Integrated microelectrode array and microfluidics for temperature clamp of sensory neurons in culture
Thomas M.
Pearce
a,
J. Adam
Wilson
a,
S. George
Oakes
b,
Shing-Yan
Chiu
b and
Justin C.
Williams
*a
aDepartment of Biomedical Engineering, University of Wisconsin-Madison, WI, USA. E-mail: jwilliams@engr.wisc.edu
bDepartment of Physiology, University of Wisconsin-Madison, WI, USA. E-mail: oakes@physiology.wisc.edu
First published on 14th September 2004
Abstract
A device for cell culture is presented that combines MEMS technology and liquid-phase photolithography to create a microfluidic chip that influences and records electrical cellular activity. A photopolymer channel network is formed on top of a multichannel microelectrode array. Preliminary results indicated successful local thermal control within microfluidic channels and control of lamina position over the electrode array. To demonstrate the biological application of such a device, adult dissociated dorsal root ganglion neurons with a subpopulation of thermally-sensitive cells are attached onto the electrode array. Using laminar flow, dynamic control of local temperature of the neural cells was achieved while maintaining a constant chemical culture medium. Recording the expected altered cellular activity confirms the success of the integrated device.
Introduction
The integration of living, biological systems into lab-on-a-chip devices is an emerging interdisciplinary field. Micro Electro Mechanical Systems (MEMS) based devices have been used in a wide variety of in vitro biological applications such as drug screening, cell separation, cell culture, enzyme-linked assays, and many others. Chemical reaction devices are widely used in biological applications, and often utilize expensive reagents. Miniaturizing these devices provides the advantage that very small reagent and sample volumes are necessary to perform various functions. In addition to low reagent volume and cost, these systems offer significant advantages in size, portability, disposability, and time savings over conventional bench-top procedures. For these reasons, there is great interest in developing technologies involving miniaturization of numerous analysis tools including mixers, valves, pumps, reaction chambers, and various forms of readouts. Microfluidic devices, designed to handle fluid samples on the microscale, benefit greatly from the aforementioned characteristics. As most biological applications are aqueous-based, microfluidic devices are the most common class of BioMEMS devices.Laminar flow is a dominating and important characteristic of microscale fluid handling devices.1 Although this may present challenges, intelligent design of these devices can make use of the inherent lack of turbulence to achieve unique capabilities. For example, physical boundaries between fluids become less important, as mixing occurs only by diffusion. It has been shown that boundary position between two streams is controlled by relative flow rate, and is easily predictable.2 This can be extended to any number of streams. Utilizing control of the shape and location of a fluid stream, it is possible to control the physical and chemical environment within specific areas in a microfluidic device. This concept is not novel, and has been used in a wide variety of applications. This characteristic, along with the scale of the fluid channels themselves, make microfluidics advantageous for use with biology.
Microdevices are well suited for biological applications, particularly on the cellular level, because the scale of the device is close to that of the cell.3 The benefits include fewer wasted reagents, more detailed control of the cellular environment, and faster response time. Selective physical control and chemical delivery has many uses in biological systems, from preparation of cellular samples via cell lysis to drug delivery. As an example of one field that can make use of this, electrophysiology investigates the electrical activity of nerve cells, and the use of drugs as a tool for exploration of the nervous system is common in the field. Neural response to drug exposure typically involves an alteration of the electrical behavior of the cell. This response can be measured in a variety of ways. As electrical behavior is a result of the flow of ions across the cell membrane, dyes selective to ion concentration can be used to image this flow. Direct measurement of electrical potential is common as well, from extracellular field potential to patch clamp to penetrating intracellular electrodes.4
In this paper, a microfluidic channel network with an integrated microelectrode array is used to demonstrate the utility of these combined microtechnologies to the field of neuroscience. To illustrate controlled modulation of neural activity via temperature control within a microfluidic device, dorsal root ganglion (DRG) neurons were used. A wide variety of sensory cell types are present within the DRG, including cell subpopulations that respond to different temperature stimuli.5,6 The cell bodies of these acutely isolated cells contain somatic receptors present on the less accessible nerve endings.6 The freshly isolated cells are spherical in shape, lack neuritic processes and can be attached to the microarray by a surface coating of polylysine. Neurons stimulated by cooling, which are of interest in this study, make up only a small percentage of the total neuron population and cannot be identified by visualization through the microscope. No method of recording electrical activity of DRG neurons and identifying the action potentials produced in these cool and cold sensitive neurons has been reported. This technology has the potential to expand the capability of conventional electrophysiology methods, and can be applied to a wide variety of cell types and experimental conditions.
