Hybrid materials. Functional properties. From Maya Blue to 21st century materials
Pedro
Gómez-Romero
a and
Clément
Sanchez
b
aMaterials Science Institute of Barcelona (ICMAB, CSIC)†, Universitat Autonoma de Barcelona, Campus UAB, 08193, Bellaterra (Barcelona), Spain. E-mail: pedro@icmab.es
bLaboratoire de Chimie de la Matière Condensée (CNRS UMR 7574)†, Université Pierre et Marie Curie, 4 place Jussieu, 75252, Paris cedex 05, France. E-mail: clems@ccr.jussieu.fr
First published on 22nd December 2004
Abstract
The field of hybrid organic-inorganic materials has reached a stage of ripeness that is beginning to make possible extraordinary new developments. On the one hand the field has witnessed a trend towards the design of functionality in hybrids materials, while broadening spectacularly the variety of hybrid combinations explored. On the other hand, improved understanding and control of the chemistry, processing and microstructure of these versatile nanocomposite systems announce a new landscape of opportunities in dealing with increasingly complex chemistries and materials.
Mayan artisans concocting in the 8th century the unique pigment we now know as Maya Blue (see Fig. 1) could have never suspected that their lively blue tones would endure more than twelve centuries of harsh jungle environment. Any modern chemist aware of the fact that the blue color was not coming from a mineral substance but from indigo, a well-known organic dye extracted from Indigofera suffruticosa (Lin.) would not have given much for the long-term stability of the pigment either. But Maya Blue is no ordinary organic dye, nor is it any simple mineral. Instead, it is a hybrid organic-inorganic nanocomposite in which the organic dye molecules are protected within palygorskite, a complex natural clay.1,2 Despite some controversy on the resting place of the indigo molecules,1i the organic dye is molecularly isolated, hydrogen-bonded and protected within the inorganic lattice. The result is a synergistic combination of vegetal color and mineral sturdiness, the best of each world.
 | ||
| Fig. 1 Computer-enhanced Mayan warrior from Bonampak. | ||
As the 20th century turned into the 21st our improved knowledge of materials science and the mastery of a wealth of physico-chemical techniques have opened the gates of chemistry to the field of molecular engineering, configuring the bottom-up road to nanoscience and nanotechnology. It is in this new and wide-open landscape of molecular and supramolecular control that the true dimension of hybrid organic-inorganic materials is beginning to be realized. Organic-inorganic admixtures have indeed been around for a long time, but it is their combination at a molecular level that is leading to remarkable new materials and improved properties.2–4
And speaking of properties, a change of emphasis has been brewing in laboratories worldwide through the last decade. From an emphasis on the design of structural hybrid materials bringing together the best properties of glass and plastics, has followed a fast growing interest in functional properties and applications.2–9 There is indeed a quickly expanding area of research on functional hybrid materials in which mechanical properties are secondary—though certainly not unimportant—and the emphasis is on chemical, electrochemical, or biochemical activity, as well as on magnetic, electronic, optical or other physical properties, or a combination of them.
Numerous new applications in the field of advanced materials science are related to functional hybrids. Thus, the combination at the nanosize level of active inorganic and organic or even bioactive components into a single material has made accessible an immense new area of materials science that has extraordinary implications in the development of multifunctional materials. The chemical nature of these emerging classes of hybrids varies wildly, from molecular and supramolecular adducts, to polymer matrix hybrids, to extended solids, mineral or biomineral phases.10–12 These functional hybrids are considered as innovative advanced materials and promising applications are expected in many fields: optics, electronics, ionics, energy storage and conversion, mechanics, membranes, protective coatings, catalysis, sensors, biology and biomedicine.2 Many interesting new materials have already been prepared with remarkable new properties. To give just a handful of examples, hybrid materials having excellent laser efficiency and good photostability, very fast photochromic response, very high and stable second-order nonlinear optical response, featuring high charge-storage capabilities, or being original pH sensors and electroluminescent diodes have been reported in the past five years (see Fig. 2). And some hybrid products have already entered the applied field and the market. Examples9 include: silane coupling agents used to tune interfaces in the reinforcement of plastics by glass fibers, the new generation of “Green Tires” developed by Michelin, organically doped sol-gel glassware sold by Spiegelau, or sol-gel entrapped enzymes sold by Fluka.
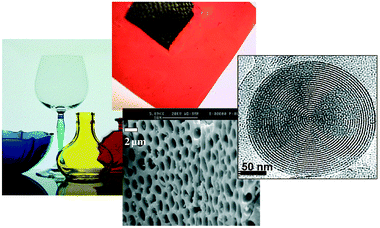 | ||
| Fig. 2 Hybrid materials permeate a very large variety of advanced technologies: from the conspicuous glassware coatings13 on the left to the proton-exchange membrane hidden in state-of-the-art polymeric fuel cells14 (top). They also allow for the design of micro-, meso- and nanostructured systems, whether hybrid or inorganic, like the biopolymer/nanoCeO2 hybrid precursors of ceria with hierarchical porosity15 shown in the middle, or the nanostructured mesoporous onion-like SiO2 structures15 on the right. | ||
Yet, the 21st century is very young and these examples only represent a small part of the tip of the iceberg, an appetizer for what it is to come when the century comes of age. Indeed, bioinspired approaches to the design of hybrid and inorganic materials10–12 will be one of the most promising scientific and technological challenges of the coming years, whereas environment and energy, microelectronics and of course biomedicine will be some of the fields most strongly benefiting from the hybrid approach to materials developments. Bioinspired hybrid materials and systems will include adaptative materials, nanoarchitectures, hierarchically structured systems, three-dimensional composites, biocompatible and environmentally benign ecomaterials. All of them should become major players in advanced technologies. Furthermore, multifunctionality will come to add more complex services to our newest devices. Selective multifunctional hybrid materials with just the right combination of properties (separation, adsorption, catalysis, sensing, bio-sensing, imaging, multitherapy, etc.) which will bring unsuspected surprises.
