Selective catalytic reduction of NOx over microporous CuAPO-5: structural characterisation by XAS and XRD
Karina
Mathisen
*a,
David G.
Nicholson
a,
Andrew N.
Fitch
b and
Michael
Stockenhuber
c
aDepartment of Chemistry, Norwegian University of Science and Technology (NTNU), N-7491 Trondheim, Norway. E-mail: Karina.Mathisen@chem.ntnu.no
bESRF, BP 220, 38043 Grenoble Cedex, France
cThe Catalysis Research Laboratory, Department of Chemistry and Physics, The Nottingham Trent University, Clifton Lane, Nottingham, UK NG11 8NS
First published on 24th November 2004
Abstract
The effects of copper source, copper content, Al : P ratio and silicon content for the hydrothermal synthesis of CuAPO-5 using tetraethylammonium hydroxide as template have been investigated. The copper to phosphorus ratio in the synthesis gel was varied from 0.04 to 0.2, and copper(II) oxide and copper(II) acetate were used as copper sources. The samples were characterised by XAS (XANES, EXAFS), XRD (X-ray diffraction), TGA (thermogravimetric analysis), SEM (scanning electron microscopy) and elemental analysis. Rietveld refinements of the structure of CuAPO-5 were carried out on synchrotron X-ray powder diffraction data. The catalytic properties of CuAPO-5 in selectively reducing NOx in the presence of hydrocarbons (SCR-HC) were studied. The results from EXAFS show that copper is present in a distorted octahedral environment, with 4 or 5 Cu–O distances at 1.95 Å and 1 or 2 Cu–Al/P distances at about 3.14 Å for the as-synthesised samples. The calcined samples still show the same four Cu–O distances at 1.95 Å, but with the composite peak at 3.14 Å being significantly reduced. The turquoise colour of the as-synthesised CuAPO-5 is consistent with the copper atoms being complexed by water molecules within the pores as the hexaaquacopper(II) complex. The extraframework complex cation is electrostatically bound to the anionic phosphate sites of the framework. The colour changes to olive green on calcination, indicating tetrahedrally coordinated framework copper. The selective catalytic reduction of NOx by propene is catalysed by CuAPO-5 to the extent of 18% conversion. The Rietveld analysis show that calcined CuAPO-5 belongs to the P6/mcc spacegroup in which Al and P are indistinguishable.
Introduction
Numerous studies show that a variety of copper materials are active towards the reduction and decomposition of nitrogen oxides (NOx). Examples include copper(II) oxide on alumina, mixtures of copper(II) oxide with nickel(II) or zirconium(II) oxide, amorphous copper alloys (e.g. copper/zirconium) and the zeolites copper-mordenite, copper-Y, copper-X, copper-β and copper-ZSM-5.1–10 In contrast to the copper-containing zeolites relatively few studies have been reported on another class of zeotypes namely copper-containing microporous aluminium phosphates (AlPO's).11–19 The fundamental difference between the two different zeotypes is that the AlPO framework is neutral. This has consequences for the incorporation of extraneous metals and so methods other than ion-exchange are necessary for introducing metals into the AlPO's.Metal cations can be introduced either by impregnation or during the hydrothermal synthesis itself. The latter method can lead to aluminium being substituted by the extraneous metal thereby generating Brønsted acid sites in the MeAPO through bridged hydroxyl bonds. The Brønsted sites often co-exist with Lewis acid sites.20–22 Including a source of silicon in the reaction mixture yields SAPO's, where silicon substitutes for phosphorus or a combination of both phosphorus and aluminium.23 The resulting framework now has a net charge that makes possible ion exchange and the creation of catalytically active sites. Introducing metals into SAPOs either directly during synthesis or through ion-exchange gives MeSAPOs.
The microporous aluminium phosphate used in this work is the large pore (7.9 Å) AlPO-5 first synthesised over twenty years ago by Wilson et al.24 The AlPO4-5 framework is known to accept a variety of cations.25–32 Introducing copper into the lattice has proven difficult because that element is partial to octahedral (with tetragonal distortion) environments rather than the tetrahedrally-based geometries of the substituted Al/P atoms. An added complication is the effect of temperature on the interaction between copper and the templating agents necessary to produce the desired AlPO. Attempts to prepare CuAPO-n by Rajić et al.33 were unsuccessful because copper(II) was reduced to metallic copper during their synthesis. The synthesis of CuAPO-11 by Lee et al.34 was also unsuccessful resulting instead in a product similar to that obtained by impregnation. In general, the choice of template as a structure directing agent is specific to the synthesis of a given AlPO4-n, and so it is difficult to incorporate copper for those systems where the metal is complexed by the template. Amine template interactions have been explained in a study on the chemistry of copper(II) in the presence of weak and strongly coordinating anions during syntheses of SAPO-5 and SAPO-11.35 At temperatures above 190 °C copper(II) undergoes a two-step process in which it is reduced to copper(I) followed by disproportionation to metallic copper and copper(II). Successful introduction of tetrahedral copper(II) into AlPO4-5 was first reported by Muñoz et al.36 who suggested that copper substitutes for phosphorus in the framework. These workers used copper oxide as the copper source in order to eliminate possible interacting anions from the gel, and the quaternary amine tetraethylammonium hydroxide (TEAOH) was chosen because this template does not complex with copper(II). The effect of different copper sources on the synthesis of CuAPO-5 is reported to give different copper environments when using the two different copper sources; copper(II) oxide and copper(II) acetate (see below).11,12
Structural and electronic characteristics of the molecular sieve MeAPO-5 and MeSAPO-5 materials make them interesting candidates for catalysis. They are catalytically active in a number of reactions by virtue of their acidic properties and redox behaviour. The acidic properties allow the materials to catalyse reactions like transalkylation, where the strength of the acid site influences competing reactions.37,38 Redox properties make possible the selective oxidation of linear alkanes and cyclohexane.39,40 Impregnated Cu:AlPO-5, Cu:SAPO-5, Cu:SAPO-11 and Cu:SAPO-34 are active in reducing NOx in the presence of CO, while ion-exchanged Cu:MeAPO-n (n = 5, 11, 34) are active for NOx decomposition.13,15–19 Studies on CuAPO-34 and CuSAPO-34 in which copper is introduced during synthesis show that they are active in decomposing N2O.14 It has been shown that the activity of CuAPO-5 in reducing NOx in the presence of ammonia achieves 53% maximum conversion.11 In the reduction of NOx in the presence of propene, CuAPO-5 prepared from copper(II) acetate and impregnated Cu:AlPO4-5 show maximum conversions of 16.8% and 11.8% respectively.12
We report here the results of a combined XAS and XRD study on CuAPO-5 materials which were prepared by varying different synthesis parameters, such as the copper source and Al : P ratio. We have also looked at the effects of using the two copper sources copper(II) oxide and copper(II) acetate because we were unconvinced by previous reports stating that only the former leads to CuAPO-5.33,36 The activity towards the selective catalytic reduction of NOx in the presence of propene and O2 by CuAPO-5 materials as a function of Cu : Al ratio and copper source is also reported.