Materials and methods
Device fabrication
The substrate of the cell culture device is the MEA-60 electrode array (ALA Science, USA). The array consists of 60 circular pads 30 µm in diameter connected via 10 µm traces to contact pads near the edge of the 49 by 49 mm slide. An array with a microfluidic channel built on top is pictured in Fig. 1. A commercially available array was used in an attempt to make this technology widely repeatable and accessible to the neuroscience field. The microfluidic system was created using the microfluidic tectonics (μFT) construction platform.7 On top of the electrode array substrate, a hybriwell (Grace BioLabs, USA) modified to fit inside the contact pads was adhered, forming a hollow cavity. A hybriwell consists of a polycarbonate sheet with an adhesive gasket at the edges, with access ports punched through the top. To modify it for this application, two sides were removed, reducing the overall length, and additional access ports were punched by hand. Along two edges, a 250 µm thick layer of adhesive maintains the overall height of the chamber. The cavity was then filled with pre-polymer (Fig. 2a), relying on capillary forces at the open ends to keep the liquid within the chamber. A photomask printed with the desired channel geometry was placed over the device, and exposed to UV light (Fig. 2b) leaving solid structures behind where exposed. In this instance, ground wires and thermocouples (Physitemp, USA) were integrated into the device to allow the temperature within the channel to be measured. The remaining pre-polymer was removed under vacuum, leaving behind the microfluidic channel network (Fig. 2c). After flushing with deionized water and methanol, the device was dried and press-on connectors were added. The device was connected via microline tubing to syringe pumps (Kent Scientific, USA), completing the flow system. The 1.4 mm × 1.4 mm square array is contained within a channel 1.75 mm in width and 250 µm in height (Fig. 2d).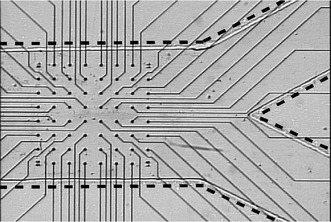 | ||
| Fig. 1 MEA-60 microelectrode array with Y-shaped microfluidic channel fabricated on top. Dashed lines are added to highlight the channel walls. The fluidics and substrate are optically transparent, allowing visualization of the array. | ||
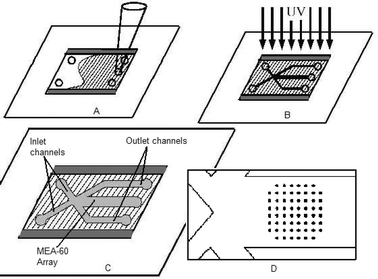 | ||
| Fig. 2 Schematic of fabrication process. The microfluidics are fabricated by pressing a hybriwell on top of the MEA-60 chip, thus forming a shallow cavity over the array. The cavity is first filled with pre-polymer (a). A mask is then placed above the cavity and ultraviolet light polymerizes the exposed monomer (b). Remaining liquid is removed under suction, leaving channels of the desired geometry (c). A blowup of the array within the outlet channel is pictured (d). | ||
Cellular methods
Adult dorsal root ganglion neurons were dissociated and concentrated using methods previously described.8 Adsorbed polylysine was used to promote cell adhesion over the electrode array. To prepare the device for polylysine adsorption, the channel was flushed with 2 N nitric acid and rinsed thoroughly with deionized water. Approximately 15 µL of polylysine solution was introduced through the outlet port, filling the outlet channel over the array. After 30 min, the solution was removed under suction and the channel was air dried. Next approximately 60 µL of the cell suspension was used to fill the channel system and connectors, leaving excess fluid covering each access port to eliminate bubbles while connecting tubing to the device. Tubing pre-filled with solution was inserted into the connectors, and the cells were allowed to settle and adhere for one hour under conditions of zero flow. To reduce the disturbance to adhered cells, bulky debris was removed gently using a total flow rate of 20 µL min−1. Once the experiment was over, the adhered cells and remaining debris were removed with Triton X-100 solution, and the channel was flushed with water and methanol. To remove the microfluidic system and reuse the electrode array, the polycarbonate top was peeled off and the chip was soaked in methanol for 24 h. The polymer layer could then be easily removed using mechanical means.Data acquisition
The chamber was placed in a custom-made holder that allowed the connection of the electrodes to a multi-channel, multiprocessor recording system (TDT System 3, Tucker-Davis Technologies, Inc., Alachua, FL). This recording system handles the acquisition and analysis of up to 64 channels of data simultaneously and allows for the sorting and classifying of action potential data. Post recording analysis was performed using a combination of TDT and custom Matlab software. Data collection was performed by using a carefully standardized method developed previously.9 The threshold was set above the noise floor (25 µV) to detect action potential production. When the threshold is exceeded a brief record is excerpted for a preset period before and after the event is detected whereby individual action potentials can be observed and analyzed. Sampling is performed at 24 kHz and triggered events are time-stamped to allow for identification.The data is analyzed off-line using a time/voltage window discrimination program written in Matlab. This enables additional noise to be removed, and allows sorting and classification of different neurons that appear on an electrode by following protocols outlined previously.10 When noise is removed from the data set, it is possible to look at the firing characteristics for individual cells under different temperature conditions.
