 CAS.
- M. W. Rembold and H. E. A. Kramer, Singlet oxygen as an intermediate in the catalytic fading of dye mixtures, J. Soc. Dyers Colour., 1978, 94, 12–17 CAS.
- H. E. A. Kramer and A. Maute, Sensitized photooxygenation: change from type I (radical) to type II (singlet oxygen) mechanism, Photochem. Photobiol, 1973, 17, 413–423 CAS.
- B. Kalyanraman, E. Perez-Reyes and R. P. Mason, Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs, Biochim. Biophys. Acta, 1980, 630, 119–130 CrossRef.
-
Topics in antibiotic chemistry, ed. P. G. Sammers, Ellis Horwood, Chichester, UK, 1978, vol. 12, Part-C Search PubMed.
-
W. A. Remers, The Chemistry of Antitumour Antibiotics, Wiley Interscience, New York, 1979, vol. 1, ch. 2 Search PubMed.
- J. Butler and B. M. Hoey, Are reduced quinones necessarily involved in the anti-tumor activity of quinone drugs, Br. J. Cancer, 1987, 55(suppl.), 53–59 CAS.
- J. Butler and B. M. Hoey, The reduction of anti-tumor diaziridinylbenzoquinones, Biochim. Biophys. Acta, 1987, 925, 144–149 CrossRef CAS.
- T. Mukherjee, Photo and radiation chemistry of quinones, Proc. Indian Natl. Sci., Acad. A, 2000, 66, 239–265 Search PubMed.
- M. Kumbhakar, S. Nath, M. C. Rath, T. Mukherjee and H. Pal, Electron transfer interaction of dihydroxy-quinones with amine quenchers: dependence of the quenching kinetics on the aliphatic and aromatic nature of the amine donors, Photochem. Photobiol., 2004, 79, 1–10 CrossRef CAS.
- S. M. Hubig and J. K. Kochi, Electron-transfer mechanisms with photoactivated quinones. The encounter complex versus the Rehm-Weller paradigm, J. Am. Chem. Soc., 1999, 121, 1688–1694 CrossRef CAS.
- S. A. doMonte and M. Braga, Electronic factor for photoinduced electron transfer in porphyrin-bridge-quinone systems, Chem. Phys. Lett., 1998, 290, 136–142 CrossRef CAS.
- H. Pal, D. K. Palit, T. Mukherjee and J. P. Mittal, Dynamics of the fluorescence quenching of 1,4-dihydroxy-, 1-amino-4-hydroxy- and 1,4-diamino-9,10-anthraquinones by aromatic hydrocarbons, J. Chem. Soc., Faraday Trans., 1993, 89, 683–691 RSC.
- M. C. Rath, H. Pal and T. Mukherjee, Excited singlet (S1) state interactions of 6,11-dihydroxy-5,12-naphthacenequinone with aromatic hydrocarbons, J. Phys. Chem. A, 2001, 105, 7945–7956 CrossRef CAS.
- D. K. Palit, H. Pal, T. Mukherjee and J. P. Mittal, Photodynamics of the S1 state of some hydroxy and amino substituted naphthoquinone and anthraquinones, J. Chem. Soc., Faraday Trans., 1990, 86, 3861–3869 RSC.
- J. P. Rasimas and G. J. Blanchard, A Study of the Fluorescence and Reorientation Dynamics of Carminic Acid in Primary Alcohols, J. Phys. Chem., 1995, 99, 11333–11338 CrossRef CAS.
- T. Yatsuhashi and H. Inoue, Molecular mechanism of radiationless deactivation of aminoanthraquinones through intermolecular hydrogen-bonding interaction with alcohols and hydroperoxides, J. Phys. Chem. A, 1997, 101, 8166–8173 CrossRef CAS.
- H. Inoue, M. Hida, N. Nakashima and K. Yoshihara, Picosecond fluorescence lifetimes of anthraquinone derivatives. Radiationless deactivation via intra- and intermolecular hydrogen bonds, J. Phys. Chem., 1982, 86, 3184–3188 CrossRef CAS.
- S. R. Flom and P. F. Barbara, Proton transfer and hydrogen bonding in the internal conversion of S1 anthraquinones, J. Phys. Chem., 1985, 89, 4489–4494 CrossRef CAS.
- H. Pal, S. Nad and M. Kumbhakar, Photophysical properties of coumarin-120: unusual behavior in nonpolar solvents, J. Chem. Phys., 2003, 119, 443–452 CrossRef CAS.
- S. Nad and H. Pal, Unusual photophysical properties of coumarin-151, J. Phys. Chem. A, 2001, 105, 1097–1106 CrossRef CAS.
-
Lange's handbook of chemistry, ed. J. A. Dean, McGraw-Hill, New York, 13th edn., 1987 Search PubMed.
- H. Masuhara, T. Hino and N. Mataga, Ionic photodissociation
of excited electron donoracceptor systems. 1. An empirical equation on the relationship between the yield and the solvent dielectric constant, J. Phys. Chem., 1975, 79, 994–1000 CrossRef CAS.
- H. Masuhara and N. Mataga, Ionic photodissociation of electron donor-acceptor systems in solution, Acc. Chem. Res., 1981, 14, 312–318 CrossRef CAS.
- S. Nath, H. Pal and A. V. Sapre, Effect of solvent polarity on the aggregation of fullerene, C60, Chem. Phys. Lett., 2000, 327, 143–148 CrossRef CAS.
- M. C. Rath, H. Pal and T. Mukherjee, Interaction of ground and excited (S1) states of C60 and C70 with aromatic amines: exciplex and charge transfer emissions, J. Phys. Chem. A, 1999, 103, 4993–5002 CrossRef CAS.
-
J. R. Lakowitz, Principle of fluorescence spectroscopy, Plenum Press, New York, 1983 Search PubMed.
-
J. B. Birks, Photo physics of aromatic molecules, Wiley-Interscience, New York, 1970 Search PubMed.
- E. Z. Lippert, Dipole moment and electronic structure of excited molecules, Z. Naturforsch., Teil A, 1957, 10, 541–545.
- N. Mataga, Y. Kaifu and M. Koizumi, Solvent effects upon fluorescence spectra and the dipole moments of excited molecules, Bull. Chem. Soc. Jpn., 1956, 29, 465–470 CAS.
-
D. V. O'Connor and D. Phillips, Time Correlated Single Photon Counting, Academic Press, New York, 1984 Search PubMed.
- J. T. Edward, Molecular volumes and the Stokes–Einstein equation, J. Chem. Educ., 1970, 47, 261–270 CrossRef CAS.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis and J. A. Montgomery, The general atomic and molecular electronic structure systems, J. Comput. Chem., 1993, 14, 1347–1363 CrossRef CAS.
Footnote |
| † Dedicated to Professor Hiroshi Masuhara on the occasion of his 60th birthday. |
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2005 |
Click here to see how this site uses Cookies. View our privacy policy here. 


