Controllable polymerization of N-carboxy anhydrides in a microreaction system†
Takeshi
Honda
a,
Masaya
Miyazaki
*ab,
Hiroyuki
Nakamura
a and
Hideaki
Maeda
*ab
aNanotechnology Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 807-1, Shuku, Tosu, Saga 841-0052, Japan. E-mail: m.miyazaki@aist.go.jp; maeda-h@aist.go.jp; Fax: +81-942-81-3657; Tel: +81-942-81-3675
bDepartment of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, Kasuga, Fukuoka 816-8580, Japan
First published on 1st July 2005
Abstract
We have developed a microfluidic system for polymerization of amino acid N-carboxyanhydride and compared the properties of the products with those obtained by batchwise system under various experimental conditions. It was found that the microreactor produced polymers with narrower molecular weight distribution compared with polymers obtained by the batchwise system. Also, the molecular mass of the polymer produced using the microreactor was simply governed by the flow (pumping) rate. These results indicated that the microreactor could be a model for synthesis of amino acid polymer with highly controllable average molecular weight and molecular weight distribution.
Introduction
For the past decade, intense research in biomaterial science has evolved to meet scientific and industrial needs. In particular, synthetic biopolymers including polypeptides and related artificial poly(amino acid)s are very important in fields such as biomedical and tissue engineering.1 Such functional polymers have been significantly developed owing to their specific properties such as various biological activities, biodegradability, and biocompatibility.2 For instance, lysine-based polymers have attracted wide attention as functional biopolymers for cell adhesion,3 DNA and drug delivery,4 chiral sources in organic synthesis, and as morphologic controllers of inorganic materials.5 Although several useful polymers were developed, it usually takes effort and is time consuming to obtain useful polymers which can be commercialized. One of the underlying problems deals with design and screening of polymer molecules. Although there have been demands for a high-throughput and combinatorial synthesis system to rapidly and effectively obtain useful polymers, it is generally difficult to control polymer properties such as average molecular weight and molecular weight distribution, thus, a method which would enable control of polymerization reactions is desirable.Microreaction systems involve microreaction apparatus that enables high controllability of chemical reactions.6 Such controllability results from efficient heat transfer, mass transport, and/or larger surface/interface area.7 Recent studies have shown the potential benefits of applying the various chemical reactions to microfluidic reactors. Several polymerization reactions have been performed using microreaction systems.8,9 Reactions using micromixing devices give better results than those of batchwise reactions. This is because the micromixer enables rapid mixing and therefore yields excellent controllability of rapid reactions.
Our group has been interested in chemical reactions and analysis methods utilizing hydrodynamic behaviors generated characteristically in microfluids, including laminar flow, secondary flow, and shearing force.10–12 We have shown that a polymer chain can be expanded and oriented in a microfluidic system using DNA as a model.12 Such behaviour of polymers demonstrates the utility of the microreactor as a novel reaction apparatus for polymers. For example, we have demonstrated that DNA expands by shearing force in a microfluidic hybridization assay using a microchannel reaction system. By using a microfluidic-based reaction system, a short PNA probe which could not bind to DNA in a batchwise reaction could efficiently do so, thus improving the analysis of DNA by overcoming the limitation of batchwise reaction. Application of this technology is expanding further.
Taking into account the properties of microfluidic systems, we have decided to apply microreaction technology in biopolymer synthesis. In the present study, we performed polymerization reactions of amino acid N-carboxyanhydride (NCA) as a model.