Temperature and flow control
Device geometry (Fig. 2c,d) is set up such that two inlet channels and three outlet channels branch off from a single point. The microelectrode array is located 3 mm from this point in the central outlet channel. Syringe pumps control the infusion rate through the inlet channels and the withdrawal rate through the two outside outlet channels. The center outlet, containing the array, is not directly controlled with a pump. Instead, flow over the array is determined from the added rates of the other four channels, allowing fluctuations in the flow rates to occur without harmful pressure building up.Before entering the device, one fluid line was heated with a 45 °C water bath. The other line was cooled to 4 °C with an ice bath. Both baths were located approximately 12 cm from the device. Temperature near the array was measured with the embedded thermocouples in the outlet channel. During cellular recording, each side outlet channel was set to withdraw fluid at 450 µL min−1, for a total withdrawal rate of 900 µL min−1. The two inlet rates varied, but the total infusion rate was held constant at 1100 µL min−1, so that over the array flow rate was a constant 200 µL min−1. To expose the cells to warm media, the warm inlet stream was set to 750 µL min−1 and the cold stream to 350 µL min−1. These flow rates were alternated to expose the cells to cool media.
Results
Using the previously described nitric acid and polylysine treatment to promote cell adhesion, the majority of the cells initially above the array adhered solidly to the substrate. After flushing debris from the channel, approximately 90 percent of the visually identified cells remained on the array. By gently removing large debris before increasing flow instead of using high flow immediately, the number of cells that were mechanically removed was decreased. After debris removal, cells showed the ability to withstand flow up to 600 µL min−1 (20 mm s−1 linear velocity) without detachment.Spatial control of position of the laminar boundary between the two inlet streams was found to be on the order of tens of microns using optical measurements. Digital images were taken after each successive boundary shift to quantify the change in boundary position. In one example, two cells approximately 30 µm apart were first bathed simultaneously in one solution (Fig. 3a), then split by the boundary layer between two solutions (Fig. 3b), and finally bathed in the second solution (Fig. 3c). As expected, it was seen that higher flow rates produced a more defined boundary. Since the flow is always in the laminar regime (Re < 20), slower flow increases mixing at the boundary because contact time between the two streams is greater, enhancing diffusion. Similarly, temperature gradients become steeper at high flow rate, as conduction time is minimized.
 | ||
| Fig. 3 Two cells, circled, are exposed to dyed solution (left) and clear solution (right). Both cells are first exposed to dyed solution (a), then each to different media (b) and finally both to clear solution (c). | ||
Thermocouples were integrated into both sides of the outlet channel, 2 mm downstream of the array. Measurements were made under flow conditions designed to keep the array in warm and cool media. Initially cells were maintained in the warm solution, measured at 35 °C. Flow was then changed over to the cold solution, measured at 16 °C. Although it was not determined precisely at the array, photographic evaluations with a similar channel (see Fig. 5) showed that temperature changes occurred in less than one second in response to flow rate changes.
During recording, the noise floor was typically about 20 µV, well below the level necessary to record action potentials. Single unit action potentials were observed with average signal to noise ratio greater than 2. The temperature of the solution was found to have a strong effect on the firing characteristics of several cells that were observed. When warm solution was flowed over the cell, within about 10 s the firing rate decreased to almost 0 spikes s−1 (mean = 0.028 spikes s−1). When cold solution was applied, the firing rate of the cell increased to about 1 spikes s−1 (mean = 0.94 spikes s−1). After analyzing the data using multiple time-voltage window filtering, action potentials from a single source were isolated. The average voltage trace and standard deviation (n = 700) are shown in Fig. 4. A raster histogram, where each data point represents an action potential, for the five trials (three cold trials alternated with two hot) is presented in Fig. 4 as well.
