Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo
Yoshinori
Sato
*a,
Atsuro
Yokoyama
b,
Ken-ichiro
Shibata
b,
Yuki
Akimoto
a,
Shin-ichi
Ogino
a,
Yoshinobu
Nodasaka
b,
Takao
Kohgo
b,
Kazuchika
Tamura
b,
Tsukasa
Akasaka
b,
Motohiro
Uo
b,
Kenichi
Motomiya
a,
Balachandran
Jeyadevan
a,
Mikio
Ishiguro
c,
Rikizo
Hatakeyama
d,
Fumio
Watari
b and
Kazuyuki
Tohji
a
aGraduate School of Environmental Studies, Tohoku University, Aoba 6-6-20, Aramaki, Aoba-ku, Sendai 980-8579, Japan. E-mail: hige@bucky1.kankyo.tohoku.ac.jp; Fax: +81 22 795 7392; Tel: +81 22 795 7392;
bGraduate School of Dental Medicine, Hokkaido University, Kita-ku, Sapporo, 060-8586, Japan
cInstitute for Materials Research, Tohoku University, Aoba-ku, Sendai, 980-8577, Japan
dGraduate School of Engineering, Tohoku University, Aoba-ku, Sendai, 980-8579, Japan
First published on 20th April 2005
Abstract
Carbon nanotubes (CNTs) are single- or multi-cylindrical graphene structures that possess diameters of a few nanometers, while the length can be up to a few micrometers. These could have unusual toxicological properties, in that they share intermediate morphological characteristics of both fibers and nanoparticles. To date, no detailed study has been carried out to determine the effect of length on CNT cytotoxicity. In this paper, we investigated the activation of the human acute monocytic leukemia cell line THP-1 in vitro and the response in subcutaneous tissue in vivo to CNTs of different lengths. We used 220 nm and 825 nm-long CNT samples for testing, referred to as “220-CNTs” and “825-CNTs”, respectively. 220-CNTs and 825-CNTs induced human monocytes in vitro, although the activity was significantly lower than that of microbial lipopeptide and lipopolysaccharide, and no activity appeared following variation in the length of CNTs. On the other hand, the degree of inflammatory response in subcutaneous tissue in rats around the 220-CNTs was slight in comparison with that around the 825-CNTs. These results indicated that the degree of inflammation around 825-CNTs was stronger than that around 220-CNTs since macrophages could envelop 220-CNTs more readily than 825-CNTs. However, no severe inflammatory response such as necrosis, degeneration or neutrophil infiltration in vivo was observed around both CNTs examined throughout the experimental period.
Introduction
Since a number of graphite-coated plastic valves in vivo have maintained an intact graphite surface with little or no clot present,1 and given that graphite has been found to be biocompatible with cells, artificial heart valves and dental implants2 consisting of carbon fiber-reinforced carbon composites (C/C composites) have been developed. Carbon nanotubes (CNTs) possess outstanding properties such as morphology, nano-spaces and a large specific surface area. Consequently, recent investigations have utilized CNTs in the development of a probe tip for scanning probe microscopy,3 a vector used for targeting therapies4–9 for drug delivery, vaccine delivery, gene delivery10,11 and selective molecular adsorption.12,13 On the other hand, CNTs were reported to be harmful to living organisms14–16 and consequently extensive nanotoxicological investigations are necessary to determine their biocompatibility and cytotoxicity before carbon nanotubes can be safely used as biomaterials.17 Generally, metal dissolution,18,19 surface functional groups20 and size effects21–25 have been commonly cited as factors indicating cytotoxicity. In particular, biocompatible PTFE21 and Ti22,25 particles were found to be increasingly cytotoxic with decreasing particle size. In an effort to be successfully applied as drug delivery systems, a few micrometer-length CNTs should be shortened to less than one micrometer to facilitate entry into cells. CNTs are long thin structures that possess diameters of a few nanometers, while the length can be up to a few micrometers. These could have unusual toxicological properties, in that they share morphological characteristics of both fibers and nanoparticles. To date, although some researchers have reported the effect of CNTs on cytotoxicity in cells,26–29 no detailed study has been carried out to determine the effect of length on CNT cytotoxicity. Here, we investigated the activation of the human acute monocytic leukemia cell line THP-1 in vitro and the response in subcutaneous tissue in vivo to CNTs of different lengths in an effort to determine the influence of length on the cytotoxicity of CNTs in these cell types.Materials and methods
