Syntheses of water-soluble N-donor ligands for aqueous catalysis using green, Michael-type addition reactions†
Hong-Chang
Liang
*a,
Sanjib K.
Das
a,
Juan R.
Galvan
a,
Suzanne M.
Sato
a,
Yonglian
Zhang
a,
Lev N.
Zakharov
b and
Arnold L.
Rheingold
b
aDepartment of Chemistry and Biochemistry, San Diego State University, 5500 Campanile Drive, San Diego, CA 92182-1030
bDepartment of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0358
First published on 14th April 2005
Abstract
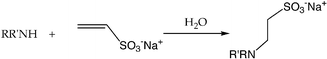
A new route for synthesizing sulfonate-containing N-donor ligands has been developed involving the Michael-type addition reaction of primary or secondary amines with sodium vinylsulfonate in water. Our reactions offer several advantages over traditional Michael-type addition reactions. For example, while most conjugate addition reactions are carried out in organic solvents in the presence of bases or acids (S. G. Davies, T. D. McCarthy, Synlett, 1995, 700–704; D. Rosenthal, G. Braundrup, K. H. Davies, M.E. Wall, J. Org. Chem., 1965, 30, 3689–3696),1 our reactions adhere to green chemistry principles and are conducted in aqueous solutions without harsh bases or acids, eliminating the need for more toxic compounds and solvents. Additionally, the new synthetic route utilizes selective conjugate addition reactions instead of unselective alkylation reactions using sodium 2-bromoethanesulfonate or similar sulfonated alkyl halides (J. March, Advanced Organic Chemistry: reactions, mechanisms and structure, Wiley, New York, 4th edn., 1992, pp. 411-413),2 which greatly decreases the amount of undesired side-products. Finally, the new synthetic route yields new, water-soluble ligands which may be utilized in aqueous catalysis involving water-soluble metal complexes.
Introduction
Water-soluble ligands, which can allow metal catalyzed reactions to take place in water, have been of significant interest in catalytic studies.3 Most ligands that are specifically designed for water-solubility contain hydrophilic groups such as carboxylate or sulfonate moieties to increase the solubility of the organic molecules in water.3 We are interested in preparing water-soluble N-donor ligands containing sulfonate groups for synthesizing metal complexes that can catalyze the hydrolysis of organic molecules such as esters, phosphate esters, and amides in water. Catalytic hydrolysis of organic molecules has been of great interest for many reasons. One motivation for studying the catalytic hydrolysis of esters, amides, and phosphoester compounds by synthetic metalloenzyme mimics comes from the desire to better understand metallohydrolases through bio-inorganic model studies.4 The hydrolysis of organophosphate compounds is also of interest as a possible means for destroying unwanted stockpiles of organophosphate-based chemical warfare nerve agents.5 Another driving force for studying the hydrolysis of organophosphate compounds by metal complexes lies in the fact that these studies may lead to better methods for the cleanup of water supplies which are contaminated by phosphotriester-based insecticides.6 In addition to better understanding the nature of nucleases that catalyze the hydrolysis of DNA or RNA and for designing artificial nucleases,7 another medically relevant metallohydrolase is β-lactamase, which catalyzes the hydrolysis of β-lactams and is responsible for bacterial resistance to β-lactam antibiotics.8The conjugate addition of nucleophiles to electron-deficient olefins is well-known in organic synthesis.9 While the addition reactions of amines10 and amides11 to vinylic compounds conjugated to neutral electron-withdrawing groups are known, there have been no reports of such conjugate addition reactions of amines towards vinylic compounds containing an anionic group such as the sulfonate moiety. The negative charge on the sulfonate group might be expected to block the conjugate addition reaction; here we report that this is not the case. We report the successful synthesis of water-soluble N-donor ligands 1–4via Michael-type additions of various amines to sodium vinylsulfonate in water and the X-ray crystal structure of a Zn(II) complex, 5, ligated by one of the new, water-soluble N-donor ligands and preliminary catalytic data on the hydrolysis of 4-nitrophenyl acetate by 5.