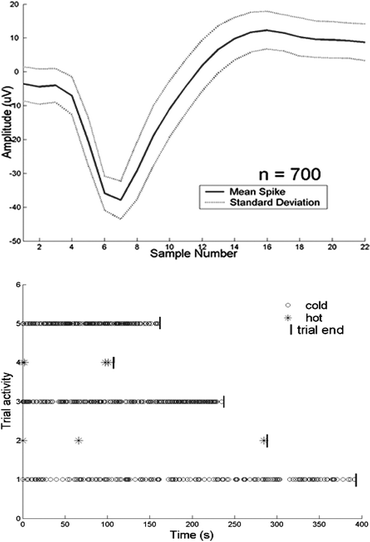 | ||
| Fig. 4 The mean and standard deviation of the action potentials are plotted (top). The firing rate of the cell responsible for this signal is strongly dependent on temperature. A raster histogram (bottom) shows the influence of temperature on firing rate during hot and cold trials. Trials 1, 3, and 5 are cool trials; 2 and 4 are warm trials. Each data point represents a single action potential. | ||
Discussion
Integrating a multichannel microelectrode array into a microfluidic device provides a method of recording electrophysiological activity. Using the flexible, inexpensive μFT construction platform allows numerous flow systems to be fabricated and tested in a short period of time. This turned out to be crucial, as a number of problems were solved through rapid prototyping and testing. An added benefit is the simplicity of the construction technique, making reproduction an easy matter. Including the array within a channel involves substituting the array for a standard microscope slide and making other minor modifications. The added fabrication time to include an array is on the order of minutes. The array adds cost to the system; however, the fluidics can be removed and the array reused in future devices, which minimizes the extra expense of the system. The combination of the two technologies allows precise control of extracellular environment by the microfluidics and subsequent recording of the change in cellular activity.Control of temperature as well as chemical environment can be necessary in electrophysiology.11 To qualitatively visualize temperature gradients within the channel, a device was built with a polymer-based aquarium thermometer embedded in the substrate underneath the outlet channel (Fig. 5a). To achieve a better understanding of the gradients, the flow system was modeled in FEMLAB (Comsol, USA). The actual device channel geometry was duplicated, and various flow rates for each inlet were simulated. Fig. 5b shows the result of a sample simulation. Plots of temperature against position across a given cross-section were generated (Fig. 5c). The results of the model were used to help determine the conditions necessary to create the desired gradient over the array. Altering the relative flow rates of the streams shifted the position of the gradient within the channel, and changing absolute flow rate changed the shape of the gradient.
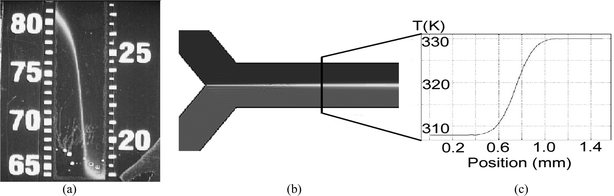 | ||
| Fig. 5 Preliminary experiments on temperature control revealed that it is possible to obtain a controllable temperature gradient using laminar flow. For visualization, an aquarium thermometer was embedded under a microfluidic channel (a). FEMLAB software was used to model the flow system (b), and Temperature vs. position plots generated from the simulation (c) showed theoretical gradients. | ||
As a demonstration, adult mouse DRG neurons, including some sensitive to temperature, were introduced into the device. Using temperature as a “drug”, the environment of the neurons was altered using microfluidic flow. In order to stimulate the cells, it is necessary to change the temperature to within a certain range.11 To ensure that the proper temperature was reached, measurements were made within the channel using the integrated thermocouples. These data led to the two-inlet, three-outlet configuration (Fig. 2b,c) used in the device, since flow rates higher than adherent cells could withstand were necessary to reduce heat loss in the inlet tubing. In addition, the continuous flow of each stream made rapid temperature switches possible simply by shifting the boundary. Without this feature, the volume between the heat/cold source and the array would need to be refreshed before thermal equilibrium was reached. Although the data from cellular recordings (Fig. 4) is merely a demonstration of the ability to alter extracellular environment and record the changes in electrical activity, many interesting features can be seen. For instance, the firing rate of the neuron increased from 0.4370 spikes s−1 to 1.0275 spikes s−1 to 1.2313 spikes s−1 sequentially during the three cool-fluid trials. It can also be seen that during exposure to warm fluid, the spikes were not evenly distributed but weighted toward the beginning and end of the trials. This may be due to the finite uncertainty in the timing of the fluid switching, or may be that the neuron adapted over a period of time before reaching a steady-state firing pattern. The durations of the trials in this demonstration were random; it is possible that the timing of temperature patterns could influence the cells. All of these questions can be addressed using the system presented here.
