DOI:
10.1039/B502586A
(Paper)
Org. Biomol. Chem., 2005,
3, 2167-2174
Radical properties governing the hypoxia-selective cytotoxicity of antitumor 3-amino-1,2,4-benzotriazine 1,4-dioxides†
Received 21st February 2005, Accepted 1st April 2005
First published on 29th April 2005
Abstract
Revealing the free radical mechanism by which the anticancer drug tirapazamine (3-amino-1,2,4-benzotriazine 1,4-dioxide) induces hypoxia-selective cytotoxicity, is seen as a way forward to develop clinically useful bioreductive drugs against chemo- and radiation-resistant hypoxic tumor cells. Our previous studies point to the formation of an active benzotriazinyl radical following the one-electron reduction of tirapazamine and its elimination of water from the initial reduction intermediate, and have suggested that this species is a cytotoxin. In this paper we have used pulse radiolysis to measure the one-electron reduction potentials of the benzotriazinyl radicals E(B˙,H+/B) of 30 analogues of tirapazamine as well as the one-electron reduction potentials of their two-electron reduced metabolites, benzotriazine 1-oxides E(B/B˙−). The redox dependencies of the back-oxidation of the one-electron reduced benzotriazine 1,4-dioxides by oxygen, their radical prototropic properties and water elimination reactions were found to be tracked in the main by the one-electron reduction potentials of the benzotriazine 1,4-dioxides E(A/A˙−). Multiple regression analysis of published aerobic and hypoxic clonogenic cytotoxicity data for the SCCVII murine tumor cell line with the physical chemistry parameters measured in this study, revealed that hypoxic cytotoxicity is dependent on E(B˙, H+/B) thus providing strong evidence that the benzotriazinyl radicals are the active cytotoxic species in hypoxia, while aerobic cytotoxicity is dependent on E(B/B˙−). It is concluded that maximizing the differential ratio between these two controlling parameters, in combination with necessary pharmacological aspects, will lead to more efficacious anticancer bioreductive drugs.
Introduction
The bioreductive anticancer prodrug tirapazamine (3-amino-1,2,4-benzotriazine 1,4-dioxide, 1) is in Phase II/III clinical trials, in combination with radiotherapy and also with cisplatin based chemotherapy.1–41 is selectively activated in hypoxic regions of tumors, thus killing treatment-resistant hypoxic cells. Activation of 1 by one-electron reductases5–9 gives rise to an oxidizing radical that induces lethal DNA double strand breaks in cells.10,11 It is known that the cytotoxicity of 1 is less sensitive to traces of O2 than the nitroaromatic12–14 and quinone15 hypoxia-selective bioreductive drugs, and it has been postulated that this may be a factor in its toxicity to tumor cells at intermediate oxygen levels, leading to a better treatment outcome.16 The basis of the hypoxia-selectivity of bioreductive drugs is postulated to be the back oxidation of their radical anion/protonated species by molecular O2, which limits their toxicity to well-oxyenated cells. However, the back oxidation of the one-electron reduced species of 1 proceeds with a rate constant similar to that for nitroaromatic compounds of comparable one-electron reduction potential.17Hence it is unlikely that the back oxidation kinetics of its radical anion (2/3), kO2, Scheme 1, can alone account for the observed large difference in sensitivity to O2 compared to other bioreductive drugs. The radical 2/3 is a reducing species18,19 since the one-electron reduction potential of 1 is E(A/A˙−)
=
−0.456 ± 0.008 V at pH 720,21 and is thus unlikely to be a toxic oxidizing species that can damage DNA, as earlier proposed.18,19,22An alternative proposal is that 3 eliminates the ˙OH radical, which is a powerful oxidant,23Scheme 1, pathway III, although spin-trap EPR experiments with bioreduced 1 were inconclusive.24Possible analogies to such a breakdown of 3 have been drawn23 from photochemical studies with N-alkoxypyridinium compounds, which rapidly release alkoxyl radicals in organic solvents upon one-electron transfer from photoexcited dyes,25,26 and N-hydroxypyrindine-2(1H)-thione, which releases 2-pyridylthiyl and ˙OH radicals both in organic27,28 and aqueous29,30 solvents. Radiation chemistry is a useful tool to produce non-photoactivated reducing radicals, which mimic reducing species in biochemistry, upon the direct scavenging of the radiolytic produced solvated electron, eaq−, or through scavenging of ˙OH and H˙ radicals by appropriate solutes to produce reducing species.31 The release of an ˙OH radical from one-electron reduced organic compounds in aqueous solution is unknown in radiation chemistry, save in the case of the eaq− adding to and splitting the O–O bond in H2O2.32 We have previously presented kinetic and spectral evidence that 3 undergoes a unimolecular reaction (in competition with its dismutation to reform 1 and the two-electron reduced product, 4, pathway I) and suggested that 3 eliminates water to form a benzotriazinyl radical, 5, pathway II.33 For 3, this reaction proceeds with a rate constant, kelim, of ca. 100 s−1 at pH 7 and is accelerated by electron donating substituents on the A-ring. Spectral studies with benzotriazine 1,4-dioxide analogues (Class A compounds) have shown that the same radical is formed on the one-electron oxidation of their equivalent benzotriazine 1-oxides (Class B compounds) by inorganic radical anions.33 The resultant benzotriazinyl radical, 5, is an oxidizing radical of neutral charge at pH 7,34E(B˙,H+/B)
=
+1.32 V, which is capable of inducing DNA radicals as modelled by 2-deoxyribose and dGMP.33 In this study we report relevant kinetic and thermodynamic factors that underpin the reactivity of this important class of bioreductive drug. |
| | Scheme 1 Pathways for the formation, back oxidation and decay of one-electron reduced 3-amino-1,2,4-benzotriazine 1,4-dioxides. | |