Experimental
Materials
N ε-Benzyloxycarbonyl-L-lysine (Z-Lys), γ-benzyl-L-glutamate (Bzl-Glu), alanine (Ala), leucine (Leu), 30% HBr solution in glacial acetic acid, m-cresol, thioanisole, trifluoroacetic acid (TFA), and trimethylsilyl bromide (TMSBr) were purchased from Watanabe Chemical Industries Ltd. (Hiroshima, Japan). Trichloromethylchloroformate (TCF) was obtained from Merck-Schuehardt (Hohenbrunn, Germany). Sigma-Aldrich Co. (St. Louis, MO, USA) supplied dichlorodimethylsiloxane, tetraethoxysilane, and anhydrous organic solvents including triethylamine (TEA), tetrahydrofuran, diethyl ether, ethyl acetate, n-hexane, ethanol and N,N-dimethylformamide (DMF). Powder charcoal used as catalyst was from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Poly(dimethylsiloxane) (PDMS) was prepared from Sylgard 184 silicone elastomer and curing agent purchased from Dow Corning Co. (Midland, MI, USA). Poly(tetrafluoroethylene) (PTFE) microtube (250 µm inner diameter) and PTFE T-shaped connector were obtained from Flon Chemical Inc. (Osaka, Japan). Cellulose acetate dialysis tube was purchased from Spectrum (Houston, TX, USA). Poly(methyl methacrylate) (PMMA) plates (3 × 7 cm) were obtained from PMT Inc. (Fukuoka, Japan).Fabrication of microreactor
The microreactor consisted of a PDMS micromixer and PTFE microtubes (Fig. 1). The design of the multi-layered fluid channel in the micromixer was previously described.13 The PDMS micromixer was attached to a PTFE microtube (length = 500 cm) and served as a polymer-propagating region. The volume of fluid channel of the micromixer was 4.4 µl, and total inner product volume of this microreactor corresponded to 250 µl.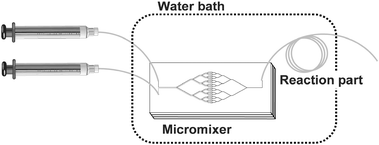 | ||
| Fig. 1 Microreaction system used in this study. The system consists of a PDMS micromixer and PTFE microtubes. Two syringes containing reagents were coupled on PDMS micromixer by PTFE microtubes (ϕ = 250 µm, 10 cm). The PDMS micromixer was attached to a PTFE microtube (ϕ = 250 µm, 500 cm) and served as a reaction region. The mixer and reaction region were kept at 30 °C in a water bath. | ||
Synthesis of amino acid N-carboxyanhydride (amino acid NCA)
Amino acid was converted into NCA as described by Katakai and Iizuka.14 The presence of impurities, in particular HCl, considerably decreases the molecular weight and strongly affects the polymerization kinetics. Therefore, to remove halogen-containing impurities, the NCAs were recrystallized three or more times from diethyl ether/n-hexane for Ala-NCA, Leu-NCA, and Bzl-Glu-NCA, and from ethyl acetate/n-hexane for Z-Lys-NCA. Nε-benzyloxycarbonyl-L-lysine was converted into NCA for less than 1 h by TCF and charcoal as a catalyst. The product was recrystallized to give a 92% yield of colorless NCA crystals. Yields of Bzl-Glu-NCA, Ala-NCA, and Leu-NCA were 88%, 53%, and 64%, respectively.Polymerization of amino acid NCAs
Polymerization of amino acid NCAs was performed and compared both in a batchwise system and microreactor. In the batch system, an equal amount of amino acid NCA dissolved in DMF (50 µl) was mixed with TEA dissolved in DMF by vigorous stirring at 1,300 rpm under dry argon. In the microreactor set up, NCA and TEA solutions were each charged into a 1 ml Hamilton Gastight Syringe (Hamilton Co., Reno, NV, USA). The solutions were supplied to the microreactor using a KDS 230 syringe pump with a parallel syringe holder (KD Scientific Inc., New Hope, PA, USA). The two syringes were set at identical pumping rates so that the final mixed solution would have a constant reagent concentration. The flow rate affects the reaction time, that is, the residence time in which the reaction mixture flowed through the microreactor. During the reaction, both NCA and TEA solutions stored in syringes were cooled at 0 °C. The reaction mixture was kept at 30 °C in a water bath, and a 100 µl product was collected. The microreactor setup operated under a dry argon atmosphere. The obtained product was immediately quenched with aqueous 0.1 M hydrochloric acid and the resulting suspension was evaporated, dried, and the final product of a white pellet was obtained.Removal of side chain-protection groups of Lys and Glu
To remove Nε-benzyloxycarbonyl group from polymer including Z-Lys, 30% HBr in AcOH solution was added to the white pellet, and stirred vigorously for 6 h at room temperature. The reaction mixture was evaporated, and then dissolved in distilled water. The poly(L-lysine) hydrobromide solution was neutralized by 0.1 M NaOH aqueous solution. The γ-benzyl group of poly(Bzl-Glu) was cleaved by TFA solution containing 12.5% (v/v) TMSBr, 11% (v/v) thioanisole, and 1% (v/v) m-cresol. The reaction was carried out for 1 h at 4 °C. Then, the reaction mixture was added with a ten fold volume of cold diethyl ether, and the resulting pellet was washed with cold diethyl ether three times. The pellet was dissolved and neutralized with 0.1 M NaOH aqueous solution. The solution was analyzed by gel filtration chromatography. A portion of the obtained aqueous solution containing each polymer was dialyzed with a dialysis tube (Molecular Weight Cut Off = 500 Da) against water to obtain a polypeptide fraction with a molecular weight of more than 500 Da.15 The dialyzed solution was evaporated and the dry weight of the resulting white pellet was measured. The yield of polymer converted from NCA was evaluated from the dry weight of the product and analysis of gel filtration chromatography.Gel filtration chromatography (GFC) analysis