Purification, cutting and separation of MWCNTs
We used the MWCNTs synthesized by the chemical vapor deposition (CVD) method from NanoLab, Inc. in this study. The purity was about 80 wt% with impurities such as amorphous carbon, Fe, Mo, Cr and Al. The diameter was in the range between 20 and 40 nm, and the length was in the range between 500 nm and 5.0 µm. One hundred milligrams of the soot was burned in air at 773 K for 90 min and the burnt soot was then introduced into a flask containing 6 M HCl to dissolve the Fe, Mo and Cr. Following this, the acid solution was filtered using a membrane filter and the filtered cake was transferred into a flask with 1.0 L of 3 M NaOH and refluxed at 373 K for 15 h to dissolve aluminium oxides. The resultant suspension was filtered using a membrane filter, and the filtered cake was washed with hot water. Finally, samples were dried in vacuo at 373 K for 24 h. The way of cutting the purified MWCNTs was as follows: 100 mg of the purified MWCNTs was suspended in a 100 mL flask containing a 3 ∶ 1 (v/v%) mixture of concentrated 95% H2SO4–60% HNO3 and exposed to ultrasonic irradiation (200 W, 39 kHz) at 313 K for 5 h. The use of sonication in the presence of an oxidizing acid (H2SO4, HNO3 or H2O2) is generally recognized as promoting attack at the point of damage which cuts the tube.30 It has been said that the open tube ends are unable to close, continued exposure to the oxidizing acid, then etches away the exposed ends at this moderate temperature. We selected the 3 ∶ 1 concentrated 95% H2SO4–60% HNO3 for the oxidizing acid because it is known to intercalate and exfoliate graphite.31 The acid-treated MWCNTs were filtered using a membrane filter, and the filtered cake was washed with water. Finally, samples were dried in vacuo at 373 K for 24 h. The procedure employed to separate the MWCNTs based on size was as follows: 20 mg of the cut MWCNTs was suspended in a flask with 400 mL of ethanol and subjected to ultrasonic irradiation for 1 h. The MWCNT aggregate was filtered by sieving through using a 37 µm aperture screen in an effort to separate the few microns long MWCNTs. The cut MWCNTs in the supernatant were then size-separated using a multi-step microfiltration process employing polycarbonate membrane filters with respective cylindrical pore diameters of 2.0, 1.2, 0.8 and 0.4 µm. Each filtered cake sample was dried in vacuo at 373 K for 24 h. We used the filtered cake samples on both 2.0 and 0.4 µm pore size membrane filters as testing samples. The sample was characterized using a scanning electron microscope (SEM; S-4100, Hitachi, Japan), transmission electron microscope (TEM; HF-2000, Hitachi, Japan), inductively coupled plasma optical emission spectrometry (ICP-OES; Thermo elemental Co. Ltd., USA), Fourier transform-infrared spectroscopy (FT-IR; Avatar 360, Thermo elemental Co. Ltd., USA) and ultraviolet-visible absorption spectroscopy (UV-VIS; U-3300, Hitachi, Japan).Chemicals
Mycoplasmal diacylated lipopeptide, referred to as FSL-1, S-(2,3-bispalmitoyloxypropyl) Cys–Gly–Asn–Asn–Asp–Glu–Ser–Asn–Ile–Ser–Phe–Lys–Glu–Lys, was synthesized according to a previously described method.32 All other chemicals were obtained from commercial sources and were of analytical or reagent grade.Activation of THP-1 cells