Experimental
Synthesis of copper-incorporated AlPO-5 molecular sieves
Various samples of CuAPO-5 were synthesised by modifying the method of Muñoz et al.36 Several samples were prepared using both copper oxide and copper acetate as copper sources and by varying the copper content in the synthesis mixture. The effects of the Cu : Al and Al : P ratios on the products were also investigated. The ten different CuAPO-5 samples included in this work are designated CuAPO-5/1 etc. and their gel compositions are given in Table 1.| Sample | Copper source | Molar composition in gel, Cu : Al : P | Elemental analysis | Cu wt.% | BET/m2 g−1 | Micro pore volume/cm3 g−1 |
|---|---|---|---|---|---|---|
| CuAPO-5/1 | CuAc2 | 0.1 : 0.85 : 1 | Cu0.20AlP0.96O4 | 4.27 | 261 | 0.094 |
| CuAPO-5/2 | CuO | 0.1 : 0.85 : 1 | Cu0.25AlP0.94O4 | 5.14 | 271 | 0.097 |
| CuAPO-5/3 | CuO | 0.15 : 0.85 : 1 | Cu0.35AlP0.92O4 | 6.93 | 223 | 0.073 |
| CuAPO-5/4 | CuO | 0.2 : 0.85 : 1 | Cu0.39AlP0.77O4 | 8.15 | 181 | 0.053 |
| CuAPO-5/5 | CuAc2 | 0.04 : 0.89 : 1 | Cu0.09Al0.85PO4 | 2.12 | 254 | 0.099 |
| CuAPO-5/6 | CuAc2 | 0.19 : 0.98 : 1 | Cu0.37Al0.83PO4 | 8.78 | 211 | 0.081 |
| CuAPO-5/7 | CuO | 0.04 : 0.82 : 1 | Cu0.10Al0.89PO4 | 2.23 | 265 | 0.099 |
| CuAPO-5/8 | CuO | 0.16 : 0.71 : 1 | Cu0.39Al0.75PO4 | 8.77 | 216 | 0.080 |
| CuAPO-5/9 | CuAc2 | 0.04 : 1 : 0.96 | Cu0.08AlP0.95O4 | 1.89 | 291 | 0.097 |
| CuAPO-5/10 | CuAc2 | 0.16 : 1 : 0.84 | Cu0.33AlP0.94O4 | 7.02 | 204 | 0.074 |
| CuAPO-5/11 | CuO | 0.04 : 1 : 0.96 | Cu0.07AlP0.91O4 | 1.61 | 252 | 0.053 |
| CuAPO-5/12 | CuO | 0.16 : 1 : 0.84 | Cu0.37AlP0.69O4 | 8.70 | 92 | 0.014 |
In a typical synthesis, the selected amount of copper(II) oxide (CuO, Merck) or copper(II) acetate (Cu(CH3COO)2, Merck) was solvated in a mixture of orthophosphoric acid (H3PO4, 85%) and water. The solution was stirred for five minutes until all the metal salt was dissolved. At this stage pseudoboehmite (AlOOH, BA Chemicals/SASOL) was added and the slurry stirred for about an hour or until the slurry was homogenous. Finally, tetraethylammonium hydroxide (TEAOH, 40%, Aldrich) was added to the homogenous gel and the mixture left under stirring for two hours.
The final gel of pH = 3 was poured into stainless steel autoclaves lined with Teflon and the syntheses carried out at 150 °C for 24 hours. After crystallisation the solid turquoise product with pH = 7 was washed with water, filtered and dried at 80 °C for 15 minutes. The template was removed by heating the dried product in air at 550 °C for 24 hours with a ramp rate of 1 °C min−1. The final product was an olive green crystalline powder.
Characterisation
X-Ray powder diffractograms were measured using a scintillation counter and a Siemens D-5005 diffractometer operated at 50 kV and 40 mA and using Ni-filtered Cu Kα radiation. The diffractograms were collected using a constant slit opening and a step size of 0.03° covering a range of 5–50° 2θ with a counting time of 6 seconds per step. Measurements of pore diameters were performed on an ASAP 2000 Micromeritics surface area and porosimetry analyser at liquid nitrogen temperature. Samples were degassed at 250 °C for 24 hours prior to measurements.The elemental analyses were performed on a Perkin Elmer Optima 3300RL Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES). Scanning electron micrographs were collected on a Zeiss DSM 940 scanning electron microscope and on a Hitachi S-3500N scanning electron microscope.
Thermogravimetric analysis was carried out at the Statoil Laboratory using a Perkin Elmer TGA. Samples were analysed in an unsealed platinum sample pan under constant airflow at 40 ml min−1. Each sample (ca. 6 mg) was heated to 550 °C at a rate of 1 °C min−1, and then held at that temperature for 6 hours while detecting mass-loss as a function of both time and temperature. The samples were also analysed with a faster rate of calcination under the same conditions up to 550 °C with no holding time and a ramp rate of 30 °C min−1.
Catalytic testing
The selective catalytic reduction of NOx in the presence of hydrocarbons (SCR-HC) over calcined CuAPO-5 was followed using a fixed bed microreactor. For comparison a sample prepared by mixing pure AlPO4-5 and CuO to give 5 wt.% of copper was also measured. The catalyst bed surrounded by silica wool was situated inside a 37.4 cm long stainless steel tube. The catalysts were calcined to 500 °C in helium using a ramp rate of 1 °C min−1 before activating in 2% oxygen in helium at the same temperature for 1 hour. The conversions were measured using a reactant gas mixture of 2000 ppm NO, 1200 ppm C3H6, 2% O2 and balance helium for a total gas flow of 300 ml min−1 at selected intervals while reducing the temperature from 500 °C to 275 °C. The catalyst was exposed to the reactant mixture for at least 20 minutes at each temperature to obtain steady state. Output from the reactor was analysed using a gas chromatograph fitted with a thermal conductivity detector (Pye Unicam PU4550) and a chemiluminescent NOx analyser (Signal Instruments Model 4000).X-Ray absorption data collection
XAS data were collected at the Swiss–Norwegian Beamline (BM1B) at the European Synchrotron Radiation Facility (ESRF) in transmission mode. The beamline is equipped with a channel cut Si(111) crystal monochromator, and chromium mirrors were used to reject higher harmonics. The ion chamber detectors were filled with the following detector gases: I0(17 cm), 97% N2 + 3% Ar, and It (31 cm) 50% N2 + 50% Ar.The ESRF provides electron beam energies of 6 GeV, and 200 mA maximum current. The amounts of the materials used were determined empirically. The powdered samples (50–150 mg) were mixed with boron nitride, and placed in aluminium sample holders to obtain a thickness of 1 mm and total cross section of 1.2 cm2. The sample was sealed using Kapton tape windows. Several scans of each sample were collected and summed to increase the signal to noise ratio.
EXAFS data analysis
The XAS data were summed and background subtracted, and the EXAFS part of the spectrum extracted to yield the χiexp(k), using the EXCALIB and EXBACK programs.41 The edge energy, Eo, was determined at the first inflection point using the derivative spectra. The curve fitting of χexp(k) to the theoretical χth(k) was carried out using the curved wave theory, and calculated ab initio phase shifts with EXCURV98.42 The EXAFS spectra were fitted using the k3 weighting scheme. The final data were Fourier filtered over a wide range (1.0–25.0 Å) using EXCURV9842 to remove low-frequency contributions to the EXAFS below 1 Å. This procedure does not smooth the spectrum or remove any noise.Copper(II) oxide,43 copper(II) hydroxide44 and the copper(II) Tutton salt45 [NH4]2[Cu(H2O)6][SO4]2 were the reference and model compounds used to verify the ab initio phase shifts and to establish the amplitude reduction parameter AFAC to be transferred to the analysis of the unknown samples.46 AFAC, sometimes known as S02, represents the average proportion of excitations which contribute to EXAFS since the Hedin–Lundqvist potential option in EXCURV98 was used AFAC should in principal be unity.42 The EXAFS data were collected over two runs, both copper(II) oxide and copper(II) hydroxide were used as model compounds to obtain AFAC, whereas the copper Tutton salt is used as a reference compound. The EXAFS spectra of the model compounds were fitted using k1 and k3 weighting schemes in order to reduce the coupling between the correlated pairs; AFAC and 2σ2 (Debye–Waller factor), and EF (refined correction of Fermi energy) and R (interatomic distance).47 An identical procedure was used for analysing the unknown samples in order to decorrelate N (multiplicities) and 2σ2 in addition to EF and R.
Synchrotron X-ray powder diffraction data collection
The synchrotron X-ray powder diffraction data were also collected at the Swiss–Norwegian Beamline (SNBL, BM1A) at the European Synchrotron Radiation Facility (ESRF) in Grenoble using a 2-circle diffractometer. The diffractometer operates with six detector chains (NaI scintillation counters) that cover the 2θ region, and resolution is enhanced by placing a Si(111) analyser crystal in front of each detector. The calcined samples were sealed in a 1.0 mm diameter capillary on a goniometer mounted on the diffractometer. Data were collected using a wavelength of λ = 0.5000 Å, over the 2θ range 1–32°.Rietveld refinements
The Rietveld program GSAS48 which was used to refine the data minimises the function Mp = Σw(Io − Ic)2 by the least squares method, where Io and Ic are the observed and calculated intensities respectively. Linear interpolation background function number 7 with 30 coefficients and peak profile number 3 for constant wavelength powder data were applied. The profile function was based on pseudo-Voight calculations. The functional pseudo-Voight calculations include 19 parameters, of which three are Gaussian and two are Lorentzian. Only the Lorentzian parameters LX and LY were fitted for all four samples. Diffractometer geometry coefficients S/L and H/L were fixed to 0.002 and 0.005 respectively for all samples, where L is the diffractometer radius and H and S are the sample and detector heights. X-Ray scattering factors and anomalous scattering coefficients are calculated in GSAS from crystallographic tables. Zero offset and histogram scale factors were also refined.Results and discussion
The turquoise colour of the as-synthesised material is a crucial piece of information about the environment of copper(II). The colour (and hence the electronic energy levels) are indicators of the geometry about the metal and the turquoise colour is characteristic of the tetragonally-distorted octahedral [Cu(H2O)6]2+ complex cation. The hexaaquacopper(II) cation is exemplified in the reference copper Tutton salt.45 We know that the colour stems from the as-synthesised product itself and not from a simple mixture of AlPO4-5 and a copper salt because the supernant solution from the hydrothermal synthesis is colourless and also because the aqueous washings from the filtered material are colourless. It follows that the hexaaquacopper(II) cation is attached to a counterion that is an integral part of the AlPO4-5 lattice. Thus the chemistry shows that copper is present as the complex hexaaquacopper(II) cation which is held in place by electrostatic forces to anionic positions within the AlPO4-5 structure. Hence, copper in as-synthesised CuAPO-5 is extraframework.On the basis of these observations, we suggest that phosphate groups at the internal surfaces (the pores) of as-synthesised CuAPO-5 contain one (or two) P–O− with the other three (or two) P–O− bonds linking Al to P thereby constituting part of the AlPO4-5 lattice. The hexaaquacopper(II) cations are kept in place (extraframework) by electrostatic attractions to the anionic sites, for example:
| […(Al–O–)3PO]−2 [Cu(H2O)6]2+ |
| […(Al–O–)2PO2]2− [Cu(H2O)6]2+ |
Calcining the as-synthesised material burns off the template and removes water, including the water ligands of hexaaquacopper(II) complex cation. The process is accompanied by the copper(II) environment rearranging. The green colour of the calcined material indicates tetrahedrally coordinated copper,49 the metal being incorporated into the framework by bonding to phosphate oxygen atoms. The net negative charges create the Brønsted acid sites necessary for catalysis.