Gel filtration chromatography analysis was performed by Alliance 2965 system (Waters Corporation, Milford, MA, USA) with UV detector under the following conditions: YMC-Pack Diol-120 and -300 columns (8.0 × 300 mm) and 50 mM phosphate buffer (pH 6.0) eluent at a flow rate of 1 ml min−1 at 30 °C. The calibration curves were obtained using poly(L-Lys) purchased from Sigma as standard. Weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity (Mw/Mn) for each polymer product were determined from GFC data.Results
Microreactor fabrication
Generally, mixing is extremely important in chemical reactions. When using microreactors for chemical reactions, micromixers were often incorporated in the microreactor for effective mixing. In this study, a multi-layered micromixer was employed for NCA-polymerizations. Mixing of NCA and base principally depends on diffusion through the interface of the two solutions. The multi-layered micromixer increases the interface area of two fluids and shortens diffusion length, resulting in an increase of mixing efficiency of reactants. Also, a characteristic of this mixer is that the mixing efficiency increases with a decrease in flow rate.13 In order to gain a long residence time required for polymerizations, low flow rate should be used in the microfluidic system. Therefore, a multi-layered micromixer is convenient for NCA-polymerizations in a microfluidic system.Fig. 1 shows the microreactor composed of micromixer and PTFE microtubes. This micromixer was made of PDMS, a siloxane based elastomer. PDMS offers many advantages for fluidics, such as transparency, flexibility, strong adhesive properties, low cost and some degree of durability.16 Although PDMS does not swell with humidity, it is incompatible with less polar organic solvents (e.g., toluene, chloroform, n-hexane, and ethyl acetate) due to its strong tendency to absorb and swell. In contrast, there is little swelling on exposure to polar organic solvents including DMF, DMSO, ethanol, acetonitrile, etc.† In this study, DMF was selected as a solvent for NCA-polymerization based on the fact that it served as convenient solvent for many polypeptides and results in homogeneous polymerization.17
Polymerization of Nε-benzyloxycarbonyl-L-lysine
The most popular approach for the synthesis of amino acid-based polymers is the α-amino acid NCA (N-carboxyanhydride) method, which was first discovered in 1906 by Leuchs.18 The anion ring-opening polymerization of NCA is a fast and efficient route for the synthesis of polypeptides.19 As shown in Scheme 1, when using a base such as tertiary amine as initiator, the initiation occurs by deprotonation of NCA. The resulting activated NCA anion reacts with another NCA molecule, producing a dimer with an electrophilic N-acyl NCA end group and a nucleophilic carbamate group. Propagation can proceed via either the carbamate or the amine mechanism. Because the initiation and NCA anion-attack were known to be relatively fast reactions,20 it is assumed that these steps are important in controlling polymerization of NCA. However, the polymerization control would be difficult in conventional batchwise systems due to the tendency to locally generate concentration gradients of the reagents. In this study, a microreactor that enables strict reaction control was employed to synthesize biopolymers.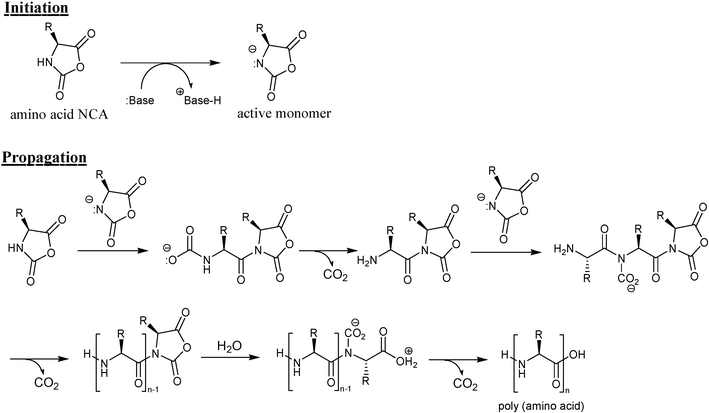 | ||
| Scheme 1 Mechanism of NCA-polymerization initiated by a base such as tertiary amine. | ||
In the experiment, polymerizations were performed in DMF and initiated by TEA. This method is known to give high molecular mass polymers and to afford more rapid reaction as compared with using a nucleophile such as primary amine.21 Both solutions of NCA and base were introduced to the microreactor (total inner volume 250 µl) by using a syringe pump. The flow rates were 1.25 to 250 µl min−1 corresponding to 200 to 1 min residence time. Fig. 2 shows the GFC data for poly(Lys) produced using the microreactor at different flow rates. The molecular weights of respective samples regularly increased with decrease in flow rates, that is, as a consequence of the increase in time required for polymerization to occur. It was found that the molecular mass of the polymer produced using the microreactor is simply governed by the flow (pumping) rate.
