Compact flame atomic absorption spectrometer based on handheld CCD for simultaneous determination of calcium and magnesium in water
Chengbin
Zheng
,
Yihua
He
,
Siyu
Wei
and
Xiandeng
Hou
*
Key Laboratory of Green Chemistry and Technology of MOE at College of Chemistry, Sichuan University, Chengdu, Sichuan 610064, China. E-mail: houxiandeng@yahoo.com.cn, houxd@scu.edu.cn; Fax: +86-28-8541 2907; Tel: +86-28-8541 5695
First published on 6th December 2004
Abstract
A simple and compact flame atomic absorption spectrometer (FAAS) was constructed to carry out simultaneous multielement determination of calcium and magnesium in water. In this instrumental system, a handheld and computer-controlled charge coupled device (CCD) spectrometer was used as the detector, and a calcium and magnesium multielement hollow cathode lamp was used as the light source. The instrumental limits of detection (LOD) were 0.5 mg l−1 and 0.03 mg l−1 for calcium and magnesium, respectively. Calcium and magnesium in a water sample were simultaneously determined, with the analytical results in good agreement with those by inductively coupled plasma optical emission spectrometry (ICP-OES). The recoveries for calcium and magnesium standards added to the water sample were in the range of 103–114%.
1. Introduction
Continuous monitoring of divalent cations such as Ca2+ and Mg2+ is important in the quality assessment (QA) of drinking water, boiler water and other industrial water. There are many atomic spectrometric techniques that can be used to measure the concentrations of calcium and magnesium in water, such as flame atomic absorption spectrometry (FAAS),1,2 inductively coupled plasma optical emission spectrometry (ICP-OES),3,4 inductively coupled plasma mass spectrometry (ICP-MS)5 and electrothermal atomization atomic absorption spectrometry (ETAAS).6,7 However, some of these instruments are very expensive and bulky, and not every company can afford them. Cost may be a key factor to persuade some small water supply companies or industries to analyze their own water samples. Compared with ICP-OES and ICP-MS, FAAS and ETAAS are much less expensive but usually lack multi-element capability. Thus, it is desired to have an inexpensive and compact atomic absorption spectrometer that is capable of the simultaneous determination of calcium and magnesium in water. During the past 30 years, the possibility of simultaneous multielement determination by AAS has been explored by several research groups.8–11 A single-channel photomultiplier tube (PMT) is usually adopted as the detector in conventional FAAS. For the purpose of simultaneous multielement determination, a multi-channel detector is necessary besides a multielement lamp. There are many types of multi-channel detector (a multiple PMT system, a photodiode array, a charge injection device, or a CCD) that can be employed in atomic absorption spectrometry.12–14 In this work, a compact FAAS instrument was constructed with a handheld CCD spectrometer, together with a Ca–Mg hollow cathode lamp (HCL), for the purpose of simultaneous determination of calcium and magnesium in water samples.2. Experimental
2.1. Instrumentation
A commercial FAAS (WYX-402, Shenyang Analytical Instrument, Shenyang, China) based on a PMT was reconstructed for this work as shown in Fig. 1. The single element HCL was replaced by a Ca–Mg multielement HCL (HL-1, Hengshui Ningqiang Source Co., Hebei, China); the detection system of the commercial FAAS instrument, including a monochromator, a PMT detector, and the related optics, was replaced with a PC-controlled handheld CCD spectrometer (USB2000, Ocean Optics, Inc., Dunedin, FL). The CCD has a spectral resolution about 0.4 nm, and is readily connected to the computer through a USB cable.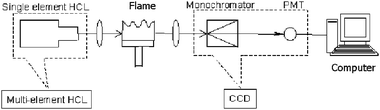 | ||
| Fig. 1 The schematic diagram of the compact FAAS instrument. | ||
An ICP-OES instrument (Iris Advantage, Thermo Jarrell Ash Corporation, MA, USA) was used to determine calcium and magnesium in the water sample to validate the accuracy of the analytical results obtained by the self-constructed multielement FAAS instrument. The major instrumental parameters were: power 1150 W; sampling rate 2.0 ml min−1; nebulizer pressure 27 psi; integration time 20 s; analytical lines 317.9 nm and 279.5 nm for the determination of calcium and magnesium, respectively. Two-line schemes were used for the background correction.
