Speciation of selenocompounds by capillary HPLC coupled with ICP-MS using multi-mode gel filtration columns
Yasumitsu Ogra* and Kazuo T. Suzuki
Graduate School of Pharmaceutical Sciences, Chiba University, Chuo, Chiba, 260-8675, Japan. E-mail: ogra@p.chiba-u.ac.jp; Fax: +81 43 226 2866; Tel: +81 43 226 2866
First published on 29th November 2004
Abstract
Multi-mode gel filtration HPLC columns of capillary size coupled with ICP-MS were evaluated as to the separation of naturally occurring selenocompounds. The major selenocompounds found in selenized garlic, i.e., Se-methylselenocysteine and γ-glutamyl-Se-methylselenocysteine, were separated on the narrower column (GS320A-M5D, 0.5 mm i.d. × 150 mm). The selenometabolites found in the urine of a selenium-toxicosis rat, i.e., selenosugar (1-β-methylseleno-N-acetyl-D-galactosamine) and trimethylselenonium, were also separated on the same column. On the other hand, the selenoamino acids, i.e., Se-methylselenocysteine and selenomethionine, were separated on the larger column (GS320A-M8E, 0.8 mm i.d. × 250 mm). These samples were introduced directly into the capillary columns without any pretreatment. The capillary HPLC separated these selenocompounds into distinct peaks with satisfactory sensitivity and a better S/N ratio than conventional HPLC despite that the sample volume was reduced to 1/200 (100 nl).
Introduction
Metallomics and metallome are newly coined terms.1,2 Metallomics is the name given to the integrated research field of biometals, and the entirety of biomolecules containing biometals are defined as the metallome. Metallomics and research on the metallome require analytical techniques that can provide information for the identification and quantification of metal species. This concept has been named speciation, and the acquisition of data according to this concept is performed with hyphenated techniques involving both separation and detection methods.3–5Hyphenated techniques for the metallome have evolved dramatically. In particular, specific detection methods are now very diversified. At first, spectroscopic methods, such as atomic absorption and emission spectrophotometry, were used for elemental speciation,6 and inductively coupled argon plasma-atomic emission spectrophotometory7,8 and -mass spectrometry (ICP-MS) are being used at present. ICP-MS has enabled multi-elemental speciation with extremely high sensitivity, and discrimination of endogenous and exogenous biometals with the use of enriched stable isotopes.9 Recently, advanced mass spectrometries coupled with electrospray ionization or matrix-assisted laser desorption ionization have been used for the molecular speciation of metal species.10–14 These methods can be used to identify unknown metal species based on their molecular information.
On the other hand, various separation techniques such as HPLC, GC, gel electrophoresis and capillary electrophoresis have been adopted as hyphenated techniques for selenium (Se) speciation.15–17 Among these separation techniques, HPLC has some advantages over other separation techniques, i.e., (1) the separating conditions are relatively similar to physiological conditions and (2) diverse methods such as gel filtration, ion exchange, affinity and reversed phase are easily selectable. Therefore, the coupling of HPLC with ICP-MS has been most frequently used for the speciation of metallome compounds.
Se is an ultra-trace essential element and is known to function as the active center of selenoproteins for redox enzymes such as glutathione peroxidases and thioredoxine reductase.18,19 It is also suggested that a low Se concentration in the diet results in an increased risk of cancer.20 In fact, Se accumulators such as garlic, onion and yeast show the cancer preventing activity.21 The absorption, tissue distribution and excretion of Se highly depend on its chemical species, resulting in dramatic changes in the metabolism of the element. Therefore, speciation of Se has become one of the hottest topics in the metallome analysis. Indeed, the major urinary selenometabolite of mammals was the unexpected selenocompound Se-containing sugar (selenosugar).12
Recently, reversed phase capillary HPLC-ICP-MS has been reported by Schaumlöffel et al.22 They showed that capillary HPLC could be coupled with ICP-MS with sufficient sensitivity. Capillary HPLC-ICP-MS is superior to conventional HPLC-ICP-MS, especially with regard to sample requirement. A nl (nanoliter) sample requirement level may allow the application of speciation to cultured cells and biopsy samples. Unfortunately, almost all commercially available capillary columns are reversed phase ones. Gel filtration columns have many advantages over other columns for the speciation of biometals. First, a gel filtration column can be used for biological fluids such as tissue supernatants, urine and blood plasma without any pretreatment. Second, the elution conditions are similar to physiological conditions, i.e., elution is normally performed with a salt-containing solution. Third, one can be used for a wide size range of biomacromolecules such as proteins and nucleic acids. Therefore, if gel filtration capillary columns were available, capillary HPLC would provide novel information for the speciation of biometals.