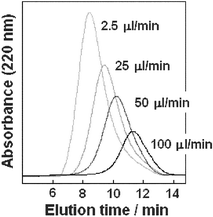 | ||
| Fig. 2 GFC analysis of poly(Lys) produced by the microreactor. The polymerizations were conducted at 0.1 M NCA and 0.01 M TEA for 2.5, 5, 10, and 100 min of reaction times corresponding to 100, 50, 25, and 2.5 µl min−1 of flow rates, respectively. | ||
Next, properties of polymers synthesized using the microreactor were compared with those using the batchwise system. The reactions were conducted at 0.3 M NCA concentration at various reaction times, and the mole ratio of monomer and initiator was 10∶1. Fig. 3 shows yield (%), number-average molecular weight (Mn), and polydispersity (Mw/Mn) of polymer products (more than 500 Da) in batchwise and microfluidic systems. In both reaction systems, the yields of poly(Lys) reached to about 57% within 50 min, and slightly increased from 50 min to 200 min (61% yield at 200 min). There were no significant differences in yields between the two systems at each reaction time (Fig. 3(A)). The values for number-average molecular weight of polymer in microfluidic system (Mn = ca. 20,000) were slightly larger in relation to those in batchwise system (Mn = ca. 18,000) at 200 min reaction time (Fig. 3(B)). It was noted that the obvious differences between the two systems were observed with respect to polydispersity values. The molecular weight distributions in the microfluidic system were narrower than those in the batchwise system (Fig. 3(C)). The NCA-polymerization using the microreactor resulted in a better molecular weight distribution control as compared to the batchwise system.
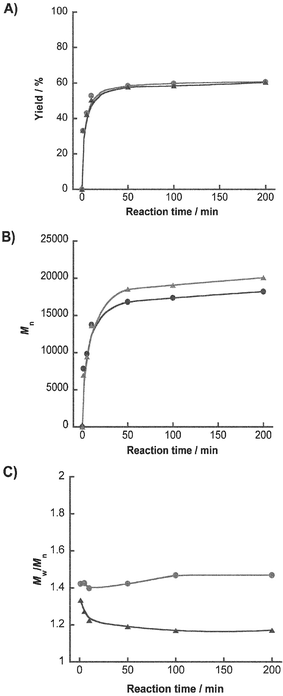 | ||
| Fig. 3 Kinetic data for polymerization of Z-Lys-NCA (0.3 M) by the batchwise (circle) and the microfluidic (triangle) systems. (A) Plots of yield, (B) number average molecular weight (Mn), and (C) polydispersity (Mw/Mn) of the obtained polymers against reaction time, respectively. | ||
When using NCA solutions of higher concentrations, controlling the molecular weight distributions would become increasingly harder because gradients of reagent concentration tend to generate locally. Thus, controllability of the microreactor for polymerization reactions was investigated at various NCA concentrations. Fig. 4 shows the properties of polymer synthesized at 0.1 M–0.5 M of Z-Lys-NCA for 200 min (monomer∶initiator ratio of 10∶1). In both batchwise and microfluidic systems, the slight increase in yields was observed at higher NCA-concentrations. There was no significant difference in yields between the two systems at all concentrations (Fig. 4(A)). The number-average molecular weights also had a tendency to increase with a rise in NCA concentration. The polymers produced using the microreactor had slightly larger number-average molecular weights than those using the batchwise system at all concentrations of NCA (Fig. 4(B)). Polydispersities of polymers increased linearly with increasing concentration of NCA (R2 = 0.9902) in the batchwise system (Fig. 4(C)). However, there was only a very slight increase in the molecular weight distributions of polymers in the microfluidic system (Fig. 4(C)). This result emphasizes one of the advantages of the microreactor. In a situation wherein control of polymerization is unfavourable, the micromixer may offer a significant role in controlling the molecular weight distribution.