X-Ray powder diffractograms of samples CuAPO-5/1 and CuAPO-5/2 (Fig. 1) exhibit good crystallinity and confirm the AlPO4-5 structure. Provided that a slow heating rate (1 °C min−1) is used, the materials are thermally stable up to 550 °C. Higher ramp rates (e.g. 20 °C min−1) often lead to the calcined material being grey instead of green. The grey material contains copper(II) oxide seen as extra peaks in the X-ray diffractograms. Evidently high ramp rates can cause copper to be expelled from the structure. The samples collapse around 800 °C into a mixture of the dense berlinite form of AlPO4 and copper(II) oxide. In comparison, pure AlPO4-5 collapses at 1100 °C. The stability of CuAPO-5 is clearly diminished by the incorporation of copper into the framework. Table 1 contains the properties and results of the elemental analyses. Elemental analyses show that increasing the copper content in the gel also increases the copper content in the final product up to 8.77 wt.% copper and that the phosphorus to aluminium ratio in the final product correlates with the molar ratios in the synthesis gel. For all samples, except for samples with small Cu/T ratios in the gel, the Cu + Al = P, or Cu + P = Al relationships are not fulfilled with the copper content being generally larger than the reduction in either Al or P. The relative excess of copper may indicate the presence of some extraframework metal additional to that in the framework. Measured surface areas and micropore volumes for the CuAPO-5 materials are given in Table 1. In the case of microporous materials the measured surface area includes pore filling, and it can be seen that increasing the copper content (and thereby reducing the crystallinity) decreases the surface areas and volumes measured.
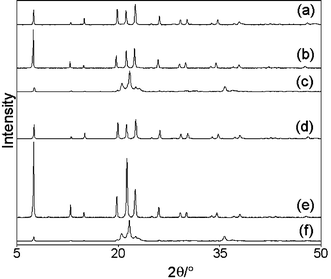 | ||
| Fig. 1 X-Ray powder diffractograms of CuAPO-5/1: (a) as-synthesised; (b) calcined at 550 °C; (c) calcined at 800 °C, and CuAPO-5/2: (d) as-synthesised; (e) calcined at 550 °C; (f) calcined at 800 °C. | ||
Scanning electron micrographs of CuAPO-5/1, CuAPO-5/5 and CuAPO-5/7 are shown in Fig. 2. The sample prepared from copper(II) acetate consists of particles that are very similar to those reported previously for CuAPO-5 prepared from copper(II) oxide. They are in the form of spherical 10–20 µm particles that are agglomerated from hexagonal crystallites.11 The lower copper-content samples 5 and 7 prepared from copper acetate and copper oxide, respectively, show the same appearance but with smaller spheres. The thermogravimetric graphs for CuAPO-5 prepared from either copper(II) acetate or copper(II) oxide, heated to 1000 °C at 1 °C min−1 (Fig. 3) show the characteristic events associated with mass loss due to dehydration and template removal. Mass losses for the samples prepared from copper(II) acetate or copper(II) oxide due to dehydration (90–100 °C) and template removal (250–500 °C) were calculated from the derivative spectra to be 8.7/4.9% and 9.0/10.3% respectively. Structural collapse between 550 °C and 800 °C is not accompanied by mass loss. The effect of the calcination ramp rate on a sample derived from copper(II) acetate is shown in Fig. 4. Mass loss due to dehydration occurs at a lower temperature at the slow heating rate. The total mass losses are independent of the heating rate, but the calcined samples heated at the slow rate (1 °C min−1) are olive green, whilst samples heated at 30 °C min−1 are grey. For both the as-synthesised and the calcined material the ability of copper to complex with ligands stronger than water was tested by adding ammonia to their aqueous suspensions. The result for both materials was the intense dark blue colour of the aqueous layer characteristic of the tetraamminecopper(II) complex, while the colour of the precipitated material remained unchanged. The colour was more intense in the as-synthesised sample. Clearly, water ligands are exchanged by ammonia in the copper complex and in the case of copper being held in place electrostatically, the tetraammine complex can then be ion-exchanged by the ammonium cations present in the solution. Copper is thereby detached from the frameworks of as-synthesised and calcined CuAPO-5 but to a larger degree in the former. Evidently in calcined CuAPO-5, copper is present in both framework and extraframework sites.
 | ||
| Fig. 2 Scanning electron micrographs of CuAPO-5/1 prepared from copper(II) acetate (top); CuAPO-5/5 prepared from copper acetate (bottom left) and CuAPO-5/7 prepared from copper oxide (bottom right). | ||
 | ||
| Fig. 3 Thermogravimetric curves for CuAPO-5 materials made from copper(II) acetate (—) and made from copper(II) oxide (- -). | ||
 | ||
| Fig. 4 Thermogravimetric curves for CuAPO-5 made from copper(II) acetate heated at 1 °C min−1 (—) and 30 °C min−1 (–). | ||
NOx conversion
All of the calcined CuAPO-5 products tested here selectively catalyse the reduction of NOx in the presence of a propene–oxygen mixture. The sample prepared by mixing AlPO4-5 and CuO did not show any activity at all under above described conditions. Clearly, the introduction of copper to the AlPO4-5 lattice improves the activity for selective catalytic reduction of NOx. The NOx conversion and analyses of gas outlet during measurements on CuAPO-5/1, CuAPO-5/2, CuAPO-5/3 and CuAPO5/4 samples are shown in Fig. 5 and 6, with the maximum conversions of NOx and propene being given in Table 2. Maximum conversion did not exceed 18% for any of the samples in the temperature region analysed. As a comparison CuAPO-5 prepared from copper(II) oxide shows 27% conversion of NOx in the presence of ammonia.11 The conversion profiles are similar for all four samples, with sample 2 showing the highest maximum conversion: 18% at 475 °C. For samples 1, 3 and 4 the maximum conversions are 15.7, 15.6 and 15.9% respectively. | ||
| Fig. 5 NOx conversion numbers for calcined copper incorporated AlPO4-5 materials: CuAPO-5/1 (—);CuAPO-5/2 (●);CuAPO-5/3 (■); CuAPO-5/4 (●). | ||
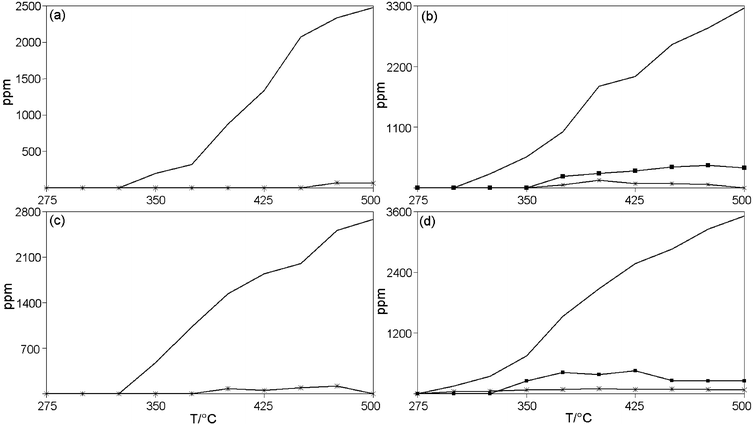 | ||
| Fig. 6 Produced gas from reaction over: (a) CuAPO-5/1; (b) CuAPO-5/2; (c) CuAPO-5/3 and (d) CuAPO-5/4 at different temperatures; CO2 (—), N2O (*) and N2 (■). | ||
| Sample | NOx conv. (%) | T/°C | C3H6 conv. (%) |
|---|---|---|---|
| CuAPO-5/1 | 15.7 | 425 | 68.8 |
| CuAPO-5/2 | 18.0 | 475 | 90.7 |
| CuAPO-5/3 | 15.6 | 425 | 74.5 |
| CuAPO-5/4 | 15.9 | 450 | 97.6 |
These maximum conversions are significantly higher than that reported for Cu:AlPO4-5 (impregnation by incipient wetness).12 It is interesting that the material with significantly higher copper content does not exhibit a corresponding higher activity. From this it is evident that copper inhabits both active and nonactive sites in CuAPO-5 with the latter dominating. The active sites could in this case be sites where copper can interact with reactants. After filling the active sites the nonactive sites continue to accept copper. This means that after a certain limit where both are being filled, copper uptake is now exclusive to the nonactive sites. The conversion of propene during reaction is lower for samples 1 and 3 indicating higher selectivity for these two samples. NO is reduced at 350 °C to form N2 and N2O over samples 2 and 4 (Fig. 6). For CuAPO-5/1 and CuAPO-5/3 NOx is reduced to form small amounts of N2O at very high temperatures. Samples 2 and 4 also show significantly higher C3H6 conversions than the two other samples for the same conversions, which means these two samples are less selective in the reduction of NOx. The converted NO is not all accounted for in the produced N2O and N2. This indicates that NOx is also adsorbed on acid sites at the catalyst surface. The catalytic activities of other MeAPO-5 materials have been reported and the strong acidity of CoAPO-5 and MnAPO-5 has been ascribed to equilibrium between Brønsted and strong Lewis sites.20,21 Following this, it seems likely that the catalytic activity of CuAPO-5 is also governed by the number of acid sites. In another study, we have investigated the oxidation state of copper in CuAPO-5 during the selective catalytic reduction of NOx using an in-situ cell.50 Since propene reduces only a small fraction of copper(II) to copper(I) these results suggest that acidity also plays a role in the deNOx activity of CuAPO-5. The EXAFS of CuAPO-5 samples after catalytic measurements show no changes. This is consistent with copper remaining in the framework.