Monocytes are differentiated into macrophages that play important roles in innate immunity by sensing and phagocytosing pathogens. We used the human acute monocytic leukemia cell line THP-1. The reason we used the THP-1 cell line was that it provides a macrophage system that not only eliminates foreign bodies but also produces cytokines that work as signaling mediators serving as initial triggers of antibody production. Additionally, THP-1 cells are a well-characterized representative of human monocytic cell lines and are frequently used for this type of experiment. The human acute monocytic leukemia cell line THP-133 was obtained from the Health Science Research Resources Bank (Osaka, Japan). Cells were grown at 310 K in a humidified 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% (v/v) FBS, penicillin G (100 units ml−1) and streptomycin (100 µg ml−1). Cells were incubated at 310 K for 24 h in the absence or presence of PMA. Following this, a 0.2 mL volume of THP-1 cell suspension (5 × 105 cells) was placed in each well of a 96-well tissue culture plate and incubated at 310 K for 16 h in culture medium containing FSL-1 supplemented with 0.1% (v/v) human serum. Also, THP-1 cells were incubated at 310 K for 16 h with the CNTs at the different doses in the same way. Culture supernatants were then collected by centrifugation at 400 × g for 10 min. The amount of TNF-α in the supernatants was determined using HU TNF-α Flexia (Biosource International, Inc., Camarillo, CA).Inflammatory response to MWCNTs
Animal experiments: Six male 6-week-old Wistar strain rats were used in this study. Incisions were made bilaterally in the thoracic region under general anesthesia. Two pockets were made in the subcutaneous tissue. 0.1 mg of clusters of the CNTs samples were implanted in the subcutaneous tissue in the thoracic region bilaterally in each rat. Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, Hokkaido University Graduate School of Dental Medicine. No rats were lost during the course of this study.Histological procedure and observation by optical microscopy and transmission electron microscopy
One and 4 weeks following surgery, segments of subcutaneous tissue including CNTs were excised and fixed. The fixed specimens were divided into two parts. One part was embedded in paraffin. Hematoxylin and eosin-stained specimens were observed by optical microscopy (AX80, Olympus, Japan). The other part was observed by transmission electron microscopy. The field observation by TEM was confirmed to be exactly consistent with that observed by optical microscopy. TEM specimens were postfixed with 1% OsO4 and routinely embedded in epoxy resin following dehydration. Ultrathin sections (ca. 80 nm) were cut using a diamond knife and stained with uranyl acetate and lead citrate. Stained sections were placed on a supporting carbon mesh grid and observed using a TEM operating at a voltage of 75 kV (H-800, Hitachi, Japan).Results and discussion
Although the elemental composition of as-grown CNTs soot consisted of C: 88.50 wt%, Al: 5.73 wt%, Fe: 4.43 wt%, Mo: 1.27 wt% and Cr: 0.07 wt% from ICP-OES, the purified CNTs consisted of C: 98.17 wt%, Al: 1.41 wt%, Fe: 0.26 wt%, Mo: 0.01 wt% and Cr: 0.15 wt% by ICP-OES and found to be highly pure. Fig. 1a and b show the SEM images (left) and corresponding size-distribution (right) of the separated MWCNTs using polycarborbonate membrane filters with cylindrical pore diameters of 0.4 and 2.0 µm, respectively. The average length was 220 nm and 825 nm, referred to as “220-CNTs” and “825-CNTs”, respectively. Fig. 1c shows the TEM photograph of 220-CNTs. Nanotube outer-layers were found to have been damaged by the cutting treatment in the mixed concentrated H2SO4 and HNO3. The damage caused by the cutting procedure affects equally short and long CNTs. Fig. 1d shows the CNTs mass concentration of the supernatant dispersion versus the sediment time in water or PBS. Both 220-CNTs and 825-CNTs are well dispersed in the water, and their precipitation is not observed. From the IR spectra of the 220- and 825-CNTs (Fig. 2), the band present at 1584 cm−1 in the two spectra were assigned to the stretching vibration of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group. The bands around 1398 cm−1 and 1240 cm−1 for both CNTs corresponded to a deformation vibration (out-of-plane) of the O–H group and a stretching vibration of the C–O carboxyl group, respectively. These bands appeared at higher wavenumbers, indicating the presence of a dimer carboxyl,34,35 or more specifically, the bands at 1398 cm−1 and 1550 cm−1 with a shoulder were assigned to the stretching vibration of the carboxylate anion. The band at 1720 cm−1 was assigned to the stretching vibration of the C