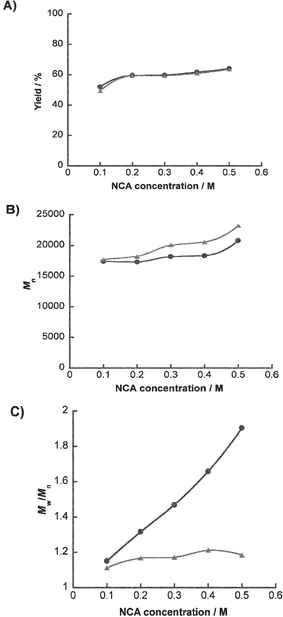 | ||
| Fig. 4 Comparisons of polymer properties in the batchwise (circle) and the microfluidic (triangle) systems at various Z–Lys–NCA concentrations. (A) Plots of yield, (B), number average molecular weight (Mn) and (C) polydispersity (Mw/Mn) of the obtained polymers against concentrations of NCA, respectively. Polymerizations were carried out for 200 min. | ||
In order to confirm the importance of the micromixer, polymerization using a microreactor attached to a simple T-shaped connector in place of the micromixer was carried out at 0.5 M of NCA (monomer∶initiator ratio of 10∶1). The polydispersity value of microreactor attached to a T-shaped connector demonstrated a higher polydispersity value (Mw/Mn = 1.75) than the micromixer, indicating poor control of molecular weight distribution by the microreactor attached to a T-shaped connector. On the other hand, lower polydispersity values of the micromixer reflected a better control of molecular weight distribution.
Polymerization and co-polymerization by other amino acid-NCAs
To verify the utility of the microreactor for polymerizations of other NCAs, the polymerization of Bzl-Glu-NCA was carried out at 0.3 M NCA for 100 min (monomer∶initiator ratio of 10∶1) in a similar fashion as Z-Lys-–NCA polymerization. Polymerization using a microreactor could not be carried out for over 2 hours because turbid substances were formed in the reaction solution as previously reported,22 and blocked flow-channels of the microreactor. The product yields of batchwise and microfluidic systems were almost the same (76% and 79%, respectively). Unlike poly(Lys), no difference in the average molecular weight was observed in poly(Glu) in both systems as shown in Table 1. The poly(Glu) synthesized using the microreactor had narrower molecular weight distribution than that obtained from the batchwise system.| M n | M w/Mn | ||
|---|---|---|---|
| Poly(Glu) | Batch | 40,200 | 1.56 |
| Microreactor | 40,000 | 1.17 | |
| Poly(Lys, Ala) | Batch | 17,700 | 2.56 |
| Microreactor | 18,800 | 1.64 | |
| Poly(Lys, Leu) | Batch | 18,100 | 2.13 |
| Microreactor | 19,200 | 1.54 |
Next, copolymerizations of NCAs were applied to the microfluidic reaction. The copolymerization mechanism is known to be more complex due to differences in the reactivities of individual NCAs.17 The properties of copolymers obtained by the microfluidic and batchwise systems were indicated in Table 1. The reactions were performed at 0.5 M of total NCA concentrations for 200 min (monomer∶initiator ratio of 10∶1). The synthesized copolymers were Lys-based polymer mixed with Ala (or Leu) with the mole ratio of Z-Lys-NCA to Ala-NCA (or Leu-NCA) at 7∶3. In a batchwise system, the product yield obtained from Lys/Leu-copolymerization (66%) was higher than that of Lys/Ala-copolymerization (59%). In the two copolymers, there were no significant differences in the yields for batch and microreactor systems (poly[Lys/Ala] 59% and 58%, respectively; poly[Lys/Leu] 66% and 67%, respectively). Lys/Ala- and Lys/Leu-copolymers as well as homo-polymerization of Z-Lys-NCA synthesized using the microreactor had slightly larger average molecular weights as compared to that of batchwise system. Batch-copolymerizations showed much poorer molecular weight distribution control than batch-homopolymerization. In terms of molecular weight distribution, copolymerization often has a negative effect. The microreactor provided optimized control of molecular weight distributions as indicated by the low value obtained from each copolymer (1.64 Mw/Mn value for Lys/Ala, 1.54 Mw/Mn value for Lys/Leu). Compared to the molecular weight distributions of homopolymers, copolymers produced by the microreactor showed lower values than those in the batchwise system.