Results and discussion
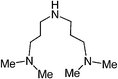
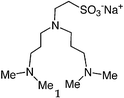
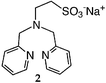
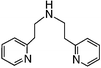
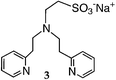
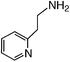
We first attempted to synthesize 1 (Table 1) by alkylating the secondary amine N-atom of 3,3′-iminobis(N,N-dimethylpropylamine) with sodium 2-bromoethanesulfonate. These reactions led to complex mixtures of products, most likely due to unselective alkylation of both the tertiary and secondary amine N-atoms, forming quaternary ammonium compounds as well as the desired tertiary amine 1.2 Wieghardt and co-workers also reported difficulties isolating the desired product when preparing 1,4,7-triazacyclononane-N,N′,N″-tris-2-ethanesulfonate by alkylating 1,4,7-triazacyclononane with sodium 2-bromoethanesulfonate, presumably due to overalkylation and the formation of quaternary ammonium compounds.12 Therefore, instead of utilizing 2-bromoethanesulfonate to achieve our synthetic goals, we developed new Michael-type reactions for the conjugate addition of amines to sodium vinylsulfonate in aqueous solutions which are selective for primary and secondary amines and do not lead to quaternary ammonium products.|
|
||||
|---|---|---|---|---|
| Entry | Starting amine | Product | Reaction time/d | Yield (%) |
| 1 |
|
|
5 | 78 |
| 2 |
|
|
3 | 74 |
| 3 |
|
|
4 | 58 |
| 4 |
|
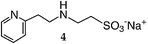
|
1 | 86 |
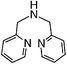
The reaction of 3,3′-iminobis(N,N-dimethylpropylamine) in a refluxing 25 wt% solution of vinylsulfonic acid sodium salt in water led to the formation of ligand 1 in 78% yield after 5 days (Table 1). Similar yields and reaction times were also obtained when other secondary amines such as di-(2-picolyl)amine and bis[2-(2-pyridyl)ethyl]amine reacted with sodium vinylsulfonate to form ligands 2 and 3, respectively. Not surprisingly, the reaction of the primary amine 2-(2-aminoethylpyridine) with sodium vinylsulfonate proceeded much more quickly than the reactions involving secondary amines, and the synthesis of the secondary amine 4 was completed within one day. No tertiary amine side-product was isolated in the synthesis of 4, probably due to the relatively slow kinetics of the formation of tertiary amines compared to secondary amines in these types of conjugate addition reactions.
The syntheses of 1–4 require longer reaction times and higher temperatures than standard Michael-type addition reactions of amines towards vinylic compounds with neutral electron withdrawing groups, which typically only require a few hours for completion. The lower reactivity of sodium vinylsulfonate compared to neutral vinylic compounds may in part be attributed to the anionic nature of the sulfonate moiety. Despite the longer reaction times and higher temperatures, our reactions offer several advantages over traditional Michael-type addition reactions. For example, while many conjugate addition reactions are carried out in organic solvents in the presence of bases or acids,1 our reactions adhere to “green synthesis” principles13 and are conducted in aqueous solutions without harsh bases or acids, eliminating the need for more toxic compounds and solvents.
Ligands 1–4 bind readily to Zn(II), Cu(II), and Ni(II) to yield water-soluble metal complexes. One of these metal complexes, synthesized by the binding of 4 to ZnCl2 to form the water-soluble complex 5 (Scheme 1), has been characterized by single crystal X-ray crystallography (Fig. 1).‡ The X-ray crystal structure of 5 (Fig. 1) shows that it is a dimer in the solid state, with distorted tetrahedral geometry around each Zn(II) center, which is ligated by a pyridyl nitrogen atom N1, an amine nitrogen atom N2, and two chloride ligands Cl1 and Cl2. One of the sulfonate O-atoms and a chloride ligand are bound to a Na+ ion, which is coordinated by two terminal (O4 and O6) and two bridging (O5 and O5A) water molecules forming a centrosymmetrical dimeric unit (Fig. 1).
