Jon-Paul Griffiths, Hui Nie, R. James Brown, Peter Day and John D. Wallis
Org. Biomol. Chem., 2005,3, 2155-2166
DOI:
10.1039/B502437D,
Paper
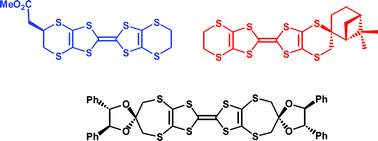
Syntheses of enantiopure organosulfur donors by three different strategies requiring only four–six steps are reported. The key step involves either double substitution of an enantiopure cyclic sulfate ester by a dithiolate, attachment of a chiral