Discussion
Many biopolymers with interesting functionalities have been developed and applied in various fields. At present, a rapid and combinatorial synthesis system with highly controllable polymerization reaction is strongly required. However, it is generally difficult to control polymerization reactions in a conventional batchwise system. In this study, we present the synthesis of amino acid polymers using a microreactor that enables a strict control of chemical reactions as a model of biopolymer synthesis. Performance of a microreaction system for polymerization was compared with that of a conventional batchwise system by analyzing the properties of the obtained polymers.First, we investigated the molecular weight of polymers synthesized by both systems. In polymer products containing lysine residue, a slight difference in average molecular weight between batchwise and microreactor was observed but product yield remained the same (Fig. 3(B), 4(B), and Table 1). Although these effects in the molecular weight might be attributable to a microfluidic environment, the reason remains unclear.
Next, we investigated the molecular weight distribution of each polymer synthesized by batchwise and microfluidic systems. In Z-Lys-NCA polymerization, Mw/Mn values of the polymers synthesized by batchwise system obviously increased with increasing concentration of NCA. Meanwhile, the Mw/Mn values in the microfluidic system remained low with high NCA concentrations (Fig. 3(C)). It was demonstrated that the microreactor provided high controllability of NCA-polymerization even at concentrations leading to poor molecular weight distribution control in the batchwise system.
The controllability of molecular weight distribution is governed by some factors. The first one would be mixing efficiency of reactants. In the batchwise system, increase of the reagent concentration tends to decrease the mixing efficiency of reagents, and leads to local generation of concentration gradient of the reagents. Also, the viscosity rise observed at highly concentrated NCA solutions would cause a decrease in the efficiency of mixing based on turbulent flow in the batchwise system. Consequently, it was assumed that such decrease in mixing efficiency induced broadening of the molecular weight distribution. In contrast, it was hypothesized that effective mixing of reactants by the attached micromixer in the microfluidic system enabled high control of molecular weight distribution. In order to investigate the direct contribution of the micromixer to the molecular weight distribution control, Z-Lys-NCA polymerization was performed using a microreactor with a simple T-shaped connector instead of the micromixer. The obtained polymer had a broad molecular weight distribution, indicating the importance of the micromixer in the control of the distribution. The interfacial area generated by laminar flow between NCA and TEA solutions was 56 mm2 per volume (1.2 µl) of micromixer region in the microreactor, and corresponded to 6 mm2 in the T-shaped connector system. This difference in areas is related directly to diffuser and mixing efficiency of NCA and TEA. In the microreactor with T-shaped connector, a decrease in mixing efficiency due to reduction of interfacial area would cause an increase in molecular weight distribution.
The multi-layered micromixer employed in this study increases the degree of mixing per unit time with decreasing flow rates.13 Therefore, this mixer is of great use in the synthesis system which requires long residence time, like NCA-polymerizations. This is in contrast to another report that increases in flow rate increases mixing efficiency.9 Although various types of micromixers have been developed and applied to various chemical reactions,23 the micromixer should be selected depending on the condition of the reaction systems.
In addition to mixing efficiency, it is conceivable that reaction temperature is one of the important factors in controlling molecular weight distribution. Many polymerization reactions are extremely sensitive to temperature and strict control of the reaction temperature is very important.24 Polymerization of NCA is also of no exception, and it was reported that the reaction rates and molecular weight distributions increase with increasing temperature.25 The strict maintenance of reaction temperature to a constant degree is important to control molecular weight distribution. During mixing of NCA and TEA in DMF solutions, heat was generated as indicated by an increase of about 2–4 °C (data not shown). The microreactor allows maintenance of a constant temperature due to effective heat transfer based on large surface-to-volume ratio of the fluid entity. The microreactor's ability to control the molecular weight distribution is attributable to its high efficiency of heat transfer. Heat transfer is less efficient in batch in comparison to the microreactor. Also, the batchwise system includes the problem of heat generation by mechanical friction produced by magnetic stirring.
Our study demonstrated the usefulness of the microreactor in controlling NCA-polymerization reaction. Furthermore, this microreaction technology offers several advantages in the production of amino acid polymers. First, polymer with low Mw/Mn values can be obtained at relatively fast reaction rate in base-initiated polymerization. In NCA-polymerization by base initiation such as tertiary amine, the NCA anions rapidly polymerize in the same way as step polymerization. On the other hand, polymerization using a nucleophile such as a primary amine occurs at slower reaction rate through nucleophilic attack on NCA. Molecular weight distributions of the obtained polymers tend to be broad in the former reaction mechanism, but narrow in the latter. NCA-polymerization using tertiary amine in combination with a microfluidic system enabled rapid production of polymer with narrower distribution. Second, the microreactor allowed narrow molecular weight distribution even with high concentration of NCA. This leads to increase in product yield per unit time, and could be of great advantage in high-throuput synthesis. In capacity of production, our microreactor could synthesize polylysine (Mw = 20,000, Mw/Mn = 1.17) at the yield of 100 mg min−1; a single reactor could produce approximately 6 g of the polylysine for 10 hours. In addition, this microreactor can be produced inexpensively and thus, could be used as a disposable device. One of the frequent problems encountered when using the microreactor is non-specific adsorption of reactants and products, especially polymers, to the wall of the reactor. Therefore, disposability is considered essential in the synthesis of a novel substance of unknown adsorption property, for instance, in a combinatorial system which produces various novel substances.