2.2. Experimental procedure
The stock solutions of Mg and Ca are prepared with MgO and CaCO3, respectively. After being dried for 2 h at 105 °C, 1.658 g MgO (AR) was dissolved in 50 ml 5 mol l−1 HCl and then diluted with deionized water to a final volume of 1 l, while 2.497 g CaCO3 (AR) was dissolved in 25 ml of 1 mol l−1 HCl and then diluted with deionized water to a final volume of 1 l; thus, 1000 mg l−1 Mg stock solution and 1000 mg l−1 Ca stock solution were obtained.15 The mixed standard solutions of Mg and Ca were prepared by serial dilution from a mixture of Mg and Ca stock solutions with deionized water. A tap water sample was collected after the tap was opened at full flow for 10 min. All containers used in this experiment were soaked in 0.2% v/v nitric acid for 24 h and rinsed with deionized water.The data collection procedure was controlled by the software OOIBase32 provided by the CCD manufacturer. The settings of the software were 50 ms per spectrum and 1 spectrum to average. The total data collection time was 1 s, which means about 20 scopes of spectra were collected during the data collection procedure. The data sets from numbers 5–15, in the middle sequence of the 20 sets of the collected data, were averaged and then used for further data processing. The sensitive lines of Mg 285.2 nm and Ca 422.7 nm were used to measure the absorbance. Fig. 2 shows a spectrum of the initial Mg–Ca HCL emission signal. As can be seen, the intensities of the two selected lines are close to each other, and the spectra are clean in the vicinity of the lines.
 | ||
| Fig. 2 The emission spectrum of the Mg–Ca HCL. | ||
3. Results and discussion
3.1. Optimization of instrumental parameters
The optimization of the height of the burner, the ratio of fuel–auxiliary gas (C2H2–air) flow rates, and the lamp current was realized by observing the changes in absorbance and relative standard deviation (RSD) of a single element (calcium or magnesium) with the height of the burner, the ratio of the gas flow rates, and the lamp current, respectively. However, ultimate operating parameters were selected with in order to find compromise “best” operating parameters for both elements. Due to the fact that the conventional FAAS instrument has been used for a long time (manufactured in 1982), the air flowmeter of the instrument has been somewhat worn-out and provides a flow rate of air fixed at 0.15 m3 min−1. Therefore, only the flow rate of acetylene was modulated in optimizing the ratio of the gas flow rates. In the optimization of the lamp current, the step width of the lamp current of the original instrument is 1 mA when using less than 6 mA but 2 mA when more than 6 mA is used, and the transmitted light intensities exceeded the measurement range of the CCD detector when the lamp current was higher than 8 mA. Therefore, the HCL current was chosen to be between 1 mA and 6 mA in the lamp current optimization experiments. Either calcium or magnesium absorbance increased as the height of burner increased up to 8 mm and then decreased, and the RSDs of both elements were almost the smallest when the height of burner was 8 mm, so 8 mm was the best height of the burner for simultaneous determination of Ca and Mg. Likewise, the flow rate of acetylene and the lamp current were 0.06 m3 h−1 and 3 mA, respectively, to ensure the best absorption signals and RSDs for both calcium and magnesium.3.2. Calibration curves, sensitivities and instrumental limits of detection
The 6-point calibration curves were constructed for calcium (0.5–50 mg l−1) and magnesium (0.03–2 mg l−1) with equations of A = 0.019 C + 0.023 and A = 0.46 C + 0.04, where A is the absorbance and C is the concentration in mg l−1, and the correlation coefficients are 0.9982 and 0.9957, respectively. The LODs (based on three-fold standard deviation) were obtained by 11 measurements of a blank solution. Sensitivities of this method can also be obtained through the slope of the calibration curves. The LODs were 0.5 mg l−1 for calcium and 0.03 mg l−1 for magnesium, and the sensitivities (slope of the calibration curve) for this method were 0.019 l mg−1 and 0.46 l mg−1 for calcium and magnesium, respectively. The LODs and sensitivities of calcium and magnesium determined by the CCD-based FAAS were inferior to those by ICP-OES (two to three orders of magnitude) and a conventional FAAS instrument (but within a factor of 5). However, it should be noted that the CCD-based FAAS instrument is much smaller than a conventional FAAS in size, so it is convenient for field applications. Although the sensitivity and LOD are not improved, this method is sensitive enough for the determination of calcium and magnesium in drinking water, environmental water and industrial water samples in the field. More importantly, it is capable of simultaneous determination of these two elements so that the sample turnaround time can be significantly reduced to about 25 s per sample.3.3. Sample analysis