These are just prospective views, not science fiction. All of these hybrid materials and systems will soon come out of the labs and into our daily lives. Just like the hybrid materials hiding in the screens of the one million TV sets sold annually by Toshiba, screens coated with hybrids made of indigo dyes embedded in a silica/zirconia matrix.16 Interestingly, a 21st century advanced hybrid material that brings us strong echoes of ancient Maya Blue.
References
-
(a) H. Van Olphen, Science, 1966, 154(3749), 645–646 CrossRef CAS
; (b) M. Miller, National Geographic, February 1995, pp. 50–69. See also http://magma.nationalgeographic.com/ngm/0207/resources_geo2.html Search PubMed
; (c) M. J. Yacamán, L. Rendon, J. Arenas and M. C. Serra, Science, 1996, 273(5272), 223–225 CrossRef CAS
; (d) L. A. Polette, N. Ugarte, M. José Yacamán and R. Chianelli, Scientific American Discovering Archaeology, July–August 2000, pp. 46–53 Search PubMed
; (e) G. Chiari, R. Giustetto, C. Reyes-Valerio and G. Richiardi, poster presented at the XXX Congresso Associazione Italiana di Cristallografia, Martina Franca, Oct. 2000; (f) L. Polette, N. Ugarte and R. Chianelli, Presentation at the Workshop on Synchrotron Radiation in Art and Archaeology, SSRL, 18 October 2000; (g) B. Hubbard, W. Kuang, A. Moser, G. A. Facey and C. Detellier, Clays Clay Miner., 2003, 51, 318–326 CrossRef CAS
; (h) D. Reinen, P. Köhl and C. Müller, Z. Anorg Allg Chem., 2003, 630(1), 97–103 CrossRef
; (i) Within the channels of palygorskite, at the openings of its nanotunnels, or in open channels on grain surfaces are some of the proposals found in the literature. All of them involve dye molecules strongly hydrogen-bonded, isolated and protected by the inorganic lattice.
-
Functional Hybrid Materials, eds. P. Gómez-Romero and C. Sánchez, Wiley–VCH, Weinheim, 2004 Search PubMed
.
- Special Issue on “Nanostructured and Functional Hybrid Organic-Inorganic Materials”, Chem. Mater., eds. H. Eckert and M. Ward, 2001, p. 13 Search PubMed.
- Special Issue on “Hybrid Organic-Inorganic Materials”, Mater. Res. Bull., ed. D. A. Loy, 2001, 26(5) Search PubMed.
- E. Ruiz-Hitzky, Adv. Mater., 1993, 5, 334–340 CrossRef CAS
.
-
(a) C. Sanchez and F. Ribot, New J. Chem., 1994, 18, 1007 Search PubMed
; (b) C. Sanchez, G. Soler-Illia, F. Ribot, T. Lalot, C. Mayer and V. Cabuil, Chem. Mater., 2001, 13, 2061 CrossRef
.
- P. Gómez-Romero, Adv. Mater., 2001, 13(3), 163–174 CrossRef CAS
.
-
P. Gómez-Romero and M. Lira-Cantú, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., New York, 2002, http://www.mrw.interscience.wiley.com/kirk/articles/hybdgome.a01/abstract.html Search PubMed
.
-
(a)
P. Gómez-Romero and C. Sánchez, in Functional Hybrid Materials, eds. P. Gómez-Romero and C. Sánchez, Wiley–VCH, Weinheim, 2004, ch. 1 Search PubMed
; (b) C. Sanchez, B. Lebeau, J.-P. Boilot, F. Chaput, in Functional Hybrid Materials, eds. P. Gómez-Romero and C. Sánchez, Wiley–VCH, Weinheim, 2004, ch. 5 and references therein Search PubMed
.
- G. J. A. A. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef
and references therein.
-
S. Mann, in Biomimetic Materials Chemistry, ed. S. Mann, VCH, Weinheim, 1989, pp. 1–40 Search PubMed
.
-
Biomimétisme et Matériaux, coordinator C. Sanchez, OFTA Ed, Paris, 2001, série Arago 25 Search PubMed
.
- G. Schottner, Chem. Mater, 2001, 13, 3422 CrossRef CAS
.
- J. A. Asensio, S. Borrós and P. Gómez-Romero, Electrochem. Commun., 2003, 5(11), 967–972 CrossRef CAS
.
- C. Sanchez, G. J. A. A. Soler-Illia, F. Ribot and D. Grosso, C. R. Chimie, 2003, 6, 1131 Search PubMed
.
- T. Itou and H. Matsuda, Key Eng. Mater., 1998, 67, 150
.
Footnote |
| † Laboratories working within the framework of the Network of Excellence FAME (Functional Advanced Materials and their Engineering). |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2005 |