We have already reported that multi-mode gel filtration columns allow unique separation of Se-containing biomolecules at the conventional size.23,24 Multi-mode gel filtration columns have not only size exclusion but also ion-exchange and reversed phase properties depending on the elution conditions.25 In this study, a newly developed technique, multi-mode gel filtration capillary HPLC was evaluated as to application to nano level speciation, which is named nano-speciation.
Experimental
Reagents
Sodium selenite, L-selenomethionine (SeMet), ammonium acetate and acetic acid were purchased from Wako Pure Chemical Industries (Osaka, Japan), while Se-methyl-L-selenocysteine (MeSeCys) was purchased from Acros Organics (Geel, Belgium). Selenized garlic fortified with sodium selenate was purchased from PhytoSelenium Research Laboratories (Kumamoto, Japan). The concentration of Se in the garlic was 180 μg g−1 wet weight.Apparatus
A HP4500 inductively coupled argon plasma mass spectrometer (ICP-MS; Yokogawa Analytical Systems, Hachiouji, Japan) was used. The instrumental conditions for the ICP-MS are summarized in Table 1. The ICP-MS was coupled to a conventionally sized HPLC system or a capillary HPLC one as a detector. The conventionally sized HPLC system consisted of an on-line degasser, a HPLC pump (PU610; GL Science Co., Ltd, Tokyo), a Rheodyne six-port injector and a column. A multi-mode gel filtration column, Shodex Asahipak GS-320 HQ, was used (Showa Denko, Tokyo, Japan). The eluate was introduced directly into the Babington nebulizer of the ICP-MS to detect Se at m/z 77 and 82.12 The elution conditions for the conventional size column are summarized in Table 2.| Conventionally sized HPLC | Capillary HPLC | |
|---|---|---|
| Plasma settings | ||
| RF power/W | 1250 | ← |
| Nebulizer type | Babington | Total consumption |
| Nebulizer gas flow/l min−1 | 1.2 | 0.6 or 1.1 |
| Auxiliary gas flow/l min−1 | 1.15 | ← |
| Plasma gas flow/l min−1 | 15.0 | ← |
| Data acquisition | ||
| Dwell time/ms | 100 | ← |
| m/z monitored | 77 and 82 | ← |
| Column | Asahipak GS-320 HQ | GS320A-M5D | GS320A-M8E |
|---|---|---|---|
| Guard column | GS-2B 7G | — | — |
| Size/mm | 7.6 id × 300 | 0.5 id × 150 | 0.8 id × 250 |
| + 7.6 id × 75 (guard) | |||
| Exclusion size | >40![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 | ← | ← |
| Theoretical plate number | 19![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 | 3000 | 8000 |
| Elution buffer | 50 mM ammonium acetate, pH 6.5 | ← | ← |
| Flow rate | 0.5 ml min−1 | 2.0 μl min−1 | 5.5 μl min−1 |
| Injection volume | 20 μl | 100 nl | 100 nl |
Capillary HPLC
The capillary HPLC system was used in conjunction with a micro-HPLC pump (MP710; GL Science Co. Ltd.). The prototype columns for capillary gel filtration, Shodex GS320A-M5D and GS320A-M8E, were kindly provided by Showa Denko. Samples, 100 nl, were injected with a 2-position sample injector of 100 nl (Valco International, Schenkon, Switzerland). All tubing comprised fused silica capillary tubing (30 μm id, 375 μm od; GL Science). The interface between the capillary HPLC and the ICP-MS was constructed according to the method of Schaumlöffel et al.22 Namely, the eluate was introduced directly into the plasma via a microflow total consumption nebulizer fitted with a low-dead volume spray chamber without a drain. The elution conditions for the capillary columns are summarized in Table 2.Sample preparation
A ramentum of the selenized garlic was homogenized in 9 times the weight of Milli-Q water (18.3 MΩ; Millipore, Tokyo, Japan) with a Polytron homogenizer (Kinematica, Lutzern, Switzerland) under an atmosphere of nitrogen with ice-water cooling. The homogenate was centrifuged at 105![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000g for 1 h at 4 °C to obtain a supernatant. The supernatant was filtered through a 0.22 μm PVDF membrane filter (MILLEX®-GV; Millipore), and then quickly chilled and stored at −20 °C prior to use.