Results and discussion
(a) One-electron reduction potentials
A widely recognized controlling parameter in the activation of several classes of oxygen-sensitive bioreductive drugs is the one-electron reduction potential, E(1)/V at pH 7, of the compounds.35,36We have previously measured the E(1) values for a series of A-ring substituted benzotriazine 1,4-dioxides,20 designated E(A/A˙−) which are presented in Table 1. We have now measured the E(A/A˙−) value of the 5-methyl substituted analogue, 5d-A, which at −0.428 V is significantly higher in one-electron reduction potential than 1, despite the electron donating effect of the methyl substituent. This result is in line with the previously reported potential of the electron-donating 5-methoxy analogue, 5c-A, and it is suggested that these substituents in the 5-position stabilize the radical anion being within van der Waals contact to the 4-oxide moiety. The benzotriazine 1-oxide metabolite of tirapazamine, SR4317, 4, has recently been found to possess an E(1) of −0.568 V,37 which is at the lower end of the range of values reported for the benzotriazine 1,4-dioxides. As it has been reported that the rate of SR4317 formation is related to cytotoxicity8,10,38 and that the addition of excess benzotriazine 1-oxides greatly enhances the cytotoxicity of 1,39 we have extended our previous measurements of E(1) values of the benzotriazine 1-oxides, E(B/B˙−),34,37 over the same series of A-ring analogues, to study possible correlations with cytotoxicity. These data are presented in Table 1 and in Fig. 1, where the experimentally derived values are treated by the Hammett ρσ equation. The E(A/A˙−) data for the benzotriazine 1,4-dioxides have been previously reported as best fitted using σp for 6- and 7-substituted compounds and σm for the 5- and 8-substituted compounds.20 An inverse pattern of dependency is seen for the benzotriazine 1-oxides, Fig. 1. Substitution on the benzotriazine 1-oxide ring at the 5- and 8-positions has little effect on E(B/B˙−), whereas regression analysis of the data for the 6- and 7- positions (including 4 in each case) gave the following relationships:
| 6-substitution: E(B/B˙−)/V =
−(0.580 ± 0.078)
+
(0.317 ± 0.025)
σm n
= 8, r2
= 0.963, F
= 156 |
| 7-substitution: E(B/B˙−)/V =
−(0.541 ± 0.008)
+
(0.199 ± 0.021)
σm n
= 8, r2
= 0.939, F
= 92 |
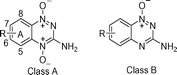
Table 1 One electron reduction potentials vs. NHE
|
|---|
| no.a | substituent | E(A/A˙−)
± 0.010/V | E(B/B˙−)
± 0.010/V | E(B˙,H+/B)
± 0.01/V |
|---|
| Letter-appended numbers refer to the regio-position of substituents common to both benzotriazine 1,4-dioxides (Class A) and benzotriazine 1-oxides (Class B). Ref 20 Ref 34 Ref 33. this work NDS not determined, insufficient solubility. |
|---|
| 1/4 | H | −0.456b | −0.568c | 1.32d |
| 5a-A/B | 5-N(Et)2 | −0.489b | −0.504e | 0.94e |
| 5b-A/B | 5-N(Me)2 | −0.481b | −0.491e | 0.97e |
| 5c-A/B | 5-OMe | −0.427b | −0.480e | 1.30e |
| 5d-A/B | 5-Me | −0.428e | −0.517e | 1.31e |
| 5e-A/B | 5-F | −0.394b | −0.393e | 1.30e |
| 5f-A/B | 5-Cl | −0.401b | −0.476e | 1.29e |
| 6a-A/B | 6-N(Et)2 | −0.671b | −0.654e | 1.20e |
| 6b-A/B | 6-N(Me)2 | −0.668b | −0.647c | 1.16c |
| 6c-A/B | 6-OMe | −0.558b | −0.533c | 1.30c |
| 6d-A/B | 6-Me | −0.493b | −0.586c | 1.30c |
| 6e-A/B | 6-F | −0.443b | −0.505e | 1.31e |
| 6f-A/B | 6-Cl | −0.391b | NDSf | NDSf |
| 6g-A/B | 6-CF3 | −0.335b | −0.455c | 1.27c |
| 6h-A/B | 6-SO2Me | −0.258b | −0.368c | 1.28c |
| 7b-A/B | 7-N(Me)2 | −0.525b | −0.567c | 0.94d |
| 7c-A/B | 7-OMe | −0.494b | −0.501e | 1.33e |
| 7d-A/B | 7-Me | −0.474b | −0.540e | 1.33e |
| 7e-A/B | 7-F | −0.400b | −0.480e | 1.31e |
| 7f-A/B | 7-Cl | −0.397b | NDSf | NDSf |
| 7g-A/B | 7-CF3 | −0.345b | −0.460e | 1.32e |
| 7h-A/B | 7-SO2Me | −0.297b | −0.436e | 1.28e |
| 7i-A/B | 7-NO2 | −0.260b | −0.381e | 1.39e |
| 8a-A/B | 8-N(Et)2 | −0.554b | −0.485e | 1.05e |
| 8b-A/B | 8-N(Me)2 | −0.545b | −0.495e | 1.02e |
| 8c-A/B | 8-OMe | −0.503b | −0.496e | 1.32e |
| 8d-A/B | 8-Me | −0.510b | −0.581e | 1.30e |
| 8e-A/B | 8-F | −0.400b | −0.472e | 1.31e |
| 8f-A/B | 8-Cl | −0.388b | −0.469e | 1.30e |
| 8g-A/B | 8-CF3 | −0.372b | −0.460e | 1.31e |
| 8h-A/B | 8-SO2Me | −0.309b | NDSf | NDSf |
 |
| | Fig. 1 Dependence of the one-electron reduction potentials of benzotriazine 1,4-dioxides, A, E(A/A˙−) (open symbols) and 1-oxides, B, (E(B/B˙−) (filled symbols) with (A) 5-, (B) 6-, (C) 7-, and (D) 8-substituents, on σp or σm. | |
The differences in the dependence on σp and σm, as well as the span in E(1) values on the A-ring, 6 > 7 ∼ 8 > 5, for the benzotriazine 1,4-dioxides and 6 ∼7 ≫ 8 ∼ 5 for the benzotriazine 1-oxides, must reflect the through-bond influence of the A-ring substituents, and/or different influences of the N-oxide substitutions, on the radical centers of one-electron reduced species of the compounds. Our recent studies point to the importance of benzotriazinyl radicals in effecting the radical-based cytotoxicity of the benzotriazine 1,4-dioxide class of bioreductive drugs.33,34Here we extend our previous measurements of the one-electron reduction potentials of benzotriazinyl radicals,33,34E(B˙,H+/B) produced by one-electron oxidation of the 1-oxides, by establishing redox equilibria with reference compounds as previously described.33 The values are presented in Table 1, and in Fig. 2 where they are plotted against the σp parameter of the substituents. The ρ value is highly positive for negative values of σp and slightly negative for positive values of σp
(E(B˙,H+/B)/V =
(1.31 ± 0.01)–(0.03 ± 0.01)
σp). This pattern of dependence has been previously related to polar effects in the stabilization of free radicals in solution.40 Compound 7i is an outlier in the above analysis, possibly due to the influence of the nitro substituent in resonance stabilization of the formed benzotriazinyl radical.