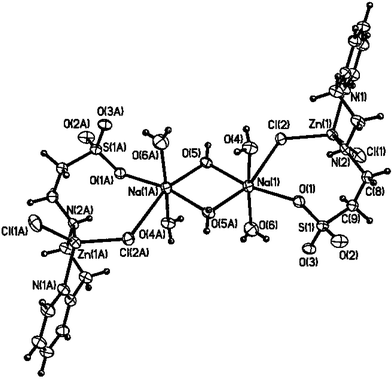 | ||
| Fig. 1 An ORTEP diagram of 5. Selected bond distances (Å) and angles (degrees): Zn1–N1, 2.0411 (17), Zn1–N2, 2.0538 (17), Zn1–Cl1, 2.2200 (6), Zn1–Cl2, 2.2326 (5), Na1–Cl2 3.009 (1), Na1–O1 2.388 (2), Na1–O4 2.399 (2), Na1–O5 2.371 (2), Na1–O5a (−x, 1 − y, −z) 2.362 (20); N1–Zn1–N2 100.12 (7), N1–Zn1–Cl1 107.13 (5), N2–Zn1–Cl1 114.02 (5), N1–Zn1–Cl2 109.63 (5), N2–Zn1–Cl2 104.42 (5), Cl1–Zn1–Cl2, 119.77 (2), Cl2–Na1–O1 89.75 (5), O5–Na1–O5a (−x, 1 − y, −z) 85.63 (6). | ||
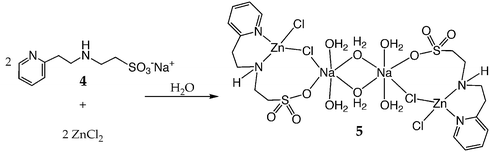 | ||
| Scheme 1 | ||
In addition to the advantages of the new, “green” synthetic routes to form 1–4 and the syntheses of new complexes such as 5, preliminary results also indicate that some of the metal complexes of 1–4 catalyze the hydrolysis of activated esters and phosphate esters such as 4-nitrophenyl acetate and bis(4-nitrophenyl) phosphate in aqueous solutions, which may also be of environmental significance. For example, complex 5 is active in catalyzing the hydrolysis of 4-nitrophenyl acetate, NA. At 25 °C, pH 7.4, I = 0.10 (NaNO3) in 10% v/v CH3CN, 5 has an observed second-order rate constant of 2.03 × 10−2 M−1 s−1 (see electronic supplementary information†). While the activity of 5 in catalyzing the hydrolysis of NA is modest, it is comparable to that of many other Zn(II) complexes reported in the literature.14 It is postulated that as we synthesize other metal complexes of 1–4 with anions that are more weakly coordinating than Cl−, we may be able to obtain more active catalysts.
Conclusion
In summary, new sulfonate-containing water-soluble ligands 1–4 have been synthesized via previously unknown Michael-type addition reactions of both primary and secondary amines with sodium vinylsulfonate. These syntheses are conducted using environmentally benign methods in water and do not require harsh bases or acids. We have also structurally characterized a new Zn(II) complex 5 using one of these new ligands. Initial experiments show that 5 catalyzes the hydrolysis of organic substrates such as 4-nitrophenyl acetate in aqueous solutions. We are currently conducting detailed investigations of catalytic reactions with other complexes and substrates and are exploring the differences in catalytic abilities of the metal complexes as a function of the varying ligands as well as examining the effects of the anions on their catalytic abilities. It is anticipated that these new types of ligands will lead to further developments in aqueous catalysis chemistry.Acknowledgements
We thank San Diego State University and the San Diego Foundation Blasker-Rose-Miah Fund for financial support. We also thank Professors Mike Bergdahl and Doug Grotjahn for helpful discussions. We are also grateful to Dr LeRoy Lafferty for help in obtaining NMR spectra.References
- (a) S. G. Davies and T. D. McCarthy, Synlett, 1995, 700–704 CrossRef CAS; (b) D. Rosenthal, G. Braundrup, K. H. Davies and M. E. Wall, J. Org. Chem., 1965, 30, 3689–3696 CrossRef CAS.