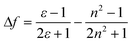
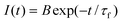





![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) ) for 2AAQ dye in different solvents and solvent mixtures
) for 2AAQ dye in different solvents and solvent mixtures
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) /cm−1
/cm−1![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) ) is expected to follow a linear relation with Δf, as suggested in the Lippert–Mataga relationship,27–30
) is expected to follow a linear relation with Δf, as suggested in the Lippert–Mataga relationship,27–30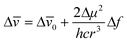
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values were estimated as the difference in energy between the absorption and fluorescence maxima for most of the solvents except the first three low polarity solvents mentioned earlier. For the latter three solvents instead of the observed absorption maxima, the vibrational band that corresponds to the absorption maxima in all other solvents were considered in the calculation of the Δ
values were estimated as the difference in energy between the absorption and fluorescence maxima for most of the solvents except the first three low polarity solvents mentioned earlier. For the latter three solvents instead of the observed absorption maxima, the vibrational band that corresponds to the absorption maxima in all other solvents were considered in the calculation of the Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values. The Δ
values. The Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values thus estimated for the dye in different solvents are listed in Table 2. Fig. 3C shows the plot of the Δ
values thus estimated for the dye in different solvents are listed in Table 2. Fig. 3C shows the plot of the Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values of the dye against the solvent polarity function Δf. It is seen from this figure that the plot is linear within experimental error for the solvents of medium to high polarities (Δf > ca. 0.1) but the data points are deviated towards lower values at Δf < ca. 0.1. Similar results were also obtained in our earlier studies with C120 dye,20 and these results indicate that the fluorophore is relatively less polar in structure in the lower polarity solvents than in the solvents of medium and high polarity.
values of the dye against the solvent polarity function Δf. It is seen from this figure that the plot is linear within experimental error for the solvents of medium to high polarities (Δf > ca. 0.1) but the data points are deviated towards lower values at Δf < ca. 0.1. Similar results were also obtained in our earlier studies with C120 dye,20 and these results indicate that the fluorophore is relatively less polar in structure in the lower polarity solvents than in the solvents of medium and high polarity.

![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) abs), (B) fluorescence maxima (
abs), (B) fluorescence maxima (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) fl) and (C) Stokes' shift of 2AAQ dye against Δf. The results substantiate the observations made in the Φf and τf measurements of the dye in different solvents.
fl) and (C) Stokes' shift of 2AAQ dye against Δf. The results substantiate the observations made in the Φf and τf measurements of the dye in different solvents.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) vs. Δf plot is linear, the data were analyzed according to eqn. (7). In this analysis, the Onsager radius r was considered to be 3.32 Å, as estimated on the basis of Edward's volume addition method.32 The Δμ value for 2AAQ is thus estimated to be ≈4.4 D, which is a substantially high value, suggesting the possible ICT character for the fluorescent state of the dye (cf. Chart 2). As the Stokes’ shifts are also higher for this solvent polarity region compared to those in the lower solvent polarity region, we suppose that the ground state of the dye also has reasonable ICT character in the former solvents. In the lower solvent polarity region, the lower than expected Δ
vs. Δf plot is linear, the data were analyzed according to eqn. (7). In this analysis, the Onsager radius r was considered to be 3.32 Å, as estimated on the basis of Edward's volume addition method.32 The Δμ value for 2AAQ is thus estimated to be ≈4.4 D, which is a substantially high value, suggesting the possible ICT character for the fluorescent state of the dye (cf. Chart 2). As the Stokes’ shifts are also higher for this solvent polarity region compared to those in the lower solvent polarity region, we suppose that the ground state of the dye also has reasonable ICT character in the former solvents. In the lower solvent polarity region, the lower than expected Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values, on the basis of the linear Δ
values, on the basis of the linear Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) vs. Δf correlation observed for the rest of the solvents, suggest that the dye structure could be relatively less polar in the former solvents than in the latter. These results are thus is accordance with our inference that the dye adopts a non-planar structure in the low solvent polarity region (cf. Chart 2).
vs. Δf correlation observed for the rest of the solvents, suggest that the dye structure could be relatively less polar in the former solvents than in the latter. These results are thus is accordance with our inference that the dye adopts a non-planar structure in the low solvent polarity region (cf. Chart 2).