 |
| | Fig. 2 Dependence of the one-electron reduction potentials of benzotriazinyl radicals, E(B+˙/B), of substituted benzotriazine 1-oxides on σp. Compound 7i-B is plotted as an outlier. | |
(b) Decay kinetics of radicals of benzotriazine 1,4-dioxides in hypoxia
Pulse radiolysis experiments were performed in N2O-saturated solutions containing sodium formate (0.15 M, pH 7.0) to rapidly produce the CO2˙− species, which in turn reduced the compounds (<10 µs) to enable the dependence of the decay kinetics of the radical anions on radiation dose to be observed. The absorption bands of the radical anions (>500 nm), decayed with mixed order kinetics, as similarly reported following reduction of the compounds by the eaq− species.33 The transients were analyzed as previously described using the expression −exp(k1t1/2)
=
(k1
+
k2[C])/(2k1
+
k2[C]) where k1 is ascribed to the unimolecular loss of water to form the benzotriazinyl radical, kelim, and k2 is the disproportionation of the radical anions, kdis.While radical-radical reactions have to be taken into account in pulse radiolysis experiments, they are likely to be only a minor component in enzymatic systems compared to the unimolecular decay pathway, due to the small steady-state concentrations of the radicals that are produced. Kinetic plots of 1/t1/2vs. radiation dose (forming an initial radical concentration, C) yielded the kelim
(k1) component for each one-electron reduced compound, which then enabled the second order, dismutation rate constant, kdis
(k2) to be calculated using the above expression for pairs of t1/2 and C. These rate constants at pH 7.0 are presented in Table 2. The kdis data exhibit a trend of decreasing rate constants with lower pKa values of the radicals (pKr), measured below. This observation is in line with a previous pH study on kdis for the radical anion of 1 where it was concluded that kdis between protonated and unprotonated radical anions is greater than between protonated radicals, and that no such reaction occurs between unprotonated radical anions.18 Our value of kdis for the radical anion of 1, 2.2 ± 0.1 × 107 M−1 s−1, is lower than that of previous reports18,41 which did not take into account the concurrent elimination reaction. The kelim data are plotted as log kelim against E(A/A˙−) in Fig. 3. Data for the 6-, 7- and 8-substituted compounds (except 8a-A and 8b-A), conform to the relationship,
| log kelim
=
−(0.71 ± 0.29)–(6.50 ± 0.66)E(A/A˙−)
n
= 23, r2
= 0.823, F
= 98 |
 |
| | Fig. 3 Dependence of the observed unimolecular reaction, kelim, following one electron reduction of the benzotriazine 1,4-dioxides, on E(A/A˙−) at pH 7. The line is fitted using linear regression, excluding the 5-substituted compounds and compounds 8a-A and 8b-A. | |
Table 2 Properties of one-electron reduced benzotriazine 1,4-dioxides (Class A compounds)
| no. | pKr | kelim/s−1 | k(A˙−+ O2) 10−6/M−1 s−1 | 10−7kdis/M−1 s−1 |
|---|
| Ref 18. Ref 33. radical pK of diethylamine substituent, N-oxide radical. NDE, not determined as kelim is too high. |
|---|
| 1 | 6.19 ± 0.05ab | 83 ± 6 | 6.20 ± 0.25a | 2.16 ± 0.08 |
| 5a-A | 8.38 ± 0.07c | 6599 ± 806 | NDEd | NDEd |
| 5b-A | 7.22 ± 0.13 | 7223 ± 867 | 18.4 ± 1.1 | NDEd |
| 5c-A | 8.85 ± 0.44 | 3577 ± 194 | 28.5 ± 3.0 | 31.4 ± 8.8 |
| 5d-A | 7.83 ± 0.05 | 511 ± 24 | 18.8 ± 0.3 | 55.6 ± 1.2 |
| 5e-A | 6.75 ± 0.05 | 6473 ± 347 | 11.3 ± 3.4 | NDEd |
| 5f-A | 6.95 ± 0.06 | 53747 ± 3535 | NDEd | NDEd |
| 6a-A | 6.73 ± 0.08 | 7389 ± 623 | 115 ± 2.4 | NDEd |
| 6b-A | 6.90 ± 0.04 | 6612 ± 919 | 92.8 ± 7.2 | NDEd |
| 6c-A | 6.10 ± 0.03 | 713 ± 38 | 16.3 ± 1.1 | 11.3 ± 1.9 |
| 6d-A | 5.98 ± 0.04 | 176 ± 2 | 9.1 ± 0.95 | 11.3 ± 0.1 |
| 6e-A | 6.02 ± 0.03 | 91 ± 4 | 8.3 ± 0.16 | 5.96 ± 0.20 |
| 6f-A | 5.85 ± 0.02 | 119 ± 20 | 3.5 ± 0.45 | 3.35 ± 0.90 |
| 6g-A | 5.68 ± 0.07 | 70 ± 35 | 1.8 ± 0.16 | 2.58 ± 1.10 |
| 6h-A | 5.32 ± 0.04 | 3 ± 1 | 0.80 ± 0.02 | 0.80 ± 0.10 |
| 7b-A | 6.45 ± 0.04 | 1341 ± 35 | 23.2 ± 0.82 | 20.4 ± 0.60 |
| 7c-A | 6.10 ± 0.07 | 192 ± 28 | 9.14 ± 1.22 | 7.83 ± 0.79 |
| 7d-A | 6.13 ± 0.04 | 106 ± 10 | 5.84 ± 0.56 | 7.90 ± 0.45 |
| 7e-A | 6.12 ± 0.02 | 34 ± 4 | 3.55 ± 0.16 | 4.17 ± 0.19 |
| 7f-A | 5.87 ± 0.08 | 39 ± 5 | 2.45 ± 0.16 | 2.46 ± 0.18 |
| 7g-A | 5.34 ± 0.13 | 38 ± 10 | 2.27 ± 0.25 | 1.16 ± 0.41 |
| 7h-A | 5.52 ± 0.05 | 8 ± 4 | 0.81 ± 0.10 | 2.30 ± 0.17 |
| 7i-A | 4.96 ± 0.04 | 34 ± 2 | 0.94 ± 0.06 | 1.88 ± 0.07 |
| 8a-A | 6.46 ± 0.08 | 0.9 ± 0.2 | 0.48 ± 0.20 | 0.28 ± 0.01 |
| 8b-A | 6.91 ± 0.07 | 60 ± 12 | 0.44 ± 0.10 | 1.98 ± 0.49 |
| 8c-A | 6.67 ± 0.05 | 223 ± 23 | 12.0 ± 0.21 | 31.3 ± 1.0 |
| 8d-A | 6.60 ± 0.04 | 901 ± 129 | 8.36 ± 0.35 | 24.6 ± 7.7 |
| 8e-A | 6.17 ± 0.03 | 66 ± 3 | 3.21 ± 0.28 | 4.82 ± 0.13 |
| 8f-A | 6.29 ± 0.04 | 39 ± 7 | 3.26 ± 0.16 | 9.20 ± 0.20 |
| 8g-A | 5.97 ± 0.04 | 69 ± 1 | 2.91 ± 0.33 | 2.45 ± 0.08 |
| 8h-A | 6.12 ± 0.08 | 116 ± 30 | 2.01 ± 0.46 | 4.79 ± 1.27 |
The kelim values of the 5-substituted compounds are all considerably higher than predicted by this relationship. Two factors are likely to result in this observed increase in kelim, (i) the close proximity of electron donating groups to the radical center destabilizing the radical, as the rates increase with decreasing σp values, and (ii) halide ion elimination being facile from the 5-position for the intermediate radical, 2/3. To test the second hypothesis we measured the radiation dose-associated release of chloride ions from 5f-A
(50 µM) following one-electron reduction by the eaq− in deaerated solutions containing 2-methylpropan-2-ol (0.2 M). The measured yield, G(Cl−)
= 0.24 ± 0.01 µM Gy−1 corresponded to the loss of 5f-A, confirming stoichiometric release of chloride ions. The small kelim values obtained for 8a-A and 8b-A most likely arise from substituent effects due to the adjacent 1-oxide moiety being forced out of the plane of the triazine ring and causing partial uncoupling from the aromatic system. The effect of pH on kelim was studied for compounds 1 and 8d-A, Fig. 4. The rate constants for both compounds were found to decrease at high pH values from plateau values at low pH. The data sets are consistent for the one-electron reduced species of each compound being in prototropic equilibrium, Kr, with only the protonated radical undergoing an elimination reaction, k1, to form the putative benzotriazinyl radical, BTZ˙. For the reduction of 1,