C group. The bands around 1398 cm−1 and 1240 cm−1 for both CNTs corresponded to a deformation vibration (out-of-plane) of the O–H group and a stretching vibration of the C–O carboxyl group, respectively. These bands appeared at higher wavenumbers, indicating the presence of a dimer carboxyl,34,35 or more specifically, the bands at 1398 cm−1 and 1550 cm−1 with a shoulder were assigned to the stretching vibration of the carboxylate anion. The band at 1720 cm−1 was assigned to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carboxyl group. We also confirmed that the band between 3120 and 3450 cm−1 corresponded to the stretching vibration of the O–H carboxyl group. The surface of the cut MWCNTs was modified by the carboxyl groups, facilitating dispersion of the material into water. Sonication in the presence of the oxidizing acid, HNO3, results in attack at the point of damage thereby cutting the tube.30 In this system, the following reaction occurs: C + 4HNO3
→ CO2
+ 4NO2
+ 2H2O. It is thought that carboxyl groups are formed when HNO3 oxidizes the carbon that constitutes CNTs. Thus, we obtained two different lengths of CNTs modified by carboxyl groups. However, we cannot estimate the amount of carboxyl groups that were formed on the surfaces of both CNTs. On the other hand, CNTs were unstable in PBS. In aqueous systems, electrolyte ions are known to coagulate colloidal sols. It is well-known that the critical coagulation concentration (ccc), the minimum concentration of ions necessary to cause rapid coagulation of colloids, follows the Schulze–Hardy rule36,37 that results from interaction between van der Waals attraction and electric double-layer repulsion. The carboxyl groups formed on the CNTs possess the property of coagulation.38 Additionally, the 825-CNTs quickly precipitate further than the 220-CNTs. This phenomenon is considered to affect the weight of CNTs as well as the amount of carboxyl groups formed on the surfaces of CNTs.
O carboxyl group. We also confirmed that the band between 3120 and 3450 cm−1 corresponded to the stretching vibration of the O–H carboxyl group. The surface of the cut MWCNTs was modified by the carboxyl groups, facilitating dispersion of the material into water. Sonication in the presence of the oxidizing acid, HNO3, results in attack at the point of damage thereby cutting the tube.30 In this system, the following reaction occurs: C + 4HNO3
→ CO2
+ 4NO2
+ 2H2O. It is thought that carboxyl groups are formed when HNO3 oxidizes the carbon that constitutes CNTs. Thus, we obtained two different lengths of CNTs modified by carboxyl groups. However, we cannot estimate the amount of carboxyl groups that were formed on the surfaces of both CNTs. On the other hand, CNTs were unstable in PBS. In aqueous systems, electrolyte ions are known to coagulate colloidal sols. It is well-known that the critical coagulation concentration (ccc), the minimum concentration of ions necessary to cause rapid coagulation of colloids, follows the Schulze–Hardy rule36,37 that results from interaction between van der Waals attraction and electric double-layer repulsion. The carboxyl groups formed on the CNTs possess the property of coagulation.38 Additionally, the 825-CNTs quickly precipitate further than the 220-CNTs. This phenomenon is considered to affect the weight of CNTs as well as the amount of carboxyl groups formed on the surfaces of CNTs.