Many biological questions can be explored with this system. For example, it is hypothesized that certain temperature sensitive neurons adapt, while others may not.12 The time course for this adaptation is much slower than the time it takes to change fluid streams, making it possible to determine not only if cells adapt, but how long it takes. Macroscale systems for the same purpose take much longer to come to thermal equilibrium, and are not as suited to answering this question. Temperature control over DRGs is not the only type of extracellular environmental control that this device is capable of. A huge variety of cell types and stimuli can be easily substituted, making the number of potential applications for this device enormous. The fact that the fluidics are disposable and the electrode array reusable makes the system even more robust.
Conclusion
The device presented here demonstrates the capacity to influence cellular behavior in interesting ways. A multichannel microelectrode array integrated into the device adds recording capability, and although not demonstrated here, can be used for stimulation purposes as well.13 Combining multichannel recording capability with microfluidic control of local cellular environment provides many opportunities for both new experiments and greater understanding of previous results in the electrophysiology field. This system combines the two technologies in a novel fashion that is simple yet powerful. In addition, laminar control of temperature is demonstrated, and recordings from thermally sensitive neurons are an illustration of the ability to alter extracellular environment with the system. Because the system is not limited to a single cell or drug type, it is amenable to many potential applications.14,15 Using a commercially available electrode array in conjunction with simple microfluidic techniques helps make this a repeatable and robust piece of technology.Acknowledgements
The authors would like to acknowledge David Beebe, Ryan Pope, Sean Kruzel, Jordan Williams, Paul Victorey and Andrew Pomerantz for their help. This work was generously supported by grant RO1-23375 from the National Institutes of Health, the University of Wisconsin – Madison Graduate School and College of Engineering.References
- D. J. Beebe, G. A. Mensing and G. M. Walker, Physics and applications of microfluidics in biology, Annu. Rev. Biomed. Eng., 2002, 4, 261–286 Search PubMed.
- P. J. Stiles and D. F. Fletcher, Hydrodynamic control of the interface between two liquids flowing through a horizontal or vertical microchannel, Lab Chip, 2004, 4(2), 121–124 RSC.
- G. M. Walker, H. C. Zeringue and D. J. Beebe, Microenvironment design considerations for cellular scale studies, Lab Chip, 2004, 4(2), 91–97 RSC.
- B. Hille, Ion Channels of Excitable Membranes, Sinauer Associates, 3rd edn. 2001 Search PubMed.
- G. Reid and M. L. Flonta, Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurones, Neurosci. Lett., 2002, 324(2), 164–168 CrossRef CAS.
- J. C. Petruska, J. Napaporn, R. D. Johnson, J. G. Gu and B. Y. Cooper, Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents, J. Neurophysiol., 2000, 84(5), 2365–2379 Search PubMed.
- D. J. Beebe, J. S. Moore, Q. Yu, R. H. Liu, M. L. Kraft, B. H. Jo and C. Devadoss, Microfluidic tectonics: a comprehensive construction platform for microfluidic systems, Proc. Natl. Acad. Sci. USA, 2000, 97(25), 13488–13493 CrossRef CAS.
- T. M. Pearce , S. G. Oakes, R. Popeand J. C. Williams, Dynamic control of extracellular environment in in vitro neural recording systems, Proceedings of the 26th IEEE-EMBS 2004, 2004, in press Search PubMed.
- J. C. Williams, R. L. Rennaker and D. R. Kipke, Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex, Brain Res. Brain Res. Protoc., 1999, 4(3), 303–313 CrossRef CAS.
- D. R. Kipke, R. J. Vetter, J. C. Williams and J. F. Hetke, Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex, IEEE Trans. Neural Syst. Rehabil. Eng., 2003, 11(2), 151–5 CrossRef.
- A. Patapoutian, A. M. Peier, G. M. Story and V. Viswanath, ThermoTRP channels and beyond: mechanisms of temperature sensation, Nat. Rev. Neurosci., 2003, 4(7), 529–539 CrossRef CAS.
- G. M. Story, A. M. Peier, A. J. Reeve, S. R. Eid, J. Mosbacher, T. R. Hricik, T. J. Earley, A. C. Hergarden, D. A. Andersson, S. W. Hwang, P. McIntyre, T. Jegla, S. Bevan and A. Patapoutian, ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures, Cell, 2003, 112(6), 819–829 CrossRef CAS.
- Y. Jimbo, N. Kasai, K. Torimitsu, T. Tateno and H. P. Robinson, A system for MEA-based multisite stimulation, IEEE Trans. Biomed. Eng., 2003, 50(2), 241–248 CrossRef.
- W. L. Rutten, Selective electrical interfaces with the nervous system, Annu. Rev. Biomed. Eng., 2002, 4, 407–52 Search PubMed.
- S. M. Potter and T. B. DeMarse, A new approach to neural cell culture for long-term studies, J. Neurosci. Methods, 2001, 110(1–2), 17–24 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