| AH˙
(3)↔ A˙−
(2)
+ H+
Kr, |
| AH˙
(3)
→ BTZ˙
(5)
+ H2O k1 |
 |
| | Fig. 4 Effect of pH on the rate of observed unimolecular reaction, kelim, following the one electron reduction of the benzotriazine 1,4-dioxides 1
(○) and 8d-A
(●). | |
The kinetic description for the observed decay of the one-electron reduced species is kelim
=
(k1
×
[H+])/([H+]
+
Kr), and by setting k1
= 300 s−1 and 2000 s−1 and also pKr
= 6.118,33 and 6.633 for one-electron reduced 1 and 8d-A respectively, a good fit to the data is obtained, Fig. 4. Hence a full description of the decay kinetics (kelim and kdis) of each of the one-electron reduced compounds over a range of pH values, requires knowledge of the prototropic equilibria of the radicals. The radical pKa value (pKr) for each compound was determined from plots of absorption changes at appropriate wavelength against pH, as previously described33and the data are presented in Table 2. Both the 5-methoxy and 5-methyl analogues (5c-A and 5d-A) have pKr values considerably higher than 7 as outliers, which supports the above contention that their radical anions are somewhat stabilized. The dependence of the other pKr values on E(A/A˙−) is displayed in Fig. 5. Regression analysis of the data yields,
| pKr
=
(4.48 ± 0.23)–(3.66 ± 0.49)E(A/A˙−)
n
= 25, r2
= 0.713, F
= 55 |
 |
| | Fig. 5 Dependence of the radical pKa of the one-electron reduced benzotriazine 1,4-dioxides (pKr) on E(A/A˙−). The line is fitted using linear regression, excluding the 5-substituted compounds. | |
(c) Reaction of one-electron reduced species with oxygen
The reactivities of the radical species with oxygen at pH 7.0 were investigated by observing the decay rates of the radical absorption spectra at > 500 nm. Relatively high concentrations of the compounds (1 mM) and oxygen (0.1–0.65 mM) were used to ensure both adequate initial electron transfer from the CO2˙− species to the compounds and subsequent exponential decay of the radicals with oxygen. The decay rates of the transients were found to be linearly dependent on oxygen concentration, from which data the second order rate constants, kO2, were calculated, Table 2. The magnitude of the rate constants inversely correlates with E(A/A˙−) values, Fig. 6, except for the radicals of analogues 8a-A and 8b-A which are outliers having low reactivity. Regression analysis of the data yields,
| log kO2
=
(4.57 ± 0.09)–(4.99 ± 0.21)E(A/A˙−)
n
= 23, r2
= 0.963, F
= 559 |
 |
| | Fig. 6 Dependence of the rate constant for reaction of oxygen (kO2) with one-electron reduced benzotriazine 1,4-dioxides, on E(A/A˙−). The line is fitted using linear regression excluding the 5- substituted compounds and 8a-A and 8b-A. | |
The measured values of kO2 and their dependency on E(A/A˙−) closely follow those of nitroarene compounds of similar one-electron reduction potential.17 As in the case of nitro radicals, there is no evidence that the one-electron reduced N-oxides of the present study form intermediate adducts with oxygen. The observed slow kinetics for 8a-A and 8b-A most likely arise from the local substituent effects of bulky groups adjacent to the triazine 1,4-dioxide ring.
(d) Loss of compounds upon steady-state γ-radiolysis
The loss of the benzotriazine 1,4-dioxides (50–100 µM), under reducing conditions at pH 7.0, was followed by monitoring the sequential change in their UV–vis absorption spectra with radiation dose to obtain G(loss)/µM Gy−1
(µmol J−1) values. Three systems were studied, (i) N2-saturated solution of 2-methylpropan-2-ol (0.1 M) where the radiolytic yield of reducing radicals, Geaq−
= 0.28 µM Gy−1, (ii) N2O-saturated solutions of sodium formate (0.1 M) where GCO2˙−
= 0.68 µM Gy−142 and (iii) N2O-saturated solutions of 2-deoxyribose (50 mM, dR) where GdR˙
≤ 0.42 µM Gy−1.34 All three systems show the same pattern of results for 6-,7- and 8-substituted analogues, Fig. 7, where G(loss) is analyzed against the Hammett σp parameter. Some data sets are arbitrarily fitted to a Gaussian distribution to illustrate that the G(loss) values pass through a maximum near to a E(A/A˙−) value of −0.5 V (the 5-substituted analogues cover too small a range in σp for such an analysis). Several G(loss) values, Table 3, exceed the radiolytic yields of reducing radicals, e.g. for 6-methyl substituted compound, 6d-A, G(loss) values are −0.50 ± 0.01, −0.91 ± 0.01 and −2.21 ± 0.07 µM Gy−1 for reduction by eaq−, dR˙ and CO2˙− radicals respectively. Short chain reactions have been reported for the loss of 1 following its one-electron reduction in the presence of formate ions18 and 2-deoxyribose,34 but not following reduction by the eaq− in the presence of 2-methylpropan-2-ol.18 Our value for G(loss) of 1 by eaq− of 0.183 ± 0.004 µM Gy−1 appears to be greater than expected for simple disproportionation of the radical to reform a half a molecule of 1 and 4 as earlier proposed.18 In view of some of the observed G(loss) values exceeding Geaq−, Table 3, we investigated this system further. In a series of control experiments with 1 and several analogues, we serially irradiated the compounds (50 µM) in N2O-saturated solutions containing 2-methylpropan-2-ol (0.1 M) and found uniformly that G(loss) of the compounds was 0.10 ± 0.01 µM Gy−1. Under these conditions the eaq− are quantitatively converted to ˙OH radicals that are in turn scavenged by 2-methylpropan-2-ol to form a largely inert, non-reducing radical. This result implies that a small loss of the compounds could occur upon their reaction with such radicals or that the radiolytically produced H-atoms (0.055 µM Gy−1) could play a role in this system, possibly by forming a radical adduct, which then removes another molecule of compound. However, even adjusting the G(loss) data for this possible “side reaction” still gives corrected values higher than Geaq− for some analogues. This could arise from some of the putative benzotriazinyl radicals that are formed oxidizing another molecule of compound. Overall, the observed G(loss) values in anaerobic solutions containing 2-methylpropan-2-ol are evidence against the suggested release of ˙OH radicals23 from one-electron reduced 1,2,4-benzotriazine 1,4-dioxides, as the ˙OH radicals would be scavenged by the 2-methylpropan-2-ol, thus preventing any chain reaction.