000g for 1 h at 4 °C to obtain a supernatant. The supernatant was filtered through a 0.22 μm PVDF membrane filter (MILLEX®-GV; Millipore), and then quickly chilled and stored at −20 °C prior to use.Animal experiment
A male Wistar rat at the age of 5 weeks was purchased from Clea Japan Inc. (Tokyo), and was fed a standard diet (CE-2; Clea Japan Inc.) and water containing sodium selenite at the concentration of 5.0 μg Se ml−1ad libitum. 24 h-urine was collected with a metabolic cage. The urine sample collected at 14 d after the beginning of the feeding was used for the analyses. The naturally occurring selenocompounds used in this study were summarized in Table 3.| Selenium species | Acronym | Structure |
|---|---|---|
| γ-Glutamyl-Se-methylselenocysteine | gGluMeSeCys |  |
| Se-methylselenocysteine | MeSeCys |  |
| Selenomethionine | SeMet | 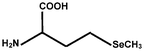 |
| 1-β-Methylseleno-N-acetyl-D-galactosamine | MeSeGalNAc |  |
| Trimethylselenonium | TMSe |  |
Calculation of chromatographic parameters
The plate number (N) and resolution (RS) for the chromatogram of the naturally occurring selenocompounds were calculated by the following equations. N = 5.55 × (Tr/Wh)2, RS = 1.18 × {(Tr2 − Tr1)/(Wh1 + Wh2)}; Tr, retention time (min); Wh, peak width at half height.Results and discussion
Separation of the major selenocompounds in selenized garlic
The two major selenocompounds in selenized garlic,26,27 that is, γ-glutamyl-Se-methylselenocysteine (gGluMeSeCys) and Se-methylselenocysteine (MeSeCys), were separated into two distinct peaks at retention times of 17.3 and 19.1 min, respectively, on a conventional size gel filtration column (GS-320 HQ) (Fig. 1(A)). gGlMeSeCys and MeSeCys on GS-320 HQ were already assigned by an ESI-MS/MS and an authentic standard, respectively (unpublished data). These two selenocompounds were also separated with comparable efficiency on a smaller capillary gel filtration column (GS320A-M5D) at retention times of 10.8 and 12.7 min, respectively (Fig. 1(B)). When GS320A-M5D was operated at nearly the same linear velocity (10.2 mm min−1, 2.0 μl min−1) as for GS-320 HQ (11.0 mm min−1, 0.5 ml min−1), it separated these two selenocompounds better than GS-320 HQ. The resolution on GS320A-M5D (3.22) was improved compared with that on GS-320 HQ (2.66) (Table 4). On comparison of the sensitivity of ICP-MS between conventional and capillary HPLC, the peaks of gGluMeSeCys on the conventional and capillary columns were found to be 1.11 × 105 and 2.67 × 104 counts, respectively. Although the sample volume was reduced to 1/200 (100 nl to 20 μl), the sensitivity was only reduced to 1/4 for GS320A-M5D. In addition, the background noise became lower when the capillary HPLC was coupled with the ICP-MS (118.8 ± 11.5 to 18.5 ± 4.5 counts). Consequently, capillary HPLC-ICP-MS allowed improvement of not only the sample requirement but also the S/N ratio for GS320A-M5D (Table 4). | ||