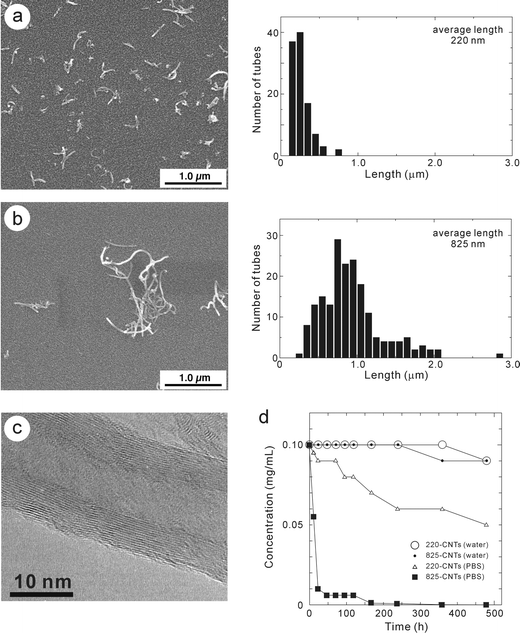 | ||
| Fig. 1 SEM (left) and size-distribution (right) of 220-CNTs (a) and 825-CNTs (b). (c) TEM photographs of 220-CNTs. (d) CNT concentration versus time plots for both 220-CNTs and 825-CNTs dispersed in water and PBS; 220-CNTs in water (○), 825-CNTs in water (•), 220-CNTs in PBS (□) and 825-CNTs in PBS (■), respectively. | ||
 | ||
| Fig. 2 IR spectra of 220-CNTs (bottom) and 825-CNTs (top). | ||
Firstly, an in vitro experiment was carried out in an effort to determine whether CNTs were capable of activating THP-1 cells, a human-derived monocytic cell line. For comparative purposes, the diacylated lipopeptide FSL-1 was used as a positive control, since FSL-1 is known to induce macrophages to produce TNF-α.32,39,40 Both 220-CNTs and 825-CNTs induced THP-1 cells to produce TNF-α in a dose-dependent manner (Fig. 3). On the other hand, the level of induction by both CNTs was much lower than that of the microbial lipopeptide, FSL-1. Furthermore, a comparison of CNT length (at the same mass concentration) with activity showed very little difference between the two sample lengths. Thus, this result demonstrated that both 220-CNTs and 825-CNTs possess induction activity toward macrophages, although their activities were much lower compared to that of a microbial antigen. It has been said that factors related to cytotoxicity are dependent on specific surface area21,41 and functional group.21,42 Actually, the specific surface area of the 220-CNTs and 825-CNTs used was 300 (±20) m2g−1 and 320 (±15) m2g−1, respectively. However, coagulation in PBS differed between the two CNTs. The stability of the CNTs was determined by measuring the dispersed concentration versus the sediment time. As shown in Fig. 1d, both CNTs were stable in water (pH 6.8) at room temperature even after 500 h. In PBS, 825-CNTs coagulated immediately (concentration of 0.1 mg mL−1 decreased to 0.01 mg mL−1 after 24 h), while 220-CNTs coagulated more gradually (concentration of 0.1 mg mL−1 decreased to 0.05 mg mL−1 after 480 h). This indicated a size effect of the CNTs. In the case of the in vitro test, we used CNT concentrations of 5 ng mL−1, 50 ng mL−1 and 500 ng mL−1 to limit any possible coagulation of the dilute CNTs solutions. Thus, there was no THP-1 cell response due to a difference in length of the 220-CNTs and 825-CNTs examined.
 | ||
| Fig. 3 Induction of THP-1 cells by 220-CNTs and 825-CNTs. The diacylated lipopeptide FSL-1 was used as a positive control. | ||
Macrophages play an important role in innate immunity by sensing the presence of invading pathogens as non-self and subsequently eradicating them through phagocytosis. This process involves ingestion and digestion by cells of solid substances such as other cells, bacteria, bits of necrosed tissue and foreign particles more than 100 nm in diameter. With the in vivo test, the cells and tissues can be observed using an optical microscope and TEM, thus permitting the investigation of the influence of length on inflammatory responses due to the presence of 220-CNTs or 825-CNTs. One week following surgery, clusters of both 220-CNTs and 825-CNTs were surrounded by granulation tissue with macrophages, foreign body giant cells and fibroblasts. Phagocytes enveloped many of the 220-CNTs (Fig. 4a). Some of the 825-CNTs were observed in the intercellular space, while others were observed within phagocytes (Fig. 4b). The inflammatory response around 220-CNTs was lower than that observed for 825-CNTs. Four weeks following surgery, the inflammatory response around 220-CNTs almost disappeared, while the response to 220-CNTs consisted mostly of macrophages and fibroblasts (Fig. 4c). There was a negligible difference in the degree of inflammation around 825-CNTs 4 weeks following surgery compared with the response observed following 1 week. The response appeared like a foreign body granuloma (Fig. 4d).