Table 3 G(loss) of benzotriazine 1,4-dioxides (in µM Gy−1) upon steady-state irradiation at pH 7.0
| NO. | formate-N2O | t-BuOH–N2 | 2-dR-N2O |
|---|
| 1 | −1.07 ± 0.07 | −0.18 ± 0.01 | −0.74 ± 0.03 |
| 5a-A | −0.42 ± 0.02 | −0.55 ± 0.05 | −0.59 ± 0.01 |
| 5b-A | −0.82 ± 0.01 | −0.64 ± 0.03 | −0.51 ± 0.01 |
| 5c-A | −1.41 ± 0.06 | −0.42 ± 0.01 | −0.26 ± 0.03 |
| 5d-A | −1.16 ± 0.01 | −0.34 ± 0.01 | −0.70 ± 0.02 |
| 5e-A | −1.81 ± 0.05 | −0.57 ± 0.01 | −0.88 ± 0.02 |
| 5f-A | −2.37 ± 0.06 | −0.65 ± 0.02 | −0.24 ± 0.01 |
| 6a-A | −0.67 ± 0.04 | −0.18 ± 0.01 | −0.18 ± 0.01 |
| 6b-A | −0.72 ± 0.04 | −0.30 ± 0.02 | −0.16 ± 0.01 |
| 6c-A | −2.12 ± 0.04 | −0.37 ± 0.01 | −0.72 ± 0.02 |
| 6d-A | −2.21 ± 0.07 | −0.50 ± 0.01 | −0.91 ± 0.02 |
| 6e-A | −1.37 ± 0.11 | −0.22 ± 0.01 | −0.23 ± 0.01 |
| 6f-A | −0.57 ± 0.01 | −0.25 ± 0.01 | −0.24 ± 0.01 |
| 6g-A | −0.58 ± 0.01 | −0.20 ± 0.01 | −0.17 ± 0.01 |
| 6h-A | −0.33 ± 0.01 | −0.17 ± 0.01 | −0.20 ± 0.01 |
| 7b-A | −0.35 ± 0.01 | −0.28 ± 0.01 | −0.29 ± 0.02 |
| 7c-A | −1.34 ± 0.03 | −0.38 ± 0.01 | −0.59 ± 0.01 |
| 7d-A | −1.32 ± 0.02 | −0.35 ± 0.01 | −0.86 ± 0.03 |
| 7e-A | −1.16 ± 0.02 | −0.17 ± 0.01 | −0.20 ± 0.01 |
| 7f-A | −1.33 ± 0.04 | −0.29 ± 0.01 | −0.30 ± 0.01 |
| 7g-A | −0.69 ± 0.01 | −0.31 ± 0.01 | −0.25 ± 0.01 |
| 7h-A | −0.34 ± 0.01 | −0.20 ± 0.02 | −0.12 ± 0.01 |
| 7i-A | −0.28 ± 0.01 | −0.45 ± 0.02 | −0.18 ± 0.01 |
| 8a-A | −0.49 ± 0.01 | −0.19 ± 0.01 | −0.05 ± 0.01 |
| 8b-A | −0.79 ± 0.02 | −0.29 ± 0.01 | −0.09 ± 0.01 |
| 8c-A | −1.56 ± 0.04 | −0.38 ± 0.02 | −0.17 ± 0.01 |
| 8d-A | −1.73 ± 0.02 | −0.47 ± 0.03 | −0.63 ± 0.02 |
| 8e-A | −1.63 ± 0.04 | −0.19 ± 0.01 | −0.40 ± 0.01 |
| 8f-A | −1.22 ± 0.03 | −0.39 ± 0.01 | −0.32 ± 0.01 |
| 8g-A | −1.21 ± 0.01 | −0.26 ± 0.01 | −0.49 ± 0.02 |
| 8h-A | −0.75 ± 0.01 | −0.10 ± 0.01 | −0.42 ± 0.02 |
 |
| | Fig. 7 Dependence of the radiation chemical loss (Gloss) of (A) 5-, (B) 6-, (C) 8- and (D) 7-substituted benzotriazine 1,4-dioxides on E(A/A˙−). Compounds were irradiated in solutions containing formate/N2O (○), deoxyribose/N2O (△) and 2-methylpropan-2-ol/N2
(□). | |
(e) Dependence of cytotoxicity on physical parameters
Possible correlations were sought between the parameters quantified by this study and the published in vitro aerobic and hypoxic cytotoxicity data for the same compounds in SCCVII tumor cells,20 measured using a clonogenic assay. This assay measures the concentration required to reduce plating efficiency to 10% of controls (C10/µM) upon exposing 106 cells under aerobic and hypoxic conditions for 1 h. As E(A/A˙−) cross correlates well with kO2 and pKr, it was selected, along with E(B/B˙−), E(B˙, H+/B) and kelim as test physical chemistry parameters in a multiple regression analysis. Only compounds for which cytotoxicity values in both hypoxic and aerobic conditions had been established were used in the analysis, some 21 compounds, see Table S1 of the electronic supplementary information (ESI).†The inclusion of either the kelim parameter or E(A/A˙−) in all combinations with E(B/B˙−) and E(B˙,H+/B) were not significant variables in these analyses. The following significant correlations are found:Hypoxic cytotoxicity (5-, 6-, 7-, 8-substituted compounds)
| C10,hypoxic/µM =
(424 ± 76)
−
(311 ± 61)E(B+˙/B)
n
= 21, r2
= 0.58, F
= 26 |
Aerobic cytotoxicity (5-, 6-, 7-, 8-substituted compounds)
| C10,aerobic/µM =
−(7890 ± 1736)
−
(18069 ± 3492)E(B/B−)
n
= 21, r2
= 0.58, F
= 27 |
The above analyses of the linear dependence of the different cytotoxicities on
E(B/B˙
−) and
E(B
+˙/B) are made without excluding any of the compounds for which complete data sets are available. Improved correlations can be obtained upon excluding the 5-substituted compounds in the analyses. These compounds undergo a fast elimination reaction following their one-electron reduction, unaffected by aeration, making it reasonable to exclude them from the above analyses.
Hypoxic cytotoxicity (6-, 7-, 8-substituted compounds)
| C10,hypoxic/μM = (675 ± 45) − (504 ± 36)E(B+˙/B)
n = 17, r2 = 0.93, F = 199 |
Aerobic cytotoxicity (6-, 7-, 8-substituted compounds)
| C10,aerobic/μM = −(7453 ± 1606) −(17609 ± 3214)E(B/B˙−) n =17, r2 = 0.67, F = 30 |
While hypoxic cytotoxicity is readily understood in terms of the benzotriazinyl radical inducing radical damage on a critical cellular structure, such as DNA,34 the reason for aerobic toxicity being related to the one electron reduction of the benzotriazine 1-oxide metabolites is not obvious. Metabolism of tirapazamine, 1, to the benzotriazine 1-oxide, 4, by the one-electron reductases is greatly inhibited by oxygen,5 which is clear from the rapid back oxidation of the radical anion 2/3. However, the back oxidation reaction is in competition with the unimolecular reaction forming the oxygen-insensitive benzotriazinyl radical. The dismutation of the benzotriazinyl radicals would produce benzotriazine 1-oxide, Scheme 1, albeit in a minor amount due to kinetic competition (kelimvs. kO2[O2]) occurring. Also, murine SCCVII tumor cells do possess the obligate two-electron reductase, DT-diaphorase,43 which is insensitive to air and it can be hypothesized that some benzotriazine 1-oxide will be formed from the benzotriazine 1,4-dioxides during the time-course of the clonogenic assay. Futile cycling of one-electron reduced tirapazamine by oxygen, Scheme 1, restores the drug and produces superoxide, which has been associated with aerobic cytotoxicity.44,45 If such a mechanism is responsible for much of the observed aerobic toxicity, then it might be expected that the E(A/A˙−) parameter would also feature in the above analysis, unless the benzotriazine 1-oxides are selectively reduced by other means, for example, upon possible direct interaction with components of the electron transport chain. Another possible mechanism of oxic cytotoxicity is disturbance of the proton gradient across the mitochondrial inner membrane. The pKr of the benzotriazine 1-oxide, 4, is 9.734 compared with 6.1 for the benzotriazine 1,4-dioxide, 118,33 and we have shown that the pKr values of analogues of 1 decrease as E(A/A˙−) increases. Hence, assuming a similar trend in pKr values with E(B/B˙−), and that the radical species have a significant lifetime, aerobic toxicity by this mechanism might occur in the case of the benzotriazine 1-oxides as their pKr values can be hypothesized to span the mitochondrial inner membrane pH of 8.0. A similar dependence for the activity of a wide range of uncouplers of oxidative phosphorylation on their pKr values, increased uncoupling with lower pKr values, has been found.46