| Fig. 1 Comparison of the separation of the major selenocompounds in selenized garlic between conventional and capillary multi-mode gel filtration HPLC. The extract of selenized garlic was applied to GS-320 HQ (A, 7.6 id × 300 mm with a guard column), GS320A-M5D (B, 0.5 id × 150 mm) or GS320A-M8E (C, 0.8 id × 250 mm). (1) γ-Glutamyl-Se-methylselenocysteine, (2) Se-Methylselenocysteine. | ||
| GS-320 HQ | GS320A-M5D | GS320A-M8E | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PNa | S/Nb | RSc | PN | S/N | RS | PN | S/N | RS | |
| a Observed plate number.b Signal-to-noise ratio.c Resolution between two peaks of the major selenocompoumds. | |||||||||
| gGluMeSeCys | 11 341 | 964 | 5711 | 1443 | 6165 | 400 | |||
| 2.66 | 3.22 | 3.72 | |||||||
| MeSeCys | 11 355 | 1165 | 6209 | 1627 | 7671 | 459 | |||
| MeSeGalNAc | 12 882 | 91.2 | 5785 | 133.8 | 4967 | 92.1 | |||
| 3.06 | 1.88 | 2.09 | |||||||
| TMSe | 17 957 | 51.6 | 8084 | 90.4 | 7992 | 60.4 | |||
The larger capillary column (GS320A-M8E) also separated these selenocompoumds into two distinct peaks with the highest resolution (Fig. 1C and Table 4). However, S/N ratio became worse than GS320A-M5D due to the increase in noise.
Separation of the selenoamino acids frequently found in plants and yeast
It has been reported that SeMet is the most abundant chemical species found in proteinous or non-proteinous biomolecules in selenized yeast, i.e., SeMet was found in SeMet-containing proteins such as SIP18 and HPS12 and non-proteinous methionine analogues such as Se-adenosylselenomethionine.14,28 Some edible mushrooms and vegetables such as wheat grain, corn, rice and soybeans also accumulate Se as SeMet.25,29 On the other hand, as mentioned in the previous section, MeSeCys and its derivatives accumulate in selenized garlic, onion and leek, and are known to be potent anti-tumor agents.21,27,29 Although SeMet and MeSeCys are highly similar to each other in chemical and physical properties, they may have different biological, pharmacological and nutritional activities in animals.30,31 Therefore, as the second step, the capillary multi-mode gel filtration columns were evaluated as to the separation of these selenoamino acids.MeSeCys and SeMet were eluted from a conventional column (GS-320 HQ) as distinct peaks at retention times of 22.9 and 24.1 min, respectively, as reported previously (Fig. 2(A)).25 However, their separation was poor on a capillary column, GS320A-M5D, as shown in Fig. 2(B). They were separated on the capillary column at retention times of 13.5 and 13.7 min, respectively, but it was not possible to separate them as distinct peaks. Then, the other capillary column (GS320A-M8E), which has a larger theoretical plate number (8000) than GS320A-M5D (3000), was evaluated. The GS320A-M8E column separated MeSeCys and SeMet with comparable efficiency to that of the conventional one, i.e., the retention times of MeSeCys and SeMet were 17.5 and 18.5 min, respectively (Fig. 2(C)). The sensitivity was also improved because GS320A-M8E could be operated at higher nebulizer gas flow due to the larger flow rate (Table 2).