XANES
Fig. 7 shows the normalised Cu K-edge XANES and their first derivatives of CuAPO-5 materials compared to copper(I) and copper(II) oxides in addition to the Cu-Tutton salt. XANES spectra are used for distinguishing between CuI and CuII as the former exhibit a strong pre-edge a few eV below the edge.51,52 In accordance with previous findings calcination does not lead to autoreduction of copper in this system.11 All samples show the low intensity pre-edge feature at about 8978–8979 eV that is associated with the formally forbidden 1s → 3d transition characteristic of copper(II) compounds. The low intensity of this feature shows that the copper environment is centrosymmetric, for example tetragonally-distorted octahedral. This feature is clearly evident in the first derivative spectra, and is also seen for the reference copper(II)-Tutton salt. The shoulder seen in the normalised spectrum of copper(II) oxide (Fig. 7) is absent in the spectra of the CuAPO-5 samples. The XANES together with the EXAFS and X-ray powder diffraction (see below) show that calcining CuAPO-5 at 550 °C does not produce copper(II) oxide. Evidently, copper is an integral part of the AlPO4-5 system as calcination does not cause copper to be ejected at this temperature, rather the extraframework copper is homogeneously incorporated into the lattice. | ||
| Fig. 7 Normalised Cu K-edge XANES and first derivatives of (a) CuAPO-5/1 as-synthesised; (b) CuAPO-5/1 calcined; (c) CuAPO-5/2 as-synthesised; (d) CuAPO-5/2 calcined; (e) CuAPO-5/3 as-synthesised; (f) CuAPO-5/3 calcined; (g) CuAPO-5/4 as-synthesised; (h) CuAPO-5/4 calcined; (i) Tutton-salt; (j) copper(II) oxide and (k) copper(I) oxide. | ||
EXAFS analysis
The k3-weighted and least squares fitted EXAFS spectra of the model compounds copper(II) oxide, copper(II) hydroxide and the copper(II) Tutton salt are shown in Fig. 8. The EXAFS results (Table 3) of the model compounds agree with the crystallographic data. Copper(II) oxide and copper(II) hydroxide have two Cu–O distances at 2.78 Å and 2.63 Å respectively. These represent the elongated Cu–O bonds that make up the tetragonally-distorted octahedral environment. The peak due to these long bonds is not apparent in the Fourier transforms of the two models and a reasonable fit could not be obtained including the axial bonds. These attempts lead to a high Debye–Waller factor, which did not affect the AFAC value to be transferred to the unknown samples. In the copper-Tutton salt copper is surrounded by six water molecules with the two axial Cu–O bonds being shorter than in copper(II) oxide and copper(II) hydroxide. There is no apparent peak representing the axial bonds in the EXAFS spectra, and they cannot be resolved from the four Cu–O equatorial bonds, however this peak was fitted in the EXAFS to give a reasonable result. | ||
| Fig. 8 Experimental (—) and calculated (- -) Fourier filtered (1–25) k3-weighted EXAFS and its Fourier transform for (a) copper(II) oxide; (b) copper(II) hydroxide; (c) copper(II)-Tutton salt. | ||
| Model | Shell | N b | r/Å | r/Å XRDb | 2σ2/Å2 | FId | R e (%) | E F/eV |
|---|---|---|---|---|---|---|---|---|
| a The EXAFS refinements give information about multiplicity (N), bonding distance (R) and thermal vibration (Debye–Waller factor, 2σ2). EF is the refined correction of Fermi energy in vacuum, compared to E0 in EXBACK. b The fixed multiplicities and crystallographic distances are taken from references. The standard deviation in the last significant digit as calculated by EXCURV98 is given in parentheses. These estimates, however, will in cases of high correlation between parameters lead to an overestimation of accuracy as the standard deviations for bonding distances are ±0.01 Å for small r-values and ±0.04 Å for r-values exceeding 3 Å. The deviation for 2σ2 is ±20%. c Not fitted. d Fit index is defined as FI = Σi(1/σi)[Exp(i) − Theory(i)]2. e The statistical R-factor is defined as R = ΣiN [1/σi (|χiexp(k) − χith(k)|)] × 100% and gives indication of the quality of fit in k-space. | ||||||||
| CuO | Cu–O | 4.0 | 1.959(6) | 1.96 | 0.008(1) | 7.26 | 38.22 | 0.84 |
| Cu–O | 2.0 | — | 2.78c | — | ||||
| Cu⋯Cu | 8.0 | 2.929(9) | 2.90 | 0.027(2) | ||||
| Cu⋯Cu | 2.0 | 3.128(7) | 3.08 | 0.007(1) | ||||
| Cu⋯Cu | 2.0 | 3.44(5) | 3.42 | 0.03(1) | ||||
| Cu(OH)2 | Cu–O | 4.0 | 1.954(4) | 1.94 | 0.0066(9) | 3.90 | 24.77 | −1.79 |
| Cu–O | 2.0 | — | 2.63c | — | ||||
| Cu⋯Cu | 2.0 | 2.949(7) | 2.95 | 0.015(2) | ||||
| Cu⋯Cu | 4.0 | 3.32(3) | 3.34 | 0.05(1) | ||||
| Cu(NH4)2(SO4)2·6H2O | Cu–O | 4.0 | 1.980(5) | 2.02 | 0.0153(8) | 4.04 | 25.57 | 4.30 |
| (Cu-Tutton) | Cu–O | 2.0 | 2.25(1) | 2.23 | 0.023(4) | |||
The spectra for the various CuAPO-5 products made from the two different copper sources and varying copper contents are shown in Fig. 9. All of the CuAPO-5 samples show the same characteristic peaks in both the as-synthesised and the calcined state with two distinct peaks in the as-synthesised spectra where the second peak at about 3.1 Å is reduced upon calcination. Results from the least square analyses are listed in Table 4, including interatomic distances, Debye–Waller factors (2σ2) and multiplicities for the CuAPO-5 samples. The as-synthesised samples have five Cu–O distances at about 1.94 Å and two Cu–T distances at about 3.1 Å. The value of N was close to five for all the samples. Different coordinations of copper in CuAPO-5 were also reported by Muñoz et al.36 on the basis on electron spin resonance (ESR) and electron spin-echo modulation (ESEM). Copper adopts four, five or six-coordination depending on the number of extraframework species. A copper complex confined within the pores is ready to interact with both framework and extraframework oxygen atoms. The EXAFS for the as-synthesised CuAPO-5 and copper Tutton salt are comparable, although the refined Cu–O bonds in the latter are somewhat longer.