Although the usefulness of this microreactor in NCA-polymerization is realized, some improvement in this system is needed. One improvement is to narrow the molecular weight distribution much more, especially in copolymerizations where control of distribution is more difficult. One of the conceived measures of improving mixing efficiency is by increasing the number of laminar layers of micromixer and narrowing the fluid channels.
Reactions involved in chemical polymerization employed for synthesis of biomaterials should be highly controlled because polymer properties such as average molecular weight and molecular weight distribution strongly affect the function of the polymer. For instance, polylysine functions are recognized in gene and drug delivery, cell adhesion, and cytotoxicity depending on the polymer size.3,4,26 When using biopolymers of less well known biological function, the molecular weight of biopolymer should be carefully selected, and the molecular weight distribution should be controlled to be as narrow as possible. It is expected that such requirements could be satisfied by using the present microfluidic system which has high controllability of molecular weight distribution and which enables simple control of molecular weight of amino acid–based biopolymers. Present-day investigations for biomaterials are directed toward the development of novel functional substances by using a high-throughput and combinatorial synthesis method. The device developed in our laboratory could be a basic model of a simple, controllable, and continuous combinatorial synthesis process with a high-throughput system for biomaterial libraries. Also, acceleration of multi-parameter optimization of the reaction conditions has been required as mentioned by some researchers.27 Our microreactor has high potential with respect to rapid optimization of polymerization reaction.
Acknowledgements
We thank Prof. Ryoichi Katakai for helpful suggestions in NCA preparation. Part of this work was supported by NEDO.References
- (a) C. H. Chiang and M. K. Yeh, Curr. Pharm. Biotechnol., 2003, 4, 323 CAS; (b) B. D. Ratner and S. J. Bryant, Annu. Rev. Biomed. Eng., 2004, 6, 41 Search PubMed.
- (a) F. Sanda and T. Endo, Macromol. Chem. Phys., 1999, 200, 2651 CrossRef CAS; (b) J. Kohn, Biodegradable Polymers as Drug Delivery Systems, ed. M. Chasin and R. Langer, Dekker, New York, 1990 Search PubMed; (c) J. M. Anderson, K. L. Spilizewski and A. Hiltner, Biocompatibility of Tissue Analogs, ed. D. F. Williams, CRC Press, Boca Raton, Florida, 1985, vol. 1 Search PubMed.
- A. Bargellesi, G. Damiani, W. M. Kuehl and M. D. Scharff, J. Cell. Physiol., 1976, 88, 247 CrossRef CAS.
- (a) I. L. Shih, Y. T. Van and M. H. Shen, Mini Rev. Med. Chem., 2004, 4, 179 Search PubMed; (b) J. P. Clarenc, G. Degols, J. P. Leonetti, P. Milhaud and B. Lebleu, Anticancer Drug Des., 1993, 8, 81 CAS; (c) W. T. Godbey and A. G. Mikos, J. Control Release., 2001, 72, 115 CrossRef CAS.
- K. M. Hawkins, S. S. S. Wang, D. M. Ford and D. F. Shantz, J. Am. Chem. Soc., 2004, 126, 9112 CrossRef CAS.
- (a) W. Enrfeld, V. Hessel and H. Lowe, Microreactors, New Technology For Modern Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) T. Schwalbe, V. Autze and G. Wille, Chimia, 2002, 56, 636 CrossRef CAS.
- K. Schubert, W. Bier, J. Brandner, M. Fichtner, C. Franz and G. Linder, Realization and testing of microstructure reactors, micro heat exchangers and micromixers for industrial applications in chemical engineering, Process Miniaturization: 2nd International Conference on Microreaction Technology, IMRET 2; Topical Conference Preprints, ed. W. Ehrfeld, I. H. Rinard, and R. S. Wegeng, , AIChE, New Orleans, USA, 1998, pp. 88–95 Search PubMed.
- T. Wu, Y. Mei, J. T. Cabral, C. Xu and K. L. Beers, J. Am. Chem. Soc., 2004, 126, 9880 CrossRef CAS.