 | ||
| Fig. 2 Comparison of the separation of selenoamino acids between conventional and capillary multi-mode gel filtration HPLC. 10.0 μg Se ml−1 of each selenoamino acid was applied to GS-320 HQ (A: 7.6 id × 300 mm with a guard column), GS320A-M5D (B: 0.5 id × 150 mm) or GS320A-M8E (C: 0.8 id × 250 mm). Se-Methylselenocysteine (upper panel), Selenomethionine (lower panel). | ||
Separation of the selenometabolites in the urine of a Se-toxicosis rat
As the third step, urinary selenometabolites of mammalian origin were used to evaluate the capillary columns. It is known that the major urinary selenometabolite is a selenosugar (1-β-methylseleno-N-acetyl-D-galactosamine, MeSeGalNAc) within the physiological or low toxic Se level.12,13,32,33 Beyond the low toxic level, a second urinary selenometabolite, i.e., trimethylselenonium (TMSe), appears.23,34 Thus, two major selenometabolites are found in the urine of a Se-toxicosis rat. These urinary selenometabolites were clearly separated on the GS-320 HQ column (Fig. 3(A)).12,35 MeSeGalNAc and TMSe on GS-320 HQ were already assigned by HPLC-ESI-MS/MS and an authentic standard, respectively.12,32–34 The resolution of the two selenometabolites was 3.06. Both capillary columns separated the two selenometabolites with better S/N ratios than the conventional one (Fig. 3 and Table 4). MeSeGalNAc and TMSe were eluted from GS320A-M5D at retention times of 13.4 and 14.8 min, respectively, and from GS320A-M8E at retention times of 17.9 and 19.9 min, respectively. The resolutions of the two selenometabolites on GS320A-M5D and GS320A-M8E were 1.88 and 2.09, respectively, suggesting that the two selenometabolites can be completely separated on either of the capillary columns. The half-height width of each peak on GS320A-M8E was slightly wider than that on GS320A-M5D, the two metabolites being eluted faster on GS320A-M5D than on GS320A-M8E. Therefore, GS320A-M5D seemed to be more suitable for routine analysis of the urinary selenometabolites than GS320A-M8E. | ||
| Fig. 3 Comparison of the separation of selenometabolites in urine of a Se-toxicosis rat on capillary multi-mode gel filtration HPLC. The urine sample was applied to GS-320 HQ (A: 7.6 id × 300 mm with a guard column), GS320A-M5D (B: 0.5 id × 150 mm), or GS320A-M8E (C: 0.8 id × 250 mm). (1) 1-β-Methylseleno-N-acetyl-D-galactosamine (selenosugar), (2) trimethylselenonium. | ||
Summarizing the present observations, the selenoamino acid and selenodipeptide present in selenized garlic were well separated on the narrower capillary column with sufficient sensitivity. Although the narrower capillary column did not separate MeSeCys and SeMet well, the other capillary column, which had a higher theoretical plate number than the narrower one, allowed comparable separation to that with the conventional column. The selenometabolites originating from animals, i.e., MeSeGalNAc and TMSe, were also well separated on the capillary columns. Consequently, this study clearly showed the advantages of the capillary HPLC over conventional HPLC, as follows: (1) the sample requirement could be reduced to as low as 100 nl; (2) the S/N ratio was improved; and (3) the time required for analysis was shortened. Multi-mode gel filtration capillary HPLC may become a key technique of the nano-speciation of the metallome.
Acknowledgements
We wish to thank Showa Denko for providing the capillary columns. We also wish to acknowledge the Grants-in-Aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan (Grant Nos. 14657587, 16689005 and 16209004).References
- R. J. P. Williams, Coord. Chem. Rev., 2001, 216, 583–595 CrossRef.
- H. Haraguchi, J. Anal. At. Spectrom., 2004, 19, 5–14 RSC.
- K. T. Suzuki, Analusis, 1998, 26, 57–60 CrossRef.
- R. Łobiński, J. Edmonds, K. T. Suzuki and P. C. Uden, Pure Appl. Chem., 2000, 72, 447–461 CrossRef CAS.
- J. Szpunar, R. Łobiński and A. Prange, Appl. Spectrosc., 2003, 57, 102A–111A CrossRef CAS.
- K. T. Suzki, Anal. Biochem., 1980, 102, 31–34 CrossRef CAS.
- M. Morita, T. Uehiro and K. Fuwa, Anal. Chem., 1980, 52, 349–351 CrossRef CAS.
- K. T. Suzuki, H. Tamagawa, S. Hirano, E. Kobayashi, K. Takahashi and N. Shimojo, Biol. Trace Elem. Res., 1991, 28, 109–121 CAS.
- K. T. Suzuki, S. Yoneda, M. Itoh and M. Ohmichi, J. Chromatogr. B, 1995, 670, 63–71 CrossRef CAS.
- M. Montes-Bayon, E. G. Yanes, C. Ponce de Leon, K. Jayasimhulu, A. Stalcup, J. Shann and J. A. Caruso, Anal. Chem., 2002, 74, 107–113 CrossRef CAS.