Conclusion
These studies indicate that large hypoxic cytotoxic ratios (HCR), for the 1,2,4-benzotriazine 1,4-dioxide class of bioreductive drugs, may be obtained by maximizing E(B˙,H+/B) above the previously suggested threshold value of 1.24 V,34 and minimising the E(B/B˙−) parameter. This conclusion is made from a radical chemistry perspective and does not consider many of the physiological and pharmacological aspects that together combine to influence drug activity. Satisfying these radical chemistry parameters, in tandem with a variety of pharmacological criteria, should determine the successful development of an improved analogue of 1.Experimental
All reagents used were of analytical grade. Sodium formate, sodium hydroxide, perchloric acid and phosphate buffers were obtained from Merck and potassium thiocyanate from Riedel-de Haen. All other reagents were obtained from Aldrich Chemical Company. All solutions were prepared in water purified by the Millipore “Milli-Q” system. Solution pH values were adjusted using the phosphate salts (5 mM) and either NaOH or HClO4 when necessary. Analyses were carried out in the Microchemical Laboratory, University of Otago, Dunedin, NZ. Melting points were determined on an Electrothermal 2300 Melting Point Apparatus. NMR spectra were obtained on a Bruker Avance 400 spectrometer at 400 MHz for 1H and 100 MHz for 13C spectra. Spectra were obtained in (CD3)2SO unless otherwise specified, and are referenced to Me4Si. Chemical shifts and coupling constants were recorded in units of ppm and Hz, respectively. Assignments were determined using COSY, HSQC, and HMBC two-dimensional experiments where appropriate. Mass spectra were determined on a VG-70SE mass spectrometer using an ionizing potential of 70 eV at a nominal resolution of 1000. High-resolution spectra were obtained at nominal resolutions of 3000, 5000, or 10000 as appropriate. Solutions in organic solvents were dried with anhydrous Na2SO4. Solvents were evaporated under reduced pressure on a rotary evaporator. Thin-layer chromatography was carried out on aluminum-backed silica gel plates (Merck 60 F254), with visualization of components by UV light (254 nm) or exposure to I2. Column chromatography was carried out on silica gel, (Merck 230–400 mesh). DCM refers to dichloromethane; DMSO refers to dimethylsulfoxide; EtOAc refers to ethyl acetate; MeOH refers to methanol; t-BuOH refers to 2-methylpropan-2-ol; MeCN refers to acetonitrile; pet. ether refers to petroleum ether, boiling range 40–60 °C. All solvents were freshly distilled. All compounds used in this study were synthesized and characterized as described previously,20 with the exception of the 1-oxides (B) of 5a, 5b, 6a, 6b, 8a, 8b.N5,N5-Diethyl-1,2,4-benzotriazine-3,5-diamine 1-oxide (5a-B)
A solution of 5-fluoro-1,2,4-benzotriazine-3-amine 1-oxide20
(5e-B)
(115 mg, 0.64 mmol) and diethylamine (3.3 cm3, 32 mmol) in DMSO (10 cm3) was stirred in a sealed pressure vessel at 100 °C for 3 days. The mixture was poured into water, extracted with EtOAc (3 × 30 cm3), the organic fraction washed with brine (50 cm3), dried, and the solvent evaporated. The residue was purified by chromatography, eluting with a gradient (0–5%) of MeOH/DCM to give 5-diethylamine 5a-B
(21 mg, 14%) as a purple gum; δH 1.09 (6 H, t, J 7.0, 2 × CH3), 3.47 (4 H, q, J 7.0, 2 × NCH2), 7.10 (1 H, dd, J 7.7 and 1.1, 6-H), 7.22–7.25 (3 H, m, 7-H, NH2), 7.87 (1 H, dd, J 8.6 and 1.1, 8-H); m/z
(EI+) 233.1270 (M+). C11H15N5O requires 233.1277.N5,N5-Dimethyl-1,2,4-benzotriazine-3,5-diamine 1-oxide (5b-B)
Similarly, reaction of fluoride205e-B
(43 mg, 0.24 mmol) with 40% aqueous dimethylamine (3 cm3) in MeOH (10 cm3) gave 5-dimethylamine 5b-B
(30 mg, 60%) as a red solid; mp (MeOH/EtOAc) 234–238 °C; δH 3.32 (6 H, s, 2 × NCH3), 7.05 (1 H, dd, J 7.8 and 1.0, 6-H), 7.17–7.25 (3 H, m, 7-H, NH2), 7.63 (1 H, dd, J 8.7 and 1.0, 8-H); m/z
(EI) 205.0966 (M+). C9H11N5O requires 205.0964.N6,N6-Diethyl-1,2,4-benzotriazine-3,6-diamine 1-oxide (6a-B)
Similarly, reaction of fluoride206e-B
(100 mg, 0.56 mmol) with diethylamine (3 cm3) in MeCN (10 cm3) gave 6-diethylamine 6a-B
(83 mg, 64%) as a purple solid (Found: C, 56.6; H, 6.6; N, 30.1. C11H15N5O requires C, 56.6; H, 6.5; N, 30.0%); mp (DCM/pet. ether) 247–251 °C; δH 1.10 (6 H, t, J 7.0, 2 × CH3) 3.47 (4 H, q, J 7.0, 2 × NCH2), 6.31 (1 H, d, J 2.8, 5-H), 6.83 (2 H, br s, NH2), 6.93 (1 H, dd, J 9.8 and 2.8, 7-H), 7.91 (1 H, d, J 9.8, 8-H); δC 12.3 (2), 44.2 (2), 99.0, 113.9, 121.2, 121.8, 150.9, 152.1, 160.6;N6,N6-Dimethyl-1,2,4-benzotriazine-3,6-diamine 1-oxide (6b-B)
Similarly, reaction of fluoride206e-B
(100 mg, 0.56 mmol) with 40% aqueous dimethylamine (3 cm3) in MeCN (10 cm3) gave 6-dimethylamine 6b-B
(93 mg, 80%) as a purple solid (Found: C, 52.5; H, 5.4; N, 34.3. C9H11N5O requires C, 52.7; H, 5.4; N, 34.1); mp (DCM/pet. ether) 264–267 °C; δH 3.09 (6 H, s, 2 × NCH3), 6.33 (1 H, d, J 2.7, 5-H), 6.88 (2 H, br s, NH2), 6.97 (1 H, dd, J 9.7 and 2.7, 7-H), 7.92 (1 H, d, J 9.7, 8-H).N8,N8-Diethyl-1,2,4-benzotriazine-3,8-diamine 1-oxide (8a-B)
Similarly, reaction of fluoride208e-B
(246 mg, 2.5 mmol) with diethylamine (13 cm3) in 10% aqueous MeOH (30 cm3) gave 8-diethylamine 8a-B