 | ||
| Fig. 4 Histology of 220-CNTs and 825-CNTs implanted in the subcutaneous tissue. 1 week: Clusters of both of 220-CNTs and 825-CNTs were surrounded by granulation tissue. (a) Many of the 220-CNTs were enveloped by macrophages (arrows). (b) Some of the 825-CNTs were observed in the intercellular space (arrows), while others were observed in macrophages (white arrows). 4 weeks: (c) Most of the 220-CNTs were observed in macrophages (arrows) and fibroblasts (white arrows). (d) There was a negligible difference in the degree of inflammation around 825-CNTs 4 weeks following surgery, compared to 1 week. All scale bars are 10 µm. | ||
Furthermore, TEM observation helped to delineate the size effect of CNTs in macrophages. Fig. 5a and b show TEM photographs 4 weeks following surgery. Most of the 220-CNTs were observed in phagocytes and many of these were recognizable in lysosomes (Fig. 5c). On the other hand, most of the 825-CNTs in macrophages aggregated in the cytoplasm and were not covered with membrane (Fig. 5c and d). Compared with 825-CNTs, the shorter 220-CNTs spread out more readily from nanotube-coagulations and collected in lysosomes since it is thought that Brownian motion acts more effectively on the short 220-CNTs. Additionally, nanotube morphology can affect the state of coagulation. 825-CNTs are not straight but bent (see Fig. 1b), and they could not spread out from the coagulation due to intertwining with each 825-CNT (see Fig. 5c and d). On the other hand, short 220-CNTs with straight shape are unable to intertwine with each other. Thus, tissue responses to CNTs are influenced by the CNT length. In our previous study,43 shortening and a change in translucence were recognized in some hat-stacked carbon nanofibers (H-CNFs). However, the 220-CNTs and 825-CNTs used were not associated with these phenomena. This is the reason why the structure of CNTs, composed of cylindrical-graphene layers, differs from the structure of H-CNFs stacked with hat-graphene toward the needle axis. It is thought that cylindrical-graphene layers are not destroyed by mechanical force in cells or hydrogen peroxide such as superoxide in lysosomes that caused the decomposition of H-CNFs since the carbon-sp2-hybrid bond is mechanically strong and chemically stable.
 | ||
| Fig. 5 TEM photographs of 220-CNTs (a) and 825-CNTs (b) implanted in the subcutaneous tissue 4 weeks following surgery. Fig. 5c is a high magnification TEM photograph at the black square part of Fig. 5a. Fig. 5d and e are high magnification TEM photographs at the black dashed square and white square part of Fig. 5b. Most of the 220-CNTs (white arrowheads in Fig. 5a and c) were observed in lysosomes (white arrows in Fig. 5a and c). Aggregation of 825-CNTs (arrows in Fig. 5b and e) was observed in the cytoplasm and was not covered by membrane. Typical structure of CNTs (white arrowheads in Fig. 5b and d) was observed in the cytoplasm. All scale bars are 300 nm. | ||
In vivo histological observations using optical microscopy and TEM helped to determine that the degree of inflammatory response around 220-CNTs was slight in comparison to that around 825-CNTs. Furthermore, no severe inflammatory response such as necrosis, degeneration or neutrophil infiltration was observed around either CNTs throughout the experimental period. These results indicated that macrophages could envelop 220-CNTs more readily than 825-CNTs. The degree of inflammation around 825-CNTs was stronger than that around 220-CNTs since some of the 825-CNTs were not enveloped by macrophages even after 4 weeks. Monteiro-Riviere et al. reported on the cytotoxicity of non-treated MWCNTs in human epidermal keratinocytes.29 They described that up to 3.2 µm length MWCNTs were inserted into cells. According to their paper, the 825-CNTs used could also be inserted into macrophages. Actually, we observed a few 825-CNTs in lysosomes using TEM 4 weeks following surgery. However, as CNTs were implanted in a powdered state in the subcutaneous tissue of rats, CNTs were already present in a coagulated form at initiation. Additionally, CNTs modified with carboxyl groups possess the property of coagulation,38 and the coagulation of 825-CNTs is far greater than that observed for 220-CNTs. Henceforth, it is thought that coagulated 825-CNTs with the larger size result in granulomatous inflammation since they were not enveloped by macrophages as easily as the 220-CNTs. In this experiment, we implanted 0.1 mg of cluster of samples in the subcutaneous tissue of rats. In contrast, in the case of implanting a large amount of the powdered samples, they widely coagulated together and became covered with fibrous connective tissue. Actually, the dose dependence is controversial. For example, while Warheit et al. have reported that pulmonary exposures to SWCNTs in rats produced a non-dose-dependent series of multifocal granulomas,15 Lam et al. have reported that all SWCNTs products in mice induced dose-dependent epithelioid granulomas and interstitial inflammation.16 Activation of immunocompetent cells by foreign bodies is thought to be largely dependent on the type and size of immunocompetent cells used, since contact between the CNTs and the cells are required for activation. The cytotoxicity response to CNTs may differ depending on the cell line and tissue types used.26,27,29,44,45 It is imperative that the cytotoxicity and metabolic pathway of CNTs be delineated for various cells and tissues in an effort to facilitate use of these materials in the development of important biomedical devices.