The accuracy of the proposed CCD-based FAAS was first validated by use of three parallel spiked water samples, with calcium and magnesium concentrations falling in the linear dynamic range of the calibration curves. In addition, the recoveries of the calcium and magnesium standards added to the water samples were found in the range of 103–114%. The accuracy of the analytical results of the water samples was furthered confirmed by ICP-OES (Table 1).As can be seen from Table 1, the analytical results agreed quite well with those by ICP-OES. Compared with the analytical results by ICP-OES, there were some positive biases for those by CCD-based FAAS, but this is reasonable because background correction was not performed with CCD-based FAAS, and this also explains why the recoveries of standard additions are rather large (103–114%). In order to construct a compact instrument for the determination of calcium and magnesium in water samples, background correction is not required unless near-line correction can be used.16 Fortunately, water samples such as drinking water are often relatively clean, thus very often the background correction is unnecessary. One problem encountered with the simultaneous determination originas from the limited linear dynamic range of the analytical elements.17,18 Many water samples have a very large concentration of one element and a low concentration of another, so that direct and simultaneous determination of both elements by CCD-based FAAS without manual dilution/addition (standard) can be impossible. ICP-OES, of course, is the best choice for simultaneous multielement determination if it is not for field use and the expense is not a factor to consider. Nevertheless, the CCD-based FAAS instrument has many advantages, compared with a conventional FAAS instrument, as summarized in Table 2. Besides, CCD-based analytical atomic spectrometry offers great opportunities for college students and junior graduate students to study simultaneous multi-element determination by atomic absorption spectrometry or atomic emission spectrometry.19
| Factors for comparison | Conventional FAAS | CCD-based FAAS |
|---|---|---|
| Detection mode | Single element | Multi-element |
| Instrumental size | Large | Compact |
| Sample turnaround time | >50 s per sample | 25 s per sample |
| Linear dynamic range | Same | Same |
| Sensitivity | Somewhat better | Good |
| Limit of detection (mg l−1) for Ca and Mg, respectively | 0.1 and 0.01 | 0.5 and 0.03 |
| Field application | Difficult | Very promising |
Acknowledgements
The authors acknowledge the financial support for this project from the Department of Science and Technology and the Department of Personnel of Sichuan Province, China. We are also grateful to Associate Professor Jiali Xie and Mr. Minzhu Chen of College of Chemistry of Sichuan University for the loan of the FAAS instrument and help with the ICP-OES measurements, respectively.References
- D. L. Giokas, E. K. Paleologos, P. G. Veltsistas and M. I. Karayannis, Talanta, 2002, 56, 415–424 CrossRef CAS.
- A. M. Jodral-Segado, M. Navarro-Alarcón, H. L. Serrana and M. L. López-Martínez, Sci. Total Environ., 2003, 312, 47–58 CrossRef CAS.
- Z. Yang, X. D. Hou, B. T. Jones, D. C. Sane, M. J. Thomas and D. C. Schwenke, Microchem. J., 2002, 72, 49–54 CrossRef CAS.
- W. Slavin, Anal. Chem., 1986, 58, 589A–597A CAS.
- Z. Arslan and A. J. Paulson, Anal. Chim. Acta, 2003, 476, 1–13 CrossRef CAS.
- Z. Kiliç, O. Acar, M. Ulasan and M. Tlim, Food Chem., 2002, 76, 107–116 CrossRef CAS.
- B. L. Gong, Y. M. Liu, Y. L. Xu and T. Z. Lin, Talanta, 1997, 44, 1003–1007 CrossRef CAS.
- J. M. Harnly, Anal. Chem., 1986, 58, 933A–943A CAS.
- T. C. O’Haver, Anal. Chem., 1991, 63, 164–169 CrossRef CAS.
- A. Salido and B. T. Jones, Talanta, 1999, 50, 649–659 CrossRef CAS.
- M. C. Hsing, Y. H. Sung and S. D. Huang, Talanta, 2004, 62, 791–799 CrossRef CAS.
- T. C. O’Haver, Analyst, 1984, 109, 211–218 RSC.
- R. Fernando, C. P. Calloway Jr. and B. T. Jones, Anal. Chem., 1992, 64, 1556–1560 CrossRef CAS.
- C. Hsiech, S. C. Petrovic and H. L. Pardue, Anal. Chem., 1990, 62, 1983–1988 CrossRef CAS.
- A. M. Li and J. Z. Wei, Yuanzi Xishou Ji Yuanzi Yingguang Guangpu Fenxi, Science Press, Beijing, 2000, pp. 330 and 355 Search PubMed.
- J. D. Batchelor, S. E. Thomas and B. T. Jones, Appl. Spectrosc., 1998, 52, 1086–1091 CAS.
- M. Hoenig and A. Cilissen, Spectrochim. Acta, Part B, 1997, 52, 1443–1449 CrossRef.
- T. Myöhänen, V. Mäntylathi, K. Koivunen and R. Matilainen, Spectrochim. Acta, Part B, 2002, 57, 1681–1688 CrossRef.
- K. Z. Zhou, X. D. Hou, X. M. Kou and Y. J. Feng, Appl. Spectrosc. Rev., 2003, 38, 295–305 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