 | ||
| Fig. 9 Experimental (—) and calculated (- -) Fourier filtered (1–25) k3-weighted EXAFS and its Fourier transform for: CuAPO-5/1 made from copper acetate with Cu/Al = 0.1 and Al/P = 0.85 (a) as-synthesised, (b) calcined; CuAPO-5/2 made from copper oxide with Cu/Al = 0.1 and Al/P = 0.85 (c) as-synthesised, (d) calcined; CuAPO-5/4 made from copper oxide with Cu/Al = 0.2 and Al/P = 0.85 (e) as-synthesised, (f) calcined. | ||
| Sample | Shell | N b | r/Å | 2σ2/Å2 | FI | R (%) | E F/eV | |
|---|---|---|---|---|---|---|---|---|
| a See footnote a of Table 3 for details. b The multiplicities are fixed to the nearest integer in the final refinements. c It is not possible to distinguish between Al and P in the composite peak. | ||||||||
| CuAPO-5/1 | As-synth. | Cu–O | 5.0 | 1.947(4) | 0.0111(6) | 3.17 | 21.24 | −1.35 |
| Cu⋯Tc | 2.0 | 3.175(9) | 0.011(2) | |||||
| Calc. | Cu–O | 5.0 | 1.945(5) | 0.0143(9) | 4.65 | 28.09 | −0.09 | |
| CuAPO-5/2 | As-synth. | Cu–O | 5.0 | 1.948(4) | 0.0108(7) | 4.29 | 27.45 | −1.39 |
| Cu⋯Tc | 2.0 | 3.18(1) | 0.013(3) | |||||
| Calc. | Cu–O | 5.0 | 1.947(5) | 0.0141(9) | 5.68 | 30.37 | −0.45 | |
| CuAPO-5/3 | As-synth. | Cu–O | 5.0 | 1.943(4) | 0.0102(6) | 3.03 | 23.63 | −0.20 |
| Cu⋯Tc | 2.0 | 3.12(2) | 0.017(3) | |||||
| Calc. | Cu–O | 5.0 | 1.946(4) | 0.0139(8) | 3.98 | 25.54 | −3.70 | |
| CuAPO-5/4 | As-synth. | Cu–O | 5.0 | 1.949(3) | 0.0111(6) | 3.20 | 18.69 | −0.77 |
| Cu⋯Tc | 2.0 | 3.11(1) | 0.017(3) | |||||
| Calc. | Cu–O | 5.0 | 1.942(4) | 0.0133(8) | 4.12 | 27.87 | 1.35 | |
Fourier filtering indicates that the second peak at 3.1 Å is a composite of several peaks and the best fit was obtained using one or two Cu⋯T distances. Distinguishing P from Al by EXAFS is impossible because the backscattered waves are almost identical. Therefore T is used to designate both Al and P. The calcined samples show five Cu–O distances ranging between 1.94–1.95 Å. The second peak at 3.1 Å is reduced in all of the calcined samples, and no reasonable fit could be obtained. It has been suggested,53 and supported in reference 11, that the presence of a second Cu⋯T peak at 3.1 Å in the EXAFS of a zeotype lattice is a diagnostic test of framework substitution. The reduction in intensity of this peak upon calcination can be rationalised in terms of the homogeneity of the as-synthesised extraframework copper environment being altered as copper enters framework sites of different symmetries. The resulting Cu⋯T distances are close and overlapping within a low intensity broad convolution. This is also supported by the colour of the as-synthesised and calcined materials, and also from the washings with ammonia.
Previous EXAFS studies on CuAPO-5 prepared from copper(II) oxide11 show that copper is present in tetragonally-distorted octahedra with four Cu–O distances at 1.92 Å and four Cu⋯T distances at 3.13 Å for the as-synthesised sample. Upon calcination the environment changes to a nontetragonally-distorted octahedron having four Cu–O distances at 1.94 Å and two elongated axial bonds at 2.15 Å in addition to the four Cu⋯T distance at 3.17 Å. These studies suggested that copper is fully integrated into the framework of calcined CuAPO-5.11 The grey of the calcined material was suggested to be consistent with the incorporation of copper into the framework. The copper environment would then be degraded to some unspecified geometry.
In the present studies, we find longer Cu–O distances for the first shell, and lower multiplicities for the second shell. We find that the colour of the calcined material depends on whether the calcination ramp rate is slow (1 °C min−1; green) or fast (20 °C min−1; grey), respectively. Also the slow ramp rate used here led to all the calcined materials being olive green or green as initially reported by Muñoz et al.36 Previous attempts to prepare CuAPO-5 from copper(II) acetate were unsuccessful, suggesting that it can not be used as a copper source.12,36 However, from our results we conclude that both copper(II) oxide and copper(II) acetate are equivalent as copper sources in the synthesis of CuAPO-5.
Rietveld refinement
Two models were used in the Rietveld refinements on CuAPO-5, both being based on the known AlPO4-5 structure (see below). The structure was refined with copper constrained to framework positions in order to establish the space group of the material. These refinements should also reveal copper through lengthened average Al/P–O distances, since these include longer Cu–O distances. The small amounts of copper preclude finding unequivocally the actual copper positions. The starting points for the structural analysis are the atomic positions reported by Bennet et al.54 and Richardson et al.55 The former workers assigned space group P6cc to as-synthesised AlPO4-5 which distinguishes between the Al and P positions, whereas the latter work assigned the higher symmetry space group P6/mcc to calcined AlPO4-5 which does not distinguish between Al and P (given the common designation: T). Subsequently Mora et al.56 found that the fourth peak in calcined AlPO-5 is split reflecting a lowering of the symmetry of from hexagonal to orthorhombic (Pcc2). This splitting (peak at 6.35° in Fig. 10) is not observed for the present CuAPO-5 materials. Computer simulations of AlPO4-5 by Hanson et al.57 match the hexagonal space group P6, but also reveal that the differences between P6 and Pcc2 are actually very small. In order to establish whether or not Al and P can be distinguished from each other, the present data were fitted in the space groups P6/mcc and P6cc. One copper atom per unit cell (corresponding to the fraction 0.08 copper) replaces in the first case a T atom and in the second case an Al or a P atom. The very weak peak at 3.1° (Fig. 11) contained in all diffractograms reveals traces of a second phase. This phase is attributed to AlPO-34 (CHA framework type). Samples 1 and 4 also contain trace amounts of a dense tridymite-like phase manifested as extraneous peaks in Fig. 10. Only the major phase was refined. | ||
| Fig. 10 Section of diffraction patterns for calcined copper incorporated AlPO4-5 materials: (a) CuAPO-5/1; (b) CuAPO-5/2; (c) CuAPO-5/3 and (d) CuAPO-5/4. | ||
 | ||
| Fig. 11 Diffraction patterns for calcined CuAPO-5 materials: (a) CuAPO-5/1; (b) CuAPO-5/2; (c) CuAPO-5/3 and (d) CuAPO-5/4 (from top to bottom). Materials are fitted to space group P6/mcc where solid line is theoretical curve, and dotted line experimental curve. Peak positions and differential curve showed at bottom. The insets show magnified patterns from 10 to 32°. | ||
Refined structural parameters for the calcined CuAPO-5 materials fitted to space group P6/mcc are shown and defined in Tables 5 to 7 with the observed and experimental diffraction patterns shown in Fig. 11. The atomic positions and thermal factors were constrained for T and Cu (assumed to replace T in framework). For sample 4, with a higher copper content, additional copper was refined to an extraframework site. (The Cu2–Cu2 distances shown in Table 7 of course do not reflect actual metal distances since copper is fractionally placed at these sites.) The unit cell volume for sample 4 is actually less than those of the other samples (Table 5). This is due to a reduction of the unit cell in the a/b direction. Up to a certain limit copper can be introduced without degrading the crystallinity (Fig. 11). Copper in excess of this limit diminishes the structural order and is identified with nonactive copper sites. The structures of the zeotypes are very flexible so the decreased volume of the material containing excess copper is envisaged as being due to Cu(O–P−)4 units arranging themselves in a manner that tightens the network across the a/b direction. Additional information is given by the EXAFS, which shows that there is no copper clustering, hence the metal atoms are widely dispersed. The refined bond distances and angles are shown in Table 7, and the T–O distances are similar for all samples, apart from sample 4 which contains the most copper where the average T–O distance is longer. The T–O–T angles are also smaller for sample 4 than the angles refined for the other samples.
| Parameters | CuAPO-5/1 | CuAPO-5/2 | CuAPO-5/3 | CuAPO-5/4 |
|---|---|---|---|---|
| a R p = Σ|Io − Ic|/ΣIo. b R wp = (Mp/ΣwIo2)1/2. c χ 2 = Mp/Nobs − Nvar. | ||||
| R p a (%) | 5.63 | 4.02 | 2.73 | 2.68 |
| R wp b (%) | 8.67 | 5.04 | 3.78 | 3.67 |
| Reduced χ2c | 32.23 | 10.76 | 20.76 | 5.34 |
| a,b/Å | 13.7613(3) | 13.7482(3) | 13.7566(2) | 13.6996(5) |
| c/Å | 8.3777(2) | 8.3742(2) | 8.3772(1) | 8.3659(3) |
| Unit cell volume/Å3 | 1373.96(7) | 1370.78(6) | 1372.94(5) | 1359.75(11) |
| No. of variables | 49 | 49 | 49 | 58 |
| No. of Bragg reflections | 315 | 385 | 385 | 390 |
| Range of 2θ/° | 1.5–30 | 1.5–32 | 1.5–32 | 2–32 |
| No. of observations | 5699 | 6099 | 6099 | 5999 |
| No. of background parameters | 30 | 30 | 30 | 35 |
| LX | 0.83(3) | 1.07(2) | 1.02(2) | 1.08(3) |
| LY | 34.1(6) | 39.5(5) | 33.7(4) | 55.4(8) |
| Zero offset/° | 0.207(11) | 0.203(9) | 0.313(7) | 0.09(1) |
| Histogram scale factor | 1.400(7) | 0.969(3) | 1.803(6) | 1.298(14) |
| Atom | x | y | z | U | F a |
|---|---|---|---|---|---|
| a Not refined. | |||||
| CuAPO-5/1 | |||||
| T | 0.4562(4) | 0.3355(4) | 0.8116(3) | 4.3(1) | 0.92 |
| O1 | 0.2105(4) | 0.4210(8) | 0.2500 | 3.6(5) | 1.00 |
| O2 | 0.4474(10) | 0.3274(12) | 0.00 | 6.9(3) | 1.00 |
| O3 | 0.3615(10) | 0.00 | 0.2500 | 1.8(7) | 1.00 |
| O4 | 0.5788(5) | 0.1576(11) | 0.2500 | 4.9(8) | 1.00 |
| Cu1 | 0.4562(4) | 0.3355(4) | 0.8116(3) | 4.3(1) | 0.08 |
| CuAPO-5/2 | |||||
| T | 0.4559(3) | 0.3341(3) | 0.8119(2) | 4.24(8) | 0.92 |
| O1 | 0.2098(3) | 0.4196(6) | 0.2500 | 3.9(3) | 1.00 |
| O2 | 0.4465(8) | 0.327(1) | 0.00 | 6.7(2) | 1.00 |
| O3 | 0.3604(8) | 0.00 | 0.2500 | 2.3(5) | 1.00 |
| O4 | 0.5762(4) | 0.1524(7) | 0.2500 | 4.4(6) | 1.00 |
| Cu1 | 0.4559(3) | 0.3341(3) | 0.8119(2) | 4.24(8) | 0.08 |
| CuAPO-5/3 | |||||
| T | 0.4561(3) | 0.3340(3) | 0.8129(2) | 4.67(8) | 0.92 |
| O1 | 0.2102(3) | 0.4204(6) | 0.2500 | 4.5(4) | 1.00 |
| O2 | 0.4489(7) | 0.3261(8) | 0.00 | 6.1(2) | 1.00 |
| O3 | 0.3623(7) | 0.00 | 0.2500 | 1.8(5) | 1.00 |
| O4 | 0.5758(4) | 0.1516(8) | 0.2500 | 5.9(6) | 1.00 |
| Cu | 0.4561(3) | 0.3340(3) | 0.8129(2) | 4.67(8) | 0.08 |
| CuAPO-5/4 | |||||
| T | 0.4559(6) | 0.3319(5) | 0.8095(6) | 8.4(3) | 0.92 |
| O1 | 0.2004(6) | 0.4009(13) | 0.2500 | 10.6(11) | 1.00 |
| O2 | 0.4501(17) | 0.3529(19) | 0.00 | 7.8(6) | 1.00 |
| O3 | 0.3301(11) | 0.00 | 0.2500 | 3.4(6) | 1.00 |
| O4 | 0.5824(5) | 0.1649(19) | 0.2500 | 6.9(11) | 1.00 |
| Cu1 | 0.4559(6) | 0.3319(5) | 0.8095(6) | 8.4(3) | 0.08 |
| Cu2 | 0.1355(22) | 0.1373(20) | 0.0866(27) | 1.3(12) | 0.08 |
| Al/P–O distance | T–T distance | T–O–T angle | |||
|---|---|---|---|---|---|
| CuAPO-5/1 fitted to P6/mcc | |||||
| T–O1 | 1.621(6) | T–T | 3.156(5) | T–O1–T | 150.0(9) |
| T–O2 | 1.5823(29) | T–T | 3.130(10) | T–O2–T | 171.6(11) |
| T–O3 | 1.600(6) | T–T/Cu | 3.057(10) | T–O3–T | 145.7(12) |
| T–O4 | 1.586(6) | T–T/Cu | 3.047(9) | T–O4–T | 147.8(11) |
| Mean | 1.597 | Mean | 3.089 | ||
| CuAPO-5/2 fitted to P6/mcc | |||||
| T–O1 | 1.608(4) | T–T | 3.151(4) | T–O1–T | 148.9(7) |
| T–O2 | 1.5795(21) | T–T | 3.097(7) | T–O2–T | 171.7(8) |
| T–O3 | 1.611(4) | T–T/Cu | 3.079(7) | T–O3–T | 145.7(9) |
| T–O4 | 1.577(4) | T–T/Cu | 3.068(7) | T–O4–T | 153.3(8) |
| Mean | 1.594 | 3.099 | |||
| CuAPO-5/3 fitted to P6/mcc | |||||
| T–O1 | 1.607(4) | T–T | 3.135(4) | T–O1–T | 149.3(6) |
| T–O2 | 1.5711(19) | T–T | 3.100(6) | T–O2–T | 172.3(7) |
| T–O3 | 1.611(4) | T–T/Cu | 3.094(6) | T–O3–T | 147.5(8) |
| T–O4 | 1.576(6) | T–T/Cu | 3.073(8) | T–O4–T | 154.2(7) |
| Mean | 1.591 | 3.101 | |||
| CuAPO-5/4 fitted to P6/mcc | |||||
| T–O1 | 1.644(7) | T–T | 3.188(10) | T–O1–T | 133.2(13) |
| T–O2 | 1.629(7) | T–T | 3.018(13) | T–O2–T | 156.3(19) |
| T–O3 | 1.782(9) | T–T/Cu1 | 3.106(15) | T–O3–T | 121.2(9) |
| T–O4 | 1.611(12) | T–T/Cu1 | 3.072(14) | T–O4–T | 145.0(18) |
| Mean | 1.667 | Mean | 3.096 | ||
| Cu2–Cu2 | 1.869(17) | Cu2–Cu2–Cu2 | 120.000(0) | ||
| Cu2–Cu2 | 2.365(32) | 113.28(28) | |||
| Cu2–Cu2 | 1.45(4) | 90.000(0) | |||
| Cu2–Cu2 | 2.73(4) | 37.8(9) | |||
| 52.2(9) | |||||
| 86.4(12) | |||||
Two refinements in P6cc were carried out with the positional and thermal parameters of copper being constrained in the two framework positions (Al or P). The origin was defined by fixing the z-parameter for oxygen number 2 to ¼. The differences in R-factors for the two models in which copper replaces either Al or P were insignificant. The best fit for each sample is shown in Tables 8 and 9. It can be seen that the R-factors obtained for samples 1, 2 and 3 are similar to those obtained from corresponding refinements in P6/mcc, while the fit for sample 4 was unsatisfactory. However, negative or near-zero thermal factors were obtained when substituting Al or P by copper, and unconstrained refinements of copper were unsuccessful. These refinements in P6cc indicate that CuAPO-5 belongs to the higher space group P6/mcc. Naturally, a consequence of incorporating copper into the lattice of the calcined materials is a lowering of symmetry.