Acknowledgements
This work was supported by a Grant-in-Aid for Basic Research #(S) 14103016, and #(S) 13852016 from the Ministry of Education, Science, Culture and Sport of Japan and #H14-nano-021 from the Ministry of Health, Labor and Welfare.References
- V. L. Gott, J. D. Whiffen and R. C. Dutton, Science, 1963, 142, 1297 CrossRef CAS.
- J. C. Bokros, Carbon, 1977, 15, 353 CrossRef.
- A. T. Woolley, C. Guillemette, C. L. Cheung, D. E. Housman and C. M. Lieber, Nat. Biotechnol., 2000, 18, 760 CrossRef CAS.
- A. Bianco and M. Prato, Adv. Mater., 2003, 15, 1765 CrossRef.
- D. Pantarotto, J. -P. Briand, M. Prato and A. Bianco, Chem. Comm., 2004, 16 RSC.
- D. Pantarotto, C. D. Partidos, J. Hoebeke, F. Brown, E. Kramer, J. P. Briand, S. Muller, M. Prato and A. Bianco, Chem. Biol., 2003, 10, 961 CrossRef CAS.
- Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, Nano Lett., 2004, 4, 2479 CrossRef.
- T. Murakami, K. Ajima, J. Miyawaki, M. Yudasaka, S. Iijima and K. Shiba, Mol. Pharm., 2004, 1, 399 CrossRef CAS.
- A. Bianco, K. Kostarelos, C. D. Partidos and M. Prato, Chem. Comm., 2005, 571 RSC.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J. P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem. Int. Ed., 2004, 43, 5242 CrossRef CAS.
- Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, Nano Lett., 2004, 4, 2473 CrossRef CAS.
- B. Fugetsu, S. Satoh, T. Shiba, T. Mizutani, Y. -B. Lin, N. Terui, Y. Nodasaka, K. Sasa, K. Shimizu, T. Akasaka, M. Shindoh, K. -I. Shibata, A. Yokoyama, M. Mori, K. Tanaka, Y. Sato, K. Tohji, S. Tanaka, N. Nishi and F. Watari, Environ. Sci. Technol., 2004, 38, 6890 CrossRef CAS.
- F. Balavoine, P. Schultz, C. Richard, V. Mallouh, T. W. Ebbesen and C. Mioskowski, Angew. Chem. Int. Ed., 1999, 38, 1912 CrossRef CAS.
- P. H. M. Hoet, A. Nemmar and B. Nemery, Nat. Biotechnol., 2004, 22, 19 CrossRef CAS.
- D. B. Warheit, B. R. Laurence, K. L. Reed, D. H. Roach, G. A. M. Reynolds and T. R. Webb, Toxicol. Sci., 2004, 77, 117 CrossRef CAS.
- C. W. Lam, J. T. James, R. McCluskey and R. L. Hunter, Toxicol. Sci., 2004, 77, 126 CrossRef CAS.
- V. L. Colvin, Nat. Biotechnol., 2004, 21, 1166.
- A. McNamara and D. F. Williams, Biomaterials, 1981, 2, 33 CrossRef CAS.
- M. Uo, F. Watari, A. Yokoyama, H. Matsuno and T. Kawasaki, Biomaterials, 2001, 22, 677 CrossRef CAS.
- B. Fubini, Environ. Health Perspect., 1997, 105, 1013 CrossRef.
- C. J. Johnston, J. N. Finkelstein, P. Mercer, N. Corson, R. Gelein and G. Oberdörster, Toxicol. Appl. Pharmacol., 2000, 168, 208 CrossRef CAS.