- T. Lindemann and H. Hintelmann, Anal. Chem., 2002, 74, 4602–4610 CrossRef CAS.
- Y. Ogra, K. Ishiwata, H. Takayama, N. Aimi and K. T. Suzuki, J. Chromatogr. B, 2002, 767, 301–312 CrossRef CAS.
- Y. Kobayashi, Y. Ogra, K. Ishiwata, H. Takayama, N. Aimi and K. T. Suzuki, Proc. Natl. Acad. Sci. USA, 2002, 99, 15932–15936 CrossRef CAS.
- J. Ruiz Encinar, L. Ouerdane, W. Buchmann, J. Tortajada, R. Łobiński and J. Szpunar, Anal. Chem., 2003, 75, 3765–3774 CrossRef.
- P. C. Uden, S. M. Bird, M. Kotrebai, P. Nolibos, J. F. Tyson, E. Block and E. Denoyer, Fresenius’ J. Anal. Chem., 1998, 362, 447–456 CrossRef CAS.
- H. Chassaigne, C. C. Chéry, G. Bordin and A. R. Rodriguez, J. Chromatogr. A, 2002, 976, 409–422 CrossRef CAS.
- C. Casiot, O. F. X. Donard and M. Potin-Gautier, Spectrochim. Acta, Part B, 2002, 57, 173–187 CrossRef.
- W. H. Cheng, G. F. Comb, Jr. and X. G. Lei, Biofactors, 1998, 7, 311–321 Search PubMed.
- P. Y. Gasdaska, M. M. Berggren, M. J. Berry and G. Powis, FEBS Lett., 1999, 442, 105–111 CrossRef CAS.
- D. G. Barceloux, J. Toxicol. Clin. Toxicol., 1999, 37, 145–172 CAS.
- C. Ip, D. J. Lisk and G. S. Stowesan, Nutr. Cancer, 1992, 17, 279–286 Search PubMed.
- D. Schaumlöffel, J. Ruiz Encinar and R. Łobiński, Anal. Chem., 2003, 75, 6837–6842 CrossRef.
- Y. Kobayashi, Y. Ogra and K. T. Suzuki, J. Chromatogr. B, 2001, 760, 73–81 CrossRef CAS.
- K. T. Suzuki, K. Ishiwata and Y. Ogra, Analyst, 1999, 124, 1749–1754 RSC.
- Y. Ogra, K. Ishiwata, J. Ruiz Encinar, R. Łobiński and K. T. Suzuki, Anal. Bioanal. Chem., 2004, 379, 861–866 CrossRef CAS.
- S. McSheehy, W. Yang, F. Pannier, J. Szpunar, R. Łobiński, J. Auger and M. Potin-Gautier, Anal. Chim. Acta, 2000, 421, 147–153 CrossRef CAS.
- Y. Dong, D. Lisk, E. Block and C. Ip, Cancer Res., 2001, 61, 2923–2928 CAS.
- S. McSheehy, J. Szpunar, V. Haldys and J. Tortajada, J. Anal. At. Spectrom., 2002, 17, 507–514 RSC.
- P. D. Whanger, J. Am. Col. Nutr., 2002, 21, 223–232 Search PubMed.
- C. Ip, Y. Dong and H. E. Ganther, Cancer Metas. Rev., 2002, 21, 281–289 Search PubMed.
- Y. R. Seo, M. R. Kelley and M. Smith, Proc. Natl. Acad. Sci. USA, 2002, 99, 14548–14553 CrossRef CAS.
- V. Díaz Huerta, J. Szpunar, R. Łobiński, M. L. Fernández Sánchez and A. Sanz-Medel, J. Anal. At. Spectrom., 2003, 18, 1471–1476 RSC.
- B. Gammelgaard and L. Bendahl, J. Anal. At. Spectrom., 2004, 19, 135–142 RSC.
- M. Itoh and K. T. Suzuki, Arch. Toxicol., 1997, 71, 461–466 CrossRef CAS.
- Y. Ogra, H. Hatano, M. Ohmichi and K. T. Suzuki, J. Anal. At. Spectrom., 2003, 18, 1252–1255 RSC.
| This journal is © The Royal Society of Chemistry 2005 |