| Parameters | CuAPO-5/1 | CuAPO-5/2 | CuAPO-5/3 |
|---|---|---|---|
| a See footnotes in Table 5 for details. | |||
| Site | Al | P | P |
| R p (%) | 5.64 | 3.87 | 2.72 |
| R wp (%) | 8.74 | 4.87 | 5.26 |
| Reduced χ2 | 32.75 | 10.708 | 20.22 |
| a,b/Å | 13.7615(3) | 13.7492(3) | 13.7571(2) |
| c/Å | 8.3778(2) | 8.3743(2) | 8.3774(1) |
| Unit cell volume/Å3 | 1374.04(7) | 1370.99(6) | 1373.07(5) |
| No. of variables | 59 | 99 | 64 |
| No. of Bragg reflections | 315 | 378 | 385 |
| Range of 2θ/° | 1.5–30 | 1.5–32 | 1.5–32 |
| No. of observations | 5699 | 6099 | 6099 |
| No. of background parameters | 30 | 30 | 34 |
| LX | 0.84(3) | 1.04(2) | 1.04(2) |
| LY | 33.5(6) | 39.8(5) | 32.4(7) |
| Zero offset/° | 0.209(12) | 0.223(9) | 0.326(7) |
| Histogram scale factor | 1.423(9) | 0.964(4) | 1.843(7) |
| Atom | x | y | z | U × 100 | F |
|---|---|---|---|---|---|
| CuAPO-5/1/Cu in Al site | |||||
| P | 0.4548(10) | 0.3271(10) | 0.0314(27) | 5.6(4) | 0.92 |
| Al | 0.4560(9) | 0.3394(8) | 0.4087(26) | 2.6(3) | 1.00 |
| O1 | 0.4229(9) | 0.1950(15) | −0.0190(34) | 3.0(5) | 1.00 |
| O2 | 0.4455(12) | 0.3318(13) | 0.2500 | 4.3(5) | 1.00 |
| O3 | 0.3671(13) | 0.3635(13) | −0.0598(37) | −1.1(7) | 1.00 |
| O4 | 0.5806(18) | 0.4418(18) | −0.0677(41) | 5.5(7) | 1.00 |
| Cu1 | 0.4560(9) | 0.3394(8) | 0.4087(26) | 2.6(3) | 0.08 |
| CuAPO-5/2/Cu in P site | |||||
| P | 0.4623(7) | 0.3349(6) | 0.0942(15) | 3.4(2) | 0.92 |
| Al | 0.4479(9) | 0.3370(7) | 0.4748(15) | 4.5(3) | 1.00 |
| O1 | 0.4226(6) | 0.2093(11) | 0.0850(25) | 5.1(4) | 1.00 |
| O2 | 0.4382(9) | 0.3157(10) | 0.2500 | 3.8(5) | 1.00 |
| O3 | 0.3675(10) | 0.3651(11) | 0.0569(27) | −0.5(3) | 1.00 |
| O4 | 0.5587(9) | 0.4069(11) | 0.0200(22) | 3.7(4) | 1.00 |
| Cu1 | 0.4623(7) | 0.3349(6) | 0.0942(15) | 3.4(2) | 0.08 |
| CuAPO-5/3/Cu in P site | |||||
| P | 0.4545(6) | 0.3355(7) | 0.0791(17) | 3.7(2) | 0.92 |
| Al | 0.4582(8) | 0.3307(10) | 0.4553(17) | 5.8(3) | 1.00 |
| O1 | 0.4210(7) | 0.2022(11) | 0.0976(38) | 4.4(4) | 1.00 |
| O2 | 0.4468(8) | 0.3266(10) | 0.2500 | 6.6(3) | 1.00 |
| O3 | 0.3653(7) | 0.3665(7) | 0.0605(32) | −0.1(3) | 1.00 |
| O4 | 0.5967(13) | 0.4037(15) | 0.0280(34) | 3.9(7) | 1.00 |
| Cu1 | 0.4545(6) | 0.3355(7) | 0.0791(17) | 3.7(2) | 0.08 |
Dependence on copper source
Earlier attempts to prepare various CuAPO-n materials33,34 were unsuccessful until Muñoz et al.36 reported that copper can be introduced into the framework of AlPO4-5 using copper(II) oxide and tetraethylammonium hydroxide as noncomplexing template. Although in previous work it appeared that the product depends on the source of copper33,36 it is clear from the present work that the final product is actually independent of whether copper(II) oxide or copper(II) acetate is used in the synthesis. Both sources give the AlPO4-5 structure with good crystallinity (Fig. 1). This is hardly surprising from a chemical viewpoint. The same conclusion is supported by the deNOx conversions measured for CuAPO-5 samples 1 and 2. The EXAFS shows that the products stemming from both sources lead to the same environment about copper, with four or five Cu–O distanced at 1.95 Å and one or two Cu⋯T distances at 3.1 Å. This is not in accordance with previous studies indicating that using copper acetate would not lead to copper being incorporated into the framework, whereas copper(II) oxide would.11,12The present work shows that both sources in fact lead to CuAPO-5. Conceivably the buffering effect of acetate ions in the synthesis gel might influence the final product. However, previous studies on the synthesis parameters of MeAPO-5 show that the addition of acetate results in no change in the pH of the initial and final gel.58 In these studies the pH of the final gel was 3, and also no changes in pH were observed for the synthesis gels where acetate was added. The relatively small amounts used do not affect the pH, and other parameters like the choice of template are crucial for the end product.
Effect of copper content
The crystallinity of CuAPO-5 is degraded as the copper content decreases (Fig. 11) however EXAFS shows that the copper environments are similar. It is also clear that copper(II) oxide is not formed in any of the calcined materials (conditional on slow ramp rate), including those with higher copper contents. EXAFS does not reveal whether copper occupies more than one position, although again no changes are observed when increasing the Cu : Al ratio in the synthesis gel. Increasing the copper content has no effect on the CuAPO-5 material's ability to catalyse the selective reduction of NOx as seen in Fig. 5 as the highest conversion numbers are obtained for the samples with the lowest Cu : Al ratio. The elemental analyses show that the Cu + Al does not equal P, usually Cu + Al is larger than P. Similarly for the samples with lower phosphorus content, the Cu + P content is larger than the Al content. This would indicate that some copper sites might be extraframework.Effect of calcination ramp rate
All of the CuAPO-5 materials are thermally stable up to 550 °C, however the thermogravimetric measurements show that low ramp rate is essential if they are to be calcined without copper ejected in the form of copper(II) oxide.Effect of Al : P ratio in the synthesis gel
The Al : P ratio ranged between 0.72 and 1.19 in the materials studied. The AlPO4-5 structure was obtained in all cases, with the elemental analyses showing that the CuAPO-5 is obtained for the ratios above and below unity. We conclude from this that the system is rather robust, and can adjust to the concentrations of aluminium and phosphorus rather than copper substituting phosphorus in addition to aluminium.Conclusion
The present work confirms that copper can be incorporated into the AlPO4-5 structure when a noncomplexing quaternary amine template is used in the synthesis. It is further shown that the synthesis of CuAPO-5 does not depend on whether copper(II) oxide or copper(II) acetate is used as copper sources. Copper(II) in the as-synthesised and calcined materials is extraframework bonded in the former and framework bonded in the latter. In as-synthesised CuAPO-5 the metal is present in the form of turquoise-coloured tetragonally-distorted octahedral hexaaquacopper(II) cations which are held in place within the pores by electrostatic forces to anionic framework phosphate groups. In the green calcined CuAPO-5, copper is tetrahedrally coordinated within the framework in a combination of catalytically active and nonactive sites. There is no clustering of copper and the metal is dispersed within the structure. Above a certain limit, the active sites are saturated with the metal but uptake of copper is possible by still filling the nonactive sites. The flexible nature of the framework is demonstrated by the decrease in cell volume of the higher copper-content CuAPO-5 which is accompanied by decreased crystallinity compared to the materials with lower metal contents. This is rationalised in terms of phosphorus–oxygen bonds swivelling into the pores, and complexing copper which has the effect of pulling the flexible framework together in the a/b directions.Acknowledgements
A grant from NorFa is greatly appreciated and we also acknowledge the Norwegian University of Science and Technology and the Norwegian Research Council for grants supporting the Swiss–Norwegian Beamlines (SNBL). The assistance of the SNBL Project Team (H. Emerich, W. van Beek and H. P. Weber) is very much appreciated. Many thanks are due to Dr A. M. Beale at the Royal Institution in London for assistance with the elemental analyses, Dr. J. M. Dickson and G. Burgess at the Catalysis Research Laboratory, Nottingham Trent University for assisting with the catalytic measurements and L. Palin for the introduction to GSAS.References
- J. Blanco, P. Avila and J. L. G. Fierro, Appl. Catal. A: Gen., 1993, 96(2), 331 CrossRef CAS.