- A. Nagaki, K. Kawamura, S. Suga, T. Ando, M. Sawamoto and J. Yoshida, J. Am. Chem. Soc., 2004, 126, 14702 CrossRef CAS.
- M. Miyazaki, K. Yamashita, Y. Yamaguchi, T. Honda, H. Nakamura, M. Fujii and H. Maeda, New J. Chem., 2004, 28, 1622 RSC.
- K. Yamashita, Y. Yamaguchi, M. Miyazaki, H. Nakamura, H. Shimizu and H. Maeda, Lab Chip, 2004, 4, 1 RSC.
- K. Yamashita, Y. Yamaguchi, M. Miyazaki, H. Nakamura, H. Shimizu and H. Maeda, Anal. Biochem., 2004, 332, 274 CrossRef CAS.
- Y. Yamaguchi, K. Ogino, K. Yamashita and H. Maeda, J. Chem. Eng. Jpn., 2004, 37, 1265 Search PubMed.
- R. Katakai and Y. Iizuka, J. Org. Chem., 1985, 50, 715 CrossRef CAS.
- W. N. E. Dijk-Wolthuis, L. Water, P. Wetering, M. J. Steenbergen, J. J. K. Bosch, W. J. W. Schuyl and W. E. Hennink, Macromol. Chem. Phys., 1997, 198, 3893 CrossRef.
- Siloxane Polymers, ed. S. J. Clarson and J. A. Semlyen, Prentice Hall, Englewood Cliffs, NJ, 1993 Search PubMed.
- Y. Shalitin and E. Katchalski, J. Am. Chem. Soc., 1960, 82, 1630 CrossRef CAS.
- H. Leuchs, Ber. Dtsch. Chem. Ges., 1906, 39, 859.
- (a) H. R. Kricheldorf, α-Amino Acid N-Carboxyanhydrides and Related Heterocycles, Springer-Verlag, Berlin, 1987, pp. 1–213 Search PubMed; (b) T. J. Deming, J. Polym. Sci. Part A: Polym. Chem., 2000, 38, 3011 CrossRef CAS.
- (a) C. H. Bamfold and H. Block, J. Chem. Soc., 1961, 4989 RSC; (b) C. H. Bamfold and H. Block, J. Chem. Soc., 1961, 4992 RSC.
- (a) E. Peggion, E. Scoffone, A. Cosani and A. Portlan, Biopolymers, 1966, 4, 695 CrossRef CAS; (b) C. H. Bamford and H. Block, in Comprehensive Chemical Kinetics, ed. C. H. Bamford and C. F. H. Tipper, Elsevier, New York, 1976, vol. 15, ch. 8 Search PubMed; (c) D. S. Poche, M. J. Moore and J. L. Bowles, Syn. Comm., 1999, 29, 843 Search PubMed.
- T. Komoto, K. Y. Kim and T. Kawai, Makromol. Chem., 1978, 179, 373 CrossRef CAS.
- (a) D. Erickson and D. Li, Langmuir, 2002, 18, 1883 CrossRef CAS; (b) G. H. Seong and M. Crooks, J. Am. Chem. Soc., 2002, 124, 13360 CrossRef CAS; A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Science, 2002, 295, 647 Search PubMed; (c) V. Mengead, J. Josserand and H. H. Girault, Anal. Chem., 2002, 74, 4279 CrossRef CAS; (d) J.-H. Kim, B.-G. Kim, M. La, J.-B. Yoon and E. Yoon, Micro-total analysis systems, ed. Y. Baba, S. Shoji and A. Berg, pp. 224–226, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002, vol. 1 Search PubMed.
- T. Iwasaki and J. Yoshida, Macromolecules, 2005, 38, 1159 CrossRef CAS.
- Y. Iwakura, K. Uno and M. Oya, J. Polymer Sci. Polym. Chem. Ed., 1968, 6, 2165 Search PubMed.
- (a) M. A. Wolfert and L. W. Seymour, Gene Ther., 1996, 3, 269 CAS; (b) S. Choksakulnimitr, S. Masuda, H. Tokuda, Y. Takakura and M. Hashida, J. Controlled Release, 1995, 34, 233 CrossRef CAS.
- R. A. Potyrailo, R. J. Wroczynski, J. P. Lemmon, W. P. Flanagan and O. P. Siclovan, J. Comb. Chem., 2003, 5, 8 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Data of swelling experiments of PDMS in several organic solvents. See http://dx.doi.org/10.1039/b505137a |
| This journal is © The Royal Society of Chemistry 2005 |