(396 mg, 68%) as a purple solid (Found: C, 56.4; H, 6.8; N, 30.2. C11H15N5O requires C, 56.6; H, 6.5; N, 30.0%); mp (MeOH) 186–188 °C; δH 1.04 (6 H, t, J 7.0, 2 × CH3), 3.17 (4 H, q, J 7.0, 2 × NCH2), 6.71 (1 H, dd, J 8.1 and 1.1, 7-H), 6.89 (1 H, dd, J 8.1 and 1.1, 5-H), 6.96 (2 H, br s, NH2), 7.49 (1 H, t, J 8.1, 6-H).N8,N8-Dimethyl-1,2,4-benzotriazine-3,8-diamine 1-oxide (8b-B)
Similarly, reaction of fluoride208e-B
(435 mg, 2.4 mmol) with 40% aqueous dimethylamine (15 cm3) in MeOH (20 cm3) gave 8-dimethylamine 8b-B
(246 mg, 50%) as a purple solid (Found: C, 52.8; H, 5.4; N, 34.4. C9H11N5O requires C, 52.7; H, 5.4; N, 34.1%); mp (MeOH) 211 °C (dec.); δH 2.80 (6 H, s, 2 × NCH3), 6.21 (1 H, dd, J 8.1 and 1.0, 7-H), 6.84 (1 H, dd, J 8.1 and 1.0, 5-H), 7.03 (2 H, br s, NH2), 7.49 (1 H, t, J 8.1, 6-H).Methods
Pulse radiolysis experiments, at room temperature (22 ± 1 °C), were carried out using a 4 MeV linear accelerator of variable pulse length (200 ns to 1.5 µs), to deliver a typical absorbed dose of 2.5 Gy for spectral studies and 2.5–10 Gy for kinetic studies. The optical detection system and method of dosimetry have been described previously.47 Steady-state radiolysis experiments were performed using a 60Co γ-source delivering a dose rate of 15 Gy min−1. Aqueous samples were evacuated and purged with appropriate O2-free gases in glass tubes for three cycles. The tubes were fitted with a side arm incorporating a supracil spectrophotometer cell for UV–vis measurements. The G(loss) values obtained by the spectrophotometric method, in which isobestic spectral points were maintained, agreed well with values obtained for a subset of compounds using HPLC analysis.34 Chloride ion concentrations were determined using HPLC, (Dionex QIC Ionchrom analyzer) that utilizes a suppressed conductivity detection system. The separation was carried out on an analytical column IonPac ASA4 (4 × 250 mm) coupled to a guard column AG4A (4 × 50 mm) with carbonate/bicarbonate (0.002 M) as eluent (flow rate = 5 mL min−1) and sulfuric acid (0.025 M) as a regenerant (flow rate = 3–4 mL min−1). NaCl was used as a standard for the quantitative analysis of Cl−.Radiation chemistry
The radiolysis of water produces three well-characterized reactive radical species used to initiate radical reactions, as well as molecular products (µM per absorbed dose of 1 Gy (J Kg−1) given in parenthesis).
H2O ![[long arrow, wavy then straight]](https://www.rsc.org/images/entities/char_e0f6.gif) eaq−(0.28)
+˙OH(0.28)
+ H˙(0.055)
+ H2(0.04)
+ H2O2(0.07)
+ H3O+(0.28) eaq−(0.28)
+˙OH(0.28)
+ H˙(0.055)
+ H2(0.04)
+ H2O2(0.07)
+ H3O+(0.28) |
One electron reductions of the benzotriazine 1,4-dioxides and 1-oxides, (A/B), were carried out by (i) the eaq−, while at the same time scavenging the oxidizing radicals with 2-methylpropan-2-ol to form an inert radical, (ii) electron transfer from the CO2˙− species (E0 CO2/CO2˙−)
=
−1.90 V48) in N2O-saturated solutions (to quantitatively convert the eaq− to ˙OH radicals) containing 0.1 M sodium formate, to convert the ˙OH radicals and H-atoms to CO2˙−, and (iii) electron transfer from ribose radicals (dR˙) formed in N2O-saturated solutions containing 50 mM 2-deoxyribose (dR).
| ˙OH(H˙)
+
(CH3)3COH →˙CH2(CH3)2COH + H2O(H2) |
| N2O + eaq−
→˙OH + OH−
+ N2 |
| ˙OH(H˙)
+ HCOO−/dR → H2O(H2)
+ CO2˙−/ dR˙ |
| CO2˙−/dR˙
+ A/B → A˙−/ B˙−
+ CO2/dR |
One-electron oxidation of the benzotriazine 1-oxides (B) was carried out by reaction with either the selenite radical (SeO3˙−) or sulfate radical (SO4˙−) produced on scavenging the eaq− by sodium selenate (50 mM) or sodium peroxidodisulfate (25 mM) in deaerated solutions containing 2-methylpropan-2-ol (0.2 M) to scavenge the ˙OH radicals.
| eaq−
+ SeO42−/S2O82−
+ H2O → SeO3˙−/SO4˙−
+ 2OH−
+/SO42− |
| SeO3˙−/SO4˙−
+ B → B˙
+ H+
+ SeO32−/SO42− |
One-electron reduction potentials
The one-electron reduction potentials of the compounds, E(A/A˙−) and E(B/B˙−), vs. NHE, were determined at pH 7.0 (5 mM phosphate buffer) by establishing redox equilibria between three mixtures of the one-electron reduced compounds and the reference compounds methylviologen (E(MV2+/MV+˙)
=
−447 ± 7 mV) or triquat (E(TQ2+/TQ+˙)
=
−548 ± 7 mV) and calculating ΔE values from the equilibrium constants, Ke, using the Nernst equation, as described in the literature.49Similarly, the one-electron reduction potentials of the benzotriazinyl radicals, E(B+˙/B), were determined between mixtures of the oxidised benzotriazine 1-oxides and the reference compound 1,2-dimethoxybenzene (E(DMB+˙/DMB)
= 1.30 ± 0.1 V50), 1,2,4-trimethoxybenzene (E(TMB+˙/TMB)
= 1.13 ± 0.02 V50) or aniline (E(AN+˙/AN)
= 1.03 V51).
Both the radical pKa values (pKr), derived by non-linear least-squares fit to a sigmoidal function, and the parameters for multiple regression analysis, were acquired using Origin software.
Acknowledgements
This work was supported by Grant 04/094 from the Health Research Council of New Zealand (RFA, SSS), Grant CA082566-04 from the National Cancer Institute (MPH) and the Auckland Division of the Cancer Society of New Zealand (WAD).References
- D. J. Lee, A. Trotti, S. Spencer, R. Rostock, C. Fisher, R. von Roemeling, E. Harvey and E. Groves, Int. J. Radiat. Oncol. Biol. Phys., 1998, 42, 811 CrossRef CAS.
- J. Del Rowe, C. Scott, M. Werner-Wasik, J. P. Bahary, W. J. Curran, R. C. Urtasun and B. Fisher, J. Clin. Oncol., 2000, 18, 1254 CAS.
- J. von Pawel, R. von Roemeling, U. Gatzemeier, M. Boyer, L. O. Elisson, P. T. D. Clark, A. Rey, T. W. Butler, V. Hirsh, I. Olver, B. Bergman, J. Ayoub, G. Richardson, D. Dunlop, A. Arcenas, R. Vescio, J. Viallet and J. Treat, J. Clin. Oncol., 2000, 18, 1351 CAS.
- D. Rischin, L. Peters, J. Smith, A. Macann, J. Denham, M. Poulsen, M. Jackson, L. Kenny, M. Penniment and R. Fisher, Proc. Am. Soc. Oncol., 2003, 22, 494 Search PubMed.
- M. I. Walton and P. Workman, Biochem. Pharmacol., 1990, 39, 1735 CrossRef CAS.