- K. Tamura, N. Takashi, R. Kumazawa, F. Watari and Y. Totsuka, Mater. Trans., 2002, 43, 3052 Search PubMed.
- J. E. Sanders, C. E. Stiles and C. L. Hayes, J. Biomed. Mater. Res., 2000, 52, 231 CrossRef CAS.
- A. M. Rodrigo, M. E. Martinez, M. L. Escudero, J. Ruiz, P. Martinez, L. Saldana, L. Gomez-Garcia, L. Fernandez, J. Cordero and L. Munuera, Biomaterials, 2001, 22, 755 CrossRef CAS.
- R. Kumazawa, F. Watari, N. Takashi, Y. Tanimura, M. Uo and Y. Totsuka, Biomaterials, 2002, 23, 3757 CrossRef CAS.
- A. Huczko and H. Lange, Fullerene Sci. Technol., 2001, 9, 247 CrossRef CAS.
- A. Huczko, H. Lange, E. Calko, H. Grubek-Jaworska and P. Droszcz, Fullerene Sci. Technol., 2001, 9, 251 CrossRef CAS.
- M. A. Correa-Duarte, N. Wagner, J. Rojas-Chapana, C. Morsczeck, M. Thie and M. Giersig, Nano Lett., 2004, 4, 2233 CrossRef CAS.
- N. A. Monteiro-Riviere, R. J. Nemanich, A. O. Inman, Y. Y. Wang and J. E. Riviere, Toxicol. Lett., 2004, 155, 377.
- J. Liu, A. G. Rinzler, H. Dai, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. Rodriguez-Macias, Y. S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley, Science, 1998, 280, 1253 CrossRef CAS.
- S. F. McKay, J. Appl. Phys., 1964, 35, 1992 CAS.
- K. -I. Shibata, A. Hasebe, T. Into, M. Yamada and T. Watanabe, J. Immunol., 2000, 165, 6538 CAS.
- S. Tsuchiya, M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno and K. Tada, Int. J. Can., 1980, 26, 171 Search PubMed.
- E. G. Palacios and A. J. Monhemius, Hydrometalurgy, 2001, 62, 135 Search PubMed.
- C. Velasco-Santos, A. L. Martinez-Hernandez, M. Lozada-Cassou, A. Alvarez and V. Castano, Nanotechnology, 2002, 13, 495 CrossRef CAS.
- H. Schulze, J. Prakt. Chem., 1882, 25, 431 Search PubMed.
- W. B. Hardy, Proc. R. Soc. London, 1900, 66, 110.
- M. Sano, J. Okamura and S. Shinkai, Langmuir, 2001, 17, 7172 CrossRef CAS.
- T. Okusawa, M. Fujita, J. -I. Nakamura, T. Into, M. Yasuda, A. Yoshimura, Y. Hara, A. Hasebe, D. T. Golenbock, M. Morita, Y. Kuroki, T. Ogawa and K. -I. Shibata, Infect. Immun., 2004, 72, 1657 CrossRef CAS.
- M. Fujita, T. Into, M. Yasuda, T. Okusawa, S. Hamahira, Y. Kuroki, A. Eto, T. Nisizawa, M. Morita and K. -I. Shibata, J. Immunol., 2003, 171, 3675 CAS.
- D. B. Warheit, Mater. Today, 2004, 7, 32 Search PubMed.
- A. Hoshino, K. Fujioka, T. Oku, M. Suga, Y. F. Sasaki, T. Ohta, M. Yasuhara, K. Suzuki and K. Yamamoto, Nano Lett., 2004, 4, 2163 CrossRef CAS.
- A. Yokoyama, Y. Sato, Y. Nodasaka, S. Yamamoto, T. Kawasaki, M. Shindoh, T. Kohgo, T. Akasaka, M. Uo, F. Watari and K. Tohji, Nano Lett., 2005, 5, 157 CrossRef CAS.
- D. Cui, F. Tian, C. S. Ozkan, M. Wang and H. Gao, Toxicol. Lett., 2005, 155, 73 CrossRef CAS.
- A. Bianco, J. Hoebeke, S. Godefroy, O. Chaloin, D. Pantarotto, J. P. Briand, S. Muller, M. Prato and C. D. Partidos, J. Am. Chem. Soc., 2005, 127, 58 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