- M. C. Kung, K. A. Bethke and H. H. Kung, Abstr. Pap. Am. Chem. Soc., 1994, 2, 207.
- G. Gopalakrishnan, P. R. Stafford, J. E. Davidson, W. C. Hecker and C. H. Bartholomew, Appl. Catal. B: Environ., 1993, 2, 165 CrossRef CAS.
- G. Mabilong and D. Durand, Catal. Today, 1993, 17(1–2), 285 CrossRef.
- A. Corma, V. Fornés and E. Palomares, Appl. Catal. B: Environ., 1997, 11(2), 233 CrossRef CAS.
- M. Dabala, P. Canu and M. Magrini, J. Alloys Compd., 2001, 317, 590 CrossRef.
- G. Centi, S. Perathoner, B. Kartheuser, D. Rohan and B. K. Hodnett, Appl. Catal. B: Environ., 1992, 1(2), 129 CrossRef CAS.
- M. Iwamoto, H. Yahiro, Y. Mine and S. Kagawa, Chem. Lett., 1989, 2, 213.
- W. Grünert, N. W. Hayes, R. W. Joyner, E. S. Shpiro, M. R. H. Siddiqui and G. N. Baeva, J. Phys. Chem, 1994, 98, 10832 CrossRef.
- M. Iwamoto, H. Yahiro, K. Tanda, N. Mizuno, Y. Mine and S. Kagawa, J. Phys. Chem., 1991, 95, 3727 CrossRef CAS.
- D. G. Nicholson and M. H. Nilsen, J. Mater. Chem., 2000, 10, 1965 RSC.
- D. Burton, J. S. J. Hargreaves, D. G. Nicholson, M. H. Nilsen and M. Stockenhuber, J. Mater. Chem., 2001, 11, 1446 Search PubMed.
- D. Panayotov, L. Dimitrov, M. Khristova, L. Petrov and D. Mehandjiev, Appl. Catal. B: Environ., 1995, 6, 61 CrossRef CAS.
- A. Frache, B. Palella, M. Cadoni, R. Pirone, P. Ciambelli, H. O. Pastore and L. Marchese, Catal. Today, 2002, 75, 359 CrossRef CAS.
- J. Dêdecêk, A. Vondrovâ and J. Čejka, Collect. Czech. Chem. Commun., 1998, 63 Search PubMed.
- J. Dêdecêk, A. Vondrovâ and J. Čejka, Collect. Czech. Chem. Commun., 2000, 65 Search PubMed.
- J. Dêdecêk, J. Čejka and B. Wicherlová, Appl. Catal. B: Environ., 1998, 15, 233 CrossRef CAS.
- H. Nishiguchi, S. Kimura, T. Ishihara and Y. Takita, Res. Chem. Intermed., 1998, 24(4), 391 CAS.
- T. Ishahara, M. Kagawa, F. Hadama and Y. Takita, J. Catal., 1997, 169, 93 CrossRef CAS.
- U. Lohse, B. Parlitz, B. Altrichter, K. Jancke, E. Löffler, E. Shreier and F. Vogt, J. Chem. Soc., Faraday Trans., 1995, 91(7), 1155 RSC.
- U. Lohse, B. Parlitz, R. Bertram, K. Jancke, E. Löffler, E. Shreier and I. Kurzawski, J. Chem. Soc. Faraday Trans., 1995, 91(7), 1163 RSC.
- G. Lischke, B. Parlitz, U. Lohse, E. Schreier and R. Fricke, Appl. Catal. A: Gen., 1998, 166, 351 CrossRef CAS.
- B. M. Lok, C. A. Messina, R. L. Patton, R. T. Patton, R. T. Gajek, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1984, 106, 6092 CrossRef CAS.
- S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannon and E. M. Flanigen, J. Am. Chem. Soc., 1982, 104, 1176.
- G. Sankar, J. M. Thomas, J. Chen, P. A. Wright, P. A. Barrett, G. N. Greaves and C. R. A. Catlow, Nucl. Instrum. Methods Phys. Res. B, 1995, 97, 37 CrossRef CAS.
- P. A. Barrett, G. Sankar, C. R. A. Catlow and J. M. Thomas, J. Phys. Chem., 1996, 100, 8977 CrossRef CAS.
- C. M. Cardile, N. J. Tapp and N. B. Milestone, Zeolites, 1990, 10, 90 CrossRef CAS.
- K. J. Chao, A. C. Wei, H. C. Wu and J. F. Lee, Catal. Today, 1999, 49, 277 CrossRef CAS.
- Z. Zhu and L. Kevan, Phys. Chem. Chem. Phys., 1999, 1, 199 RSC.
- D. J. Parrillo, C. Pereira, G. T. Kokotailo and R. J. Gorte, J. Catal., 1992, 138, 377 CrossRef CAS.
- N. Ulagappan and V. Krishnasamy, J. Chem. Soc., Chem. Commun., 1995, 373 RSC.
- N. J. Tapp and C. M. Cardile, Zeolites, 1990, 10, 680 CrossRef CAS.
- N. Rajić, D. Stojaković and V. Kaučič, Zeolites, 1991, 11, 612 CAS.
- C. W. Lee, G. Brouet, X. Chen and L. Kevan, Zeolites, 1993, 13, 565 CrossRef CAS.
- A. Moen and D. G. Nicholson, J. Chem. Soc., Faraday Trans., 1995, 91, 3529 RSC.
- T. Muñoz, A. M. Prakash, L. Kevan and K. J. Balkus, Jr., J. Phys. Chem. B, 1998, 102, 1379 CrossRef CAS.
- G. Lischke, B. Parlitz, U. Lohse, E. Schreier and R. Fricke, Appl. Catal. A: Gen., 1998, 166, 351 CrossRef CAS.
- E. Dimitru, C. Giumon, V. Hulea, D. Lutic and I. Fechete, Appl. Catal. A: Gen., 2002, 237, 211 CrossRef.
- J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Nature, 1999, 198, 227 CrossRef CAS.
- R. Raja, G. Sankar and J. M. Thomas, J. Am. Chem. Soc., 1999, 121, 11926 CrossRef CAS.
- N. Binsted, J. W. Cambell, S. J. Gurman and P. C. Stephenson, SERC Daresbury Laboratory, EXCALIB and EXBACK programs, 1990.
- N. Binsted, EXCURV98, CCLRC, Daresbury Laboratory, 1998.
- S. Åsbrink and L. J. Norrby, Acta Crystallogr., Sect. B, 1969, 25, 676 CrossRef CAS.
- H. Jaggi Von and H. R. Oswals, Acta Crystallogr., Sect, 1961, 14, 1041 CrossRef.
- G. M. Brown and R. Chidambari, Acta Crystallogr., Sect. B, 1969, 25, 676 CrossRef CAS.
- S. J. Gurman, N. Binsted and I. Ross, J. Phys. Chem. C, 1984, 17, 143 Search PubMed.
- F. W. H. Kampers, C. W. R. Engelen, J. H. C. van Hoff and D. C. Koningsberger, J. Phys. Chem., 1990, 94, 8574 CrossRef CAS.
- A. C. Larsen and R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR, 1994, 86–743.
- L. Trouillet, T. Toupance, F. Villain and C. Louis, Phys. Chem. Chem. Phys., 2000, 2, 2005 RSC.
- K. Mathisen, D. G. Nicholson, A. M. Beale, M. Sanchez-Sanchez, G. Sankar, S. Nikitenko and W. Bras, 2004, to be submitted.
- L.-S. Kau, D. J. Spira-Solomon, J. E. Penner-Hahn, K. O. Hodgson and E. I. Solomon, J. Am. Chem. Soc., 1987, 109, 6433 CrossRef CAS.
- G. Lamble, A. Moen and D. G. Nicholson, J. Chem. Soc., Faraday Trans., 1994, 90, 2211 RSC.
- A. R. Heinrich and Ch. Baerlocher, Acta Crystallogr., Sect. C, 1991, 47, 237 CrossRef.
- J. M. Bennet, J. P. Cohen, E. M. Flanigen, J. J. Pluth and J. V. Smith, ACS Symp. Ser., 1983, 218, 109 CAS.
- W. J. Richardson, Jr., J. J. Pluth and J. V. Smith, Acta Crystallogr., Sect. C, 1987, 43, 1469 CrossRef.
- A. J. Mora, A. N. Fitch, M. Cole, R. Goyal, R. H. Jones, H. Jobic and S. W. Carr, J. Mater. Chem., 1996, 6(11), 1831 RSC.
- N. J. Hanson, A. K. Cheetham and J. D. Gale, Chem. Mater., 1996, 8, 664 CrossRef CAS.
- S. Ahn and H. Chon, Microporous Mater., 1997, 8, 113 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