- M. I. Walton, C. R. Wolf and P. Workman, Biochem. Pharmacol., 1992, 44, 251 CrossRef CAS.
- S. A. Fitzsimmons, A. D. Lewis, R. J. Riley and P. Workman, Carcinogenesis, 1994, 15, 1503 CrossRef CAS.
- A. V. Patterson, H. M. Barham, E. C. Chinje, G. E. Adams, A. L. Harris and I. J. Stratford, Br. J. Cancer, 1995, 72, 1144 CAS.
- A. V. Patterson, M. P. Saunders, E. C. Chinje, L. H. Patterson and I. J. Stratford, Anti-Cancer Drug Des., 1998, 13, 541 CAS.
- K. A. Biedermann, J. Wang, R. P. Graham and J. M. Brown, Br. J. Cancer, 1991, 63, 358 CAS.
- J. M. Brown and L.-H. Wang, Anti-Cancer Drug Des., 1998, 13, 529 CAS.
- Y. C. Taylor and A. M. Rauth, Radiat. Res., 1982, 91, 104 CrossRef CAS.
- R. T. Mulcahy, Cancer Res., 1984, 44, 4409 CAS.
- B. G. Siim, G. J. Atwell and W. R. Wilson, Br. J. Cancer, 1994, 70, 596 CAS.
- R. S. Marshall and A. M. Rauth, Cancer Res., 1988, 48, 5655 CAS.
- J. Koch, Cancer Res., 1993, 53, 3992.
- P. Wardman, M. F. Dennis, S. A. Everett, K. B. Patel, M. R. L. Stratford and M. Tracy, Biochem. Soc. Symp., 1995, 61, 171 Search PubMed.
- K. Laderoute, P. Wardman and A. M. Rauth, Biochem. Pharmacol., 1988, 37, 1487 CrossRef CAS.
- R. V. Lloyd, D. R. Duling, G. V. Rumyantseva, R. P. Mason and P. K. Bridson, Mol. Pharmacol., 1991, 40, 440 CAS.
- M. P. Hay, S. A. Gamage, M. Kovacs, F. B. Pruijn, R. F. Anderson, A. V. Patterson, W. R. Wilson, J. M. Brown and W. A. Denny, J. Med. Chem., 2003, 46, 169 CrossRef CAS.
- K. I. Priyadarsini, M. Tracy and P. Wardman, Free Rad. Res., 1996, 25, 393 CrossRef CAS.
- J. M. Brown, Br. J. Cancer, 1993, 67, 1163 CAS.
- J. S. Daniels and K. S. Gates, J. Am. Chem. Soc., 1996, 118, 3380 CrossRef CAS.
- L. H. Patterson and F. A. Taiwo, Biochem. Pharmacol., 2000, 60, 1933 CrossRef CAS.
- I. R. Gould, D. Shukla, D. Giesen and S. Farid, Helv. Chim. Acta, 2001, 84, 2796 CrossRef CAS.
- D. Lorance, W. H. Kramer and I. R. Gould, J. Am. Chem. Soc., 2002, 124, 15225 CrossRef.
- J. Boivin, E. Crépon and S. Z. Zard, Tetrahedron Lett., 1990, 31, 6869 CrossRef CAS.
- D.H. R. Barton, J. C. Jaszberenyi and A. I. Morrell, Tetrahedron Lett., 1991, 32, 311 CrossRef CAS.
- K. M. Hess and T. A. Dix, Anal. Biochem., 1992, 206, 309 CrossRef CAS.
- K. J. Reszka and C. F. Chignell, Photochem. Photobiol., 1995, 61, 269 CrossRef CAS.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513 CAS.
- D. J. Currie and F. S. Dainton, Trans. Faraday Soc., 1965, 61, 1156 RSC.
- R. F. Anderson, S. S. Shinde, M. P. Hay, S. A. Gamage and W. A. Denny, J. Am. Chem. Soc., 2003, 125, 748 CrossRef CAS.
- S. S. Shinde, R. F. Anderson, M. P. Hay, S. A. Gamage and W. A. Denny, J. Am. Chem. Soc., 2004, 126, 7865 CrossRef CAS.
- P. Wardman, Environ. Health Perspect., 1985, 64, 309 CrossRef CAS.
- M. V. Orna and R. P. Mason, J. Biol. Chem., 1989, 264, 12379 CAS.
- R. F. Anderson, T. A. Harris, M. P. Hay and W. A. Denny, Chem. Res. Toxicol., 2003, 16, 1477 CrossRef CAS.
- A. K. Costa, M. A. Baker, J. M. Brown and J. R. Trudell, Cancer Res., 1989, 49, 925 CAS.
- B. G. Siim, F. B. Pruijn, J. R. Sturman, M. P. Hay, J. M. Brown and W. R. Wilson, Cancer Res., 2004, 64, 736 CAS.
- A. Russell, in Free Radicals, ed. J. K. Koch, John Wiley & Sons, New York, 1973, vol. 1, p. 275 Search PubMed.
- P. Wardman, K. I. Priyadarsini, M. F. Dennis, S. A. Everett, M. A. Naylor, K. B. Patel, I. J. Stratford, M. R. L. Stratford and M. Tracy, Br. J. Cancer, 1996, 74, S70 CAS.
- Q. G. Mulazzani, M. Venturi, M. Z. Hoffman and M. A. J. Rodgers, J. Phys. Chem., 1986, 90, 5347 CrossRef CAS.
- J. Wang, K. A. Biedermann, C. R. Wolf and J. M. Brown, Br. J. Cancer, 1993, 67, 321 CAS.
- S. W. Tuttle, L. Hazard, C. J. Koch, J. B. Mitchell, C. N. Coleman and J. E. Biaglow, Int. J. Radiat. Oncol. Biol. Phys., 1994, 29, 357 CAS.
- J. H. Elwell, B. G. Siim, J. W. Evans and J. M. Brown, Biochem. Pharmacol., 1997, 54, 249 CrossRef CAS.
- K. B. Patel and R. F. Anderson, Annual Report, Cancer Research Campaign Gray Laboratory, 1987, 79 Search PubMed.
- R. F. Anderson, W. A. Denny, W. Li, J. E. Packer, M. Tercel and W. R. Wilson, J. Phys.
Chem. A, 1997, 101, 9704 CrossRef CAS.
- A. Schwarz and R. W. Dodson, J. Phys. Chem., 1989, 93, 409 CrossRef CAS.
- P. Wardman, J. Phys. Chem. Ref. Data, 1989, 18, 1637 CAS.
- M. Jonsson, J. Lind, T. Reitberger, T. E. Eriksen and G. Mérenyi, J. Phys. Chem., 1993, 97, 11278 CrossRef CAS.
- M. Jonsson, J. Lind, T. E. Eriksen and G. Mérenyi, J. Am. Chem. Soc., 1994, 116, 1423 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2005 |
Click here to see how this site uses Cookies. View our privacy policy here. 







![[long arrow, wavy then straight]](https://www.rsc.org/images/entities/char_e0f6.gif) eaq−(0.28)
+˙OH(0.28)
+ H˙(0.055)
+ H2(0.04)
+ H2O2(0.07)
+ H3O+(0.28)
eaq−(0.28)
+˙OH(0.28)
+ H˙(0.055)
+ H2(0.04)
+ H2O2(0.07)
+ H3O+(0.28)