- J. March, Advanced Organic Chemistry: reactions, mechanisms and structure, Wiley, New York, 4th edn., 1992, pp. 411–413 Search PubMed.
- For reviews on water-soluble ligands, see: (a) P. Kalck and F. Monteil, Adv. Organomet. Chem., 1992, 34, 219–284 CAS; (b) W. A. Herrmann and C. W. Kohlpaintner, Angew. Chem., Int. Ed. Engl., 1993, 32, 1524–1544 CrossRef.
- (a) J. Chin, Acc. Chem. Res., 1991, 24, 145–153 CrossRef CAS; (b) E. Kimura and T. Koike, J. Am. Chem. Soc., 1991, 113, 8935–8941 CrossRef CAS; (c) N. V. Kaminskaia, C. He and S. J. Lippard, Inorg. Chem., 2000, 39, 3365–3373 CrossRef CAS.
- (a) F. M. Menger, L. H. Gan, E. Johnson and D. H. Durst, J. Am. Chem. Soc., 1987, 109, 2800–2803 CrossRef CAS; (b) P. S. Zurer, Chem. Eng. News, 2002, 80, 34–35.
- (a) L. Y. Kuo and N. M. Perera, Inorg. Chem., 2000, 39, 2103–2106 CrossRef CAS; (b) J. M. Smolen and A. T. Stone, Environ. Sci. Technol., 1997, 31, 1664–1673 CrossRef CAS.
- S. J. Lippard, Metals in Medicine, in Bioinorganic Chemistry, ed. I. Bertini, H. B. Gray, S. J. Lippard, J. Valentine, University Science Books, Sausalito, 1994; pp. 505–583 Search PubMed.
- M. L. Cohen, Science, 1992, 257, 1050–1055 CrossRef CAS.
- P. Perlmutter, Conjugated Addition Reactions in Organic Synthesis, Pergamon Press, Oxford, 1992 Search PubMed.
- (a) N. S. Shaikh, V. H. Deshpande and A. V. Bedekar, Tetrahedron, 2001, 57, 9045–9048 CrossRef CAS; (b) B. C. Ranu, S. S. Dey and A. Hajra, Arkivoc, 2002,(7), 76–81 Search PubMed.
- B. Mekonnen and H. Ziffer, Tetrahedron Lett., 1997, 38, 731–734 CrossRef CAS.
- K. Wieghardt, U. Bossek and M. Guttmann, Z. Naturforsch., 1983, 38B, 81–89 Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 2000 Search PubMed.
- (a) T. Koike, S. Kajitani, I. Nakamura, E. Kimura and M. Shiro, J. Am. Chem. Soc., 1995, 117, 1210–1219 CrossRef CAS; (b) E. Kimura, I. Nakamura, T. Loike, M. Shionoya, Y. Kodama, T. Ikeda and M. Shiro, J. Am. Chem. Soc., 1994, 116, 4764–4771 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and results of kinetic studies of the hydrolysis of 4-nitrophenyl acetate by complex 5. See http://www.rsc.org/suppdata/gc/b5/b500264h/ |
| ‡ Compound 5. C9 H19 Cl2 N2 Na O6 S Zn, M = 442.58, monoclinic, a = 18.3353(10), b = 7.6965(4), c = 12.7734(7) Å, U = 1747.88(16) Å3, T =150(2) K, space group P21/c (no. 14), Z = 4, µ(Mo–Ka) = 1.880 mm−1, 10766 reflections measured, 4038 unique (Rint = 0.0220) which were used in all calculations. The final R1 and wR2 were 0.0302 and 0.0723(I>2σI).CCDC reference number 268563. See http://www.rsc.org/suppdata/gc/b5/b500264h/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |