A comparison of antimony and lead profiles over the past 2500 years in Flanders Moss ombrotrophic peat bog, Scotland†
Joanna M.
Cloy
a,
John G.
Farmer
*a,
Margaret C.
Graham
a,
Angus B.
MacKenzie
b and
Gordon T.
Cook
b
aSchool of GeoSciences, The University of Edinburgh, West Mains Road, Edinburgh, UK EH9 3JJ. E-mail: J.G.Farmer@ed.ac.uk; Fax: +44 131 6504757; Tel: +44 131 6504757
bScottish Universities Environmental Research Centre, East Kilbride, UK G75 0QF
First published on 8th November 2005
Abstract
Two cores collected in 2001 and 2004 from Flanders Moss ombrotrophic peat bog in central Scotland were dated (14C, 210Pb) and analysed (ICP-OES, ICP-MS) to derive and compare the historical atmospheric deposition records of Sb and Pb over the past 2500 years. After correction, via Sc, for contributions from soil dust, depositional fluxes of Sb and Pb peaked from ca. 1920–1960 A.D., with >95% of the anthropogenic inventories deposited post-1800 A.D. Over the past two centuries, trends in Sb and Pb deposition have been broadly similar, with fluctuations in the anthropogenic Sb/Pb ratio reflecting temporal variations in the relative input from emission sources such as the mining and smelting of Pb ores (in which Sb is commonly present, as at Leadhills/Wanlockhead in southern Scotland), combustion of coal (for which the Sb/Pb ratio is approximately an order of magnitude greater than in Pb ores) and exhaust emissions (Pb from leaded petrol) and abrasion products from the brake linings (Sb from heat-resistant Sb compounds) of automobiles. The influence of leaded petrol has been most noticeable in recent decades, firstly through the resultant minima in Sb/Pb and 206Pb/207Pb ratios (the latter arising from the use of less radiogenic Australian Pb in alkylPb additives) and then, during its phasing out and the adoption of unleaded petrol, complete by 2000 A.D., the subsequent increase in both Sb/Pb and 206Pb/207Pb ratios. The extent of the 20th century maximum anthropogenic enrichment of Sb and Pb, relative to the natural Sc-normalised levels of the Upper Continental Crust, was similar at ∼50- to 100-fold. Prior to 1800 A.D., the influence of metallurgical activities on Sb and Pb concentrations in the peat cores during both the Mediaeval and Roman/pre-Roman periods was discernible, small Sb and Pb peaks during the latter appearing attributable, on the basis of Pb isotopic composition, to the mining/smelting of Pb ores indigenous to Britain.
 John G. Farmer John G. Farmer | John Farmer, in whose group Joanna Cloy is a PhD research student, was born in the UK in 1947. He received his PhD in Radionuclide and Stable Isotope Geochemistry from the University of Glasgow, UK, in 1972. After two post-doctoral years at Woods Hole Oceanographic Institution, USA, he returned to Glasgow as a Research Fellow in Forensic Medicine/Environmental Health, with interests in the environmental geochemistry and human health effects of potentially harmful elements. A move to the University of Edinburgh, UK, as Lecturer (1987), Senior Lecturer (1990) and Reader (1997) in Environmental Chemistry and then Professor (2004) of Environmental Geochemistry, facilitated major ongoing research efforts on the speciation and behaviour of lead, arsenic, chromium, manganese, antimony and uranium in the environment. A recurring theme is the study of environmental change via the analysis of dated freshwater lake and coastal marine sediments, peat bogs, archival moss, tree rings and human teeth. |
Introduction
In recent years ombrotrophic peat bogs have been used to study the changing rates and sources of atmospheric metal deposition as they receive all their nutrient, pollutant and water inputs solely by dry and wet deposition from the atmosphere. Cores from such bogs have proved especially useful as archives of atmospheric Pb deposition as Pb is essentially immobile in ombrotrophic peat.1–3 Considerable research has been carried out using ombrotrophic peat bogs as well as other archives to characterize historical trends in the extent and sources of environmental Pb pollution,3–18 including previous work on the Flanders Moss site under investigation in the study reported here.1,19,20 In contrast, there have been only a few studies of Sb in ombrotrophic peat bogs,21–24 although similarities with the distribution of Pb, even back to Roman times,21,24 have suggested that Sb too is essentially immobile in ombrotrophic peat. In a recent study24 of Swiss peat extending back to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 14C y BP, the lowest “background” values of Sb occurred in peat dating from 8030 to 5320 14C y BP. A comparison with those in recent peat samples suggested that the natural levels of Sb in the atmospheric environment have previously been overestimated by a factor of ten and that the global atmospheric Sb cycle may have been affected to at least the same extent as that of Pb. In view of the comparable toxicity of Sb to that of Pb, Shotyk et al.24,25 have suggested that the potential threat of environmental Sb to human health may have been overlooked.
370 14C y BP, the lowest “background” values of Sb occurred in peat dating from 8030 to 5320 14C y BP. A comparison with those in recent peat samples suggested that the natural levels of Sb in the atmospheric environment have previously been overestimated by a factor of ten and that the global atmospheric Sb cycle may have been affected to at least the same extent as that of Pb. In view of the comparable toxicity of Sb to that of Pb, Shotyk et al.24,25 have suggested that the potential threat of environmental Sb to human health may have been overlooked.
As Sb is a chalcophilic trace element and is found in practically all sulfide minerals (including galena and other Pb minerals) and coals,24,25 it might be expected that anthropogenic Sb deposition from the atmosphere has closely followed that of the Pb emanating from mining/smelting and coal combustion emissions in the past. During the second half of the 20th century, however, there were significant emissions of Pb to the atmosphere from vehicle exhausts as a consequence of the use of anti-knock alkylPb additives in petrol. As a result of reductions in the maximum permitted concentration of lead in petrol and the introduction of unleaded petrol in 1986, however, such emissions in the UK declined from a maximum of ca. 8500 tonnes Pb in 1973 essentially to zero at the time of the outright ban in 2000.26 During the 20th century and the early years of the 21st century, such changes in Pb source have been reflected in variations in the isotopic composition (e.g.206Pb/207Pb) of atmospheric27,28 and deposited3–20 Pb. Meanwhile, especially in recent years, the uses (and potential releases) of Sb compounds have expanded to include Sb2S3 as a lubricant in automobile brake linings and Sb2O3 as a fire retardant in plastics etc.25
The aim of this study was therefore to investigate the extent and nature of past and present relationships between Sb and Pb emissions to the environment by comparing trends in new age-dated profiles of Sb and Pb concentrations and Pb isotopic composition in cores from Flanders Moss ombrotrophic peat bog in central Scotland.
Materials and methods
Sampling site
Two peat cores were collected from the southwest dome of Flanders Moss ombrotrophic peat bog, which lies 16 km to the west of Stirling in central Scotland (Fig. 1). The largest remaining lowland ombrotrophic peat bog in Britain with 548 ha of active bog, Flanders Moss was formed on top of Carse clay deposits after the Menteith glacier melted over 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years ago. Vegetation is currently dominated by Sphagnum mosses, lichens, heather, fens and grasses with some scattered birch.29,30
000 years ago. Vegetation is currently dominated by Sphagnum mosses, lichens, heather, fens and grasses with some scattered birch.29,30
 | ||
| Fig. 1 Location and map of Flanders Moss, showing the sampling site on the southwest dome. | ||
Sample collection
A 106 cm long peat core (01CM-1) of cross-sectional dimensions 5 cm × 5 cm was collected in September 2001 using a Cuttle and Malcolm corer,31 which was pushed vertically into the bog after ∼7 cm of grassy vegetation was first removed with a knife to enable penetration of the corer. A 33 cm long monolith peat core (04-1-M) of cross-sectional dimensions 20 cm × 10 cm, was collected in October 2004 using a monolith tin, which was inserted into the vertical face of a freshly dug pit. Cores 01CM-1 and 04-1-M were cut into 1 cm and 3 cm sections, respectively, in the field using a serrated stainless steel knife and sections were packed into polyethylene bags and transported to the laboratory.Sample preparation
The wet peat sections were weighed, air-dried at 30 °C for ∼30 days on drying trays, re-weighed and then ground using a mortar and pestle. The remaining moisture content of each air-dried peat section was determined on sub-samples by oven-drying at 105 °C and the residual ash content was estimated by weighing air-dried sub-samples (subsequently corrected for moisture content) before and after ashing at 450 °C for 4 hours. Sub-samples (∼0.25 g) of air-dried peat (subsequently corrected for moisture content) were digested using a modified US EPA Method 3052 Protocol microwave-assisted HF–HNO3 digestion method.32,33 Digests were evaporated to 1 ml on a hotplate and then made up to 25 ml with 2% (v/v) HNO3. All reagents used in sample preparation were of the highest analytical quality available, i.e. Aristar HNO3 (69%) and HF (48%) and high purity water (18.2 MΩ cm) from a Milli-Ro water system (Millipore, Watford, UK).Sample analysis
With the exception of the highest Pb concentrations (01CM-1, 0–33 cm; 04-1-M, 0–33 cm), which were measured using ICP-OES, Sb, Pb and Sc concentrations were determined in the prepared 2% v/v HNO3 solutions using ICP-MS. ICP-OES measurements of concentration were carried out once on duplicate sectional samples from 01CM-1 using a Thermo Jarrell Ash IRIS instrument (Thermo Electron, Cambridge, UK), equipped with a cross-flow nebuliser and autosampler. For single sectional samples from 04-1-M, ICP-OES measurements were carried out once, using a Perkin Elmer Optima 5300 DV instrument (Perkin Elmer, Beaconsfield, UK) equipped with a gem-cone cross-flow nebuliser and AS 93 plus autosampler. ICP-MS measurements of concentration were carried out once on single sectional samples from both cores using a PlasmaQuad (PQ) 3 instrument (Thermo Electron, Winsford, UK), equipped with a Meinhard nebuliser, nickel sampler and skimmer cones, Gilson autosampler and a Gilson Minipuls 3 peristaltic pump (Anachem, Luton, UK). Calibration solutions were prepared daily by adequate dilution of the relevant standard solutions containing 10 mg l−1 of the element of interest. Overall analytical precision (±1 RSD) for Pb determination in duplicate samples by ICP-OES was ±5%, while analytical precision for ICP-MS determination of Sb, Pb and Sc in single samples was ≤±8%.For the determination of Pb isotopic ratios by ICP-MS, a solution of the National Institute of Standards and Technology (NIST) common Pb isotopic reference standard SRM 981 (206Pb/207Pb = 1.093, 208Pb/206Pb = 2.168, 208Pb/207Pb = 2.370) was used for calibration and mass bias correction.27 Analytical precision on these ratios was typically <±0.3%.
In addition to the above, a sample of galena (PbS) from Wanlockhead lead mines, southern Scotland, was also analysed for Sb, Pb and 206Pb/207Pb following HNO3–HF hot plate digestion of ∼0.1 g sub-samples. Sb concentrations were also determined in a selection of British coal samples (n = 19) that had previously been analysed for both Pb concentration and isotopic composition.34
To ensure the quality of analytical procedures and data, six different reference materials were analysed along with the samples: Ombrotrophic Peat (NIMT/UOE/FM001),32 Canadian Peat (1878 P),35 Bush Branches and Leaves (DC73349), Peach Leaves (GBW 08501), Coal (BCR CRM No. 4) and Coal (NBS SRM 1635). Good agreement between measured and certified values was obtained (Table 1). The isotope ratios 206Pb/207Pb, 208Pb/206Pb and 208Pb/207Pb determined in the ombrotrophic peat reference material as 1.176 ± 0.002, 2.091 ± 0.004 and 2.460 ± 0.006, respectively, (n = 58, ±1 SD) were in good agreement with corresponding “information only” values of 1.176 ± 0.001, 2.092 ± 0.002 and 2.461 ± 0.003.32
| Material | Measured Pb concentration/mg kg−1 | Certified Pb concentration/mg kg−1 | Measured Sb concentration/mg kg−1 | Certified Sb concentration/mg kg−1 | Measured Sc concentration/mg kg−1 | Certified Sc concentration/mg kg−1 |
|---|---|---|---|---|---|---|
| (±1 SD) | (±1 SD) | (±1 SD) | ||||
| a Information only values. b Information only value for Sb,35 but additional information only value of 0.305 ± 0.018 mg kg−1 was determined by Q-ICP-MS.36 | ||||||
| Ombrotrophic Peat | 173 ± 11 | 174 ± 8 | 2.30 ± 0.33 | — | 0.82 ± 0.15 | — |
| (NIMT/UOE/FM001) | (n = 67) | (n = 59) | (n = 59) | |||
| Canadian Peat | 70.9 ± 4.7 | 78.8 ± 2.9 | 0.33 ± 0.07 | 0.34 ± 0.05b | 0.83 ± 0.04 | 1.04a |
| (1878 P) | (n = 10) | (n = 11) | (n = 11) | |||
| Bush Branches and | 44 ± 3 | 47 ± 3 | 0.100 ± 0.009 | 0.095 ± 0.014 | 0.30 ± 0.02 | 0.32 ± 0.04 |
| Leaves (DC73349) | (n = 14) | (n = 13) | (n = 11) | |||
| Peach Leaves | 0.99 ± 0.10 | 0.99 ± 0.08 | — | — | — | — |
| (GBW 08501) | (n = 10) | |||||
| Coal | 23.0 ± 2.0 | 24.2 ± 1.7 | 4.24 ± 0.19 | — | 5.56 ± 0.36 | 6.25 ± 0.52a |
| (BCR CRM No. 40) | (n = 5) | (n = 5) | (n = 5) | |||
| Coal | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.14 ± 0.01 | 0.14a | 0.65 ± 0.06 | 0.63a |
| (NBS SRM 1635) | (n = 5) | (n = 5) | (n = 5) | |||
Age dating
The constant rate of supply (CRS) model37 was applied to 210Pb inventories (total 2.53 ± 0.05 kBq m−2) calculated from the unsupported 210Pb data (Fig. 2) to generate age dates for sections in 04-1-M. In the case of the 01CM-1 core, 210Pb dates were obtained by extrapolation, aided by matching of 206Pb/207Pb profiles, from data from the sister core, 01CM-2, collected on the same date.20
 | ||
| Fig. 2 210Pb specific activity profile in Flanders Moss core 04-1-M. | ||
Results
01CM-1
 | ||
| Fig. 3 Profiles of Sb, Pb and Sc concentrations and 206Pb/207Pb ratio from 0–33 cm and 33–106 cm in the 210Pb- and 14C-dated Flanders Moss core 01CM-1. | ||
04-1-M (Fig. 4)
Sb and Pb concentrations increased from 0.12 and 18 mg kg−1 at 30–33 cm to maximum values of 3.7 mg kg−1 and 193 mg kg−1, respectively, at 12–15 cm, which was dated at 1943–1968 A.D., the section mid-point of 13.5 cm corresponding to 1955 ± 3 A.D. Above these peaks, concentrations of Sb and Pb decreased towards the top of the core to concentrations of 0.50 mg kg−1 and 15 mg kg−1 in the 0–3 cm section, which was dated at 1997–2004 A.D. The 206Pb/207Pb ratios remained constant at a mean value of 1.175 ± 0.002 from 30 to 18 cm (1910 ± 8 A.D.) and then declined to 1.170 ± 0.001 at 15–18 cm, which was dated at 1910–1943 A.D., the section mid-point of 16.5 cm corresponding to 1926 ± 6 A.D. Above 15 cm, the 206Pb/207Pb ratio declined to a minimum of 1.130 ± 0.002 at 6–9 cm (1982–1990 A.D., with mid-point of 7.5 cm at 1986 ± 2 A.D.) before increasing to 1.139 ± 0.002 at 0–3 cm (1997–2004 A.D.). This reversal in the 206Pb/207Pb ratio was absent from the top of the 01CM-1 core because of the loss of some surface material during core collection.20 The Sc concentrations, averaging 0.20 ± 0.03 mg kg−1 from 33 to 24 cm, increased less markedly than those of Sb and Pb, as also seen in the 01CM-1 core, reaching a maximum of 1.6 mg kg−1 at 12–15 cm, above which values declined to 0.21 mg kg−1 in the surface 0–3 cm section. | ||
| Fig. 4 Profiles of Sb, Pb and Sc concentrations and 206Pb/207Pb ratio in the 210Pb-dated Flanders Moss core 04-1-M. | ||
Coal and Pb ore
In 19 samples of British coal that had been previously analysed for Pb and 206Pb/207Pb,34 Sb concentrations ranged from 0.07 to 4.6 mg kg−1 (mean 0.75 ± 1.02 mg kg−1, median 0.49 mg kg−1), with a mean Sb/Pb ratio of 0.036 ± 0.032 (median 0.027). For all the British coals (n = 60) previously investigated, the mean 206Pb/207Pb ratio was 1.182 ± 0.009 (Scottish, 1.181 ± 0.006, n = 30), with average Pb concentrations of 23.9 mg kg−1 and 11.0 mg kg−1 for coals from Scotland and England & Wales, respectively.34 In the Pb ore sample from Wanlockhead, the Sb/Pb ratio was 0.0056 ± 0.0004, approximately one order of magnitude lower than in coal. The measured 206Pb/207Pb ratio in the Pb ore was 1.172 ± 0.003, in agreement with the value of 1.170 ± 0.003 found for Leadhills/Wanlockhead Pb ore.39–41Discussion
The use of Sc as an indicator of soil dust input of Sb and Pb
Conservative lithogenic elements such as Al, Ti, Sc and Zr have at times been taken to indicate variations in the atmospheric deposition of soil dust to peat bogs.23,42 In contrast to the other conservative elements, Sc is especially favoured as it exhibits no preference for specific mineral phases and thus tends to be uniformly distributed throughout the environment. The aim is to enable distinction between anthropogenic inputs (for example from mining, smelting, coal burning and vehicle emissions) of heavy metals (e.g. Sb, Pb) and natural, sometimes climate-related, inputs via wind-entrained soil dust.23,42 There can of course also be human-related impacts upon soil dust input, for example arising from the clearance of land for agricultural purposes. Although there can sometimes be specific-element-related problems with this approach,43 the use of metal/Sc concentration ratios, such as Sb/Sc and Pb/Sc here, in peat profiles can be of assistance in estimating the extent of Sb and Pb contributions from sources that are unrelated to direct anthropogenic inputs to the observed variations in the Sb and Pb concentration profiles. The lowest Sb/Sc and Pb/Sc ratios in the 01CM-1 core occurred over the depth region 67–75 cm, with mean values of 0.066 ± 0.016 and 5.3 ± 2.5, respectively, close to the corresponding ratios of 0.044 and 2.43 for the Earth’s Upper Continental Crust (UCC).44 It is most unlikely, however, that Sb and Pb concentrations are unaffected by anthropogenic inputs of these metals at any depth over the ca. 2500 years represented by 01CM-1. In Switzerland, Shotyk et al.24 found the lowest Sb (0.008 ± 0.003 mg kg−1) and Pb (0.23 ± 0.04 mg kg−1) concentrations (a factor of 2–5 lower than those in the zones of minimum Sb and Pb concentrations in Flanders Moss 01CM-1) in peats dating from 5320 to 8020 14C y BP, with corresponding Sb/Sc, Pb/Sc and Sb/Pb ratios of 0.105, 3.0 and 0.035 ± 0.014, respectively. For the UCC, the corresponding Sb/Sc, Pb/Sc and Sb/Pb ratios of 0.044, 2.43 and 0.018 demonstrate the variability that can occur in such ratios of natural elemental concentrations.Pre-Roman and Roman atmospheric Sb and Pb sources and deposition
In 01CM-1, it seems very likely that the elevated Sb/Sc (mean 0.29 ± 0.05) and Pb/Sc (mean 19.8 ± 6.1) ratios (Fig. 5) associated with the elevated Sb and Pb concentrations (Fig. 3) from 96–86 cm, dated at 210–40 B.C. to 20–220 A.D., can be attributed to pre-Roman and Roman metallurgical activities. The Roman Pb mining industry is well documented45,46 but, as the Romans did not occupy Britain until 43 A.D., these elevations in metal concentrations from 210 B.C. predate the Roman occupation by two centuries, suggesting the existence of a pre-Roman Pb extraction industry in the UK.17,47 Indeed, despite lower Sb and Pb concentrations over the earlier 96–102 cm depth interval, the corresponding Sb/Sc and Pb/Sc ratios were also enhanced at 0.19 ± 0.05 and 20.5 ± 4.1. In the UK, evidence has been found for the addition of Pb in varying amounts to mixtures of Cu and Sn during the late Bronze Age (ca. 900–500 B.C.) as a new technique to make bronze that flowed more freely during the casting process48 and the mines of Alderley Edge in Cheshire, west-central England, are believed to have been worked mainly for Cu from the early Bronze Age (ca. 2000 B.C.) and possibly Pb by the late Bronze Age.45,46 Iron working was also introduced ca. 500 B.C. and was well established before the arrival of the Romans.48 Copper and Fe metallurgy are also sources of Sb and Pb to the environment given the association of Sb and Pb with sulfide minerals.25 Later, the Mendip Pb mines in southwest England were worked by 49 A.D.,45,46 only 6 years after the Roman conquest, further suggesting the prior existence of a local mining industry before the arrival of the Romans. The Pb deposits of northeast Wales were worked by the late 1st century A.D. as were those of Derbyshire, central England, by 117–138 A.D.45,46 In Swiss peat cores from Etang de la Gruère, Shotyk et al.24,25 have found enrichments of Sb in association with those of Pb at ca. 2000 14C y BP and have attributed the latter to ancient Pb mining and long-range transport of aerosols from the Iberian Peninsula during the period of greatest Roman mining.6 | ||
| Fig. 5 Profiles of Sb/Sc, Pb/Sc and anthropogenic Sb/Pb ratios from 0–33 cm and 33–106 cm in the 210Pb- and 14C-dated Flanders Moss core 01CM-1. | ||
When Pb isotope ratios of the 01CM-01 peat samples for the 210–40 B.C. to 20–220 A.D. period are plotted along with those of various Pb ores (Fig. 6), they are in much better agreement with those of British origin than with those from the major Spanish mines of Rio Tinto and Murcia,49 which were widely exploited during the Iron Age and Roman times (ca. 200 B.C.–400 A.D.).45 This supports the finding by Le Roux et al.,17 based on research on Lindow Bog in NW England, that Pb contamination in Britain during this period resulted primarily from the mining and smelting of British ores.
 | ||
| Fig. 6 Plot of 208Pb/206Pb versus206Pb/207Pb ratios for samples from 210 B.C. to 220 A.D. in Flanders Moss core 01CM-1 and for galena in various British and Spanish Pb ores. | ||
From 86 to 76 cm, i.e. corresponding to 20–220 A.D. to 120–260 A.D., Pb concentrations (Fig. 3) and Pb/Sc ratios (mean 17.4 ± 5.2) (Fig. 5) were still elevated, but those of Sb and Sb/Sc (mean 0.11 ± 0.04) much less so. The mean 206Pb/207Pb ratio of 1.175 ± 0.003, however, was very similar to those for 86–96 cm (1.177 ± 0.003) and 96–102 cm (1.173 ± 0.003) and indeed for 71–102 cm (1.176 ± 0.003). While it is possible to estimate the soil dust Pb contribution (mean of 0.38 ± 0.15 mg kg−1 over 71–102 cm), and by difference the anthropogenic Pb contribution, to the total Pb concentration via the use of the Pb/Sc ratios in peat and the UCC, it is not possible to do likewise for the 206Pb/207Pb ratio of the specifically anthropogenic Pb, as the Pb isotopic composition of the soil dust Pb is unknown.
Over the various depth intervals from 71 to 102 cm, the mean measured Sb/Pb ratios were 0.013 ± 0.003 (71–76 cm), 0.006 ± 0.002 (76–86 cm), 0.013 ± 0.005 (86–96 cm) and 0.010 ± 0.001 (96–102 cm), with an overall average (71–102 cm) of 0.010 ± 0.004. The soil dust contribution of Sb can be estimated in similar fashion to that of Pb, enabling calculation of the anthropogenic contribution to both Sb and Pb. The corresponding calculated mean anthropogenic Sb/Pb ratios were 0.010 ± 0.004 (71–76 cm), 0.004 ± 0.002 (76–86 cm), 0.012 ± 0.006 (86–96 cm) and 0.009 ± 0.001 (96–102 cm), with an overall average of 0.008 ± 0.005. This is similar to the value of 0.0056 ± 0.0004 obtained for the Sb/Pb ratio in the Pb ore sample from Wanlockhead, southern Scotland, and in general is in line with the <0.8% Sb content in the galena (PbS) of Pb ores.25
Post-Roman and Mediaeval atmospheric Sb and Pb sources and deposition
Sb and Pb concentrations (Fig. 3) and the corresponding Sb/Sc (0.15 ± 0.07; 53–71 cm) and Pb/Sc (10.5 ± 5.6; 53–71 cm) ratios, with a calculated mean anthropogenic Sb/Pb ratio of 0.013 ± 0.004 (excluding the single outlier peak of 0.048 at 66–67 cm), were at their lowest towards the end of (ca. 410 A.D.) and after the Roman occupation of Britain until ca. 650 A.D. (Fig. 5). Thereafter, to 33 cm, Sb concentrations rose more rapidly than those of Pb (Fig. 3), reflected in a mean Sb/Sc ratio of 0.29 ± 0.009 compared with a value of 11.5 ± 3.4 for Pb/Sc from 53 to 33 cm (Fig. 5). The corresponding mean anthropogenic Sb/Pb ratio was also higher, averaging 0.028 ± 0.009 (excluding the single outlier peak of 0.061 at 48–49 cm). The mean 206Pb/207Pb ratio for the period was 1.168 ± 0.004, compared with a value of 1.164 ± 0.006 from 53 to 71 cm. It is interesting to speculate that these less radiogenic values could reflect deposition of Pb from Rio Tinto, at least to the early Middle Ages (cf. 840 A.D. in Monna et al.,50 corresponding to ∼38 cm in 01CM-1), although such low values of 206Pb/207Pb were not observed in Lindow Bog, NW England. The higher anthropogenic Sb/Pb ratios, which indeed averaged 0.032 ± 0.008 from 41 to 33 cm, could reflect influences from continental Europe perhaps due to the developing Ag industry associated with the well known Mediaeval German Ag mining district in the Harz, which brought about the revival of mining in Europe by the 11th century.51 A modified method of cupellation involving the melting of Cu ores with Pb to recover Ag was introduced in the mid-15th century and by ca. 1500 A.D. both this and the Pb mining and smelting industry were prominent in Europe.45,46 It is interesting to note that Klaminder et al.13 found 206Pb/207Pb minima of ∼1.155–1.17 in peat sections dated at ca. 1500 A.D. from Dumme Mosse and Traneröd Mosse in southern Sweden, close to the height of Mediaeval metal production in Europe.51Industrial and post-industrial atmospheric Sb and Pb sources and deposition
Above 33 cm in 01CM-1, a depth for which no age-date is available, both Sb and Pb concentrations (Fig. 3) and the corresponding Sb/Sc (0.67 ± 0.15; 33-23 cm) and Pb/Sc (42 ± 12) ratios increased to a depth of 23 cm (Fig. 5). The corresponding anthropogenic Sb/Pb ratio was lower than from 53 to 33 cm, however, averaging 0.017 ± 0.004, while the 206Pb/207Pb ratio was fairly constant at 1.173 ± 0.002. It seems significant that the indigenous Scottish Pb mining industry developed during the 17th century52 at Leadhills and Wanlockhead as emissions from there would have had the effect of increasing atmospheric Sb and Pb concentrations, while lowering the anthropogenic Sb/Pb ratio from its former higher value in Mediaeval times. Furthermore, the 206Pb/207Pb ratio of Pb ore from Leadhills and Wanlockhead is 1.170 ± 0.003,39–41 similar to the value of 1.173 ± 0.002 observed from 33 to 23 cm in 01CM-1 at Flanders Moss.The depth of 23 cm in 01CM-1, based on a match of 206Pb/207Pb values and associated 210Pb dates in the overlying upper regions of 01CM-1 and 04-1-M, probably corresponds to ca. 1800 A.D. The 210Pb date of 1876 ± 19 A.D. for 17.5 cm in 01CM-1 is in agreement with that of 1877 ± 8 A.D. for 22.5 cm in 04-1-M, for which the earliest 210Pb date is 1832 ± 19 A.D. at 27 cm. It seems likely that a depth of 33 cm in 04-1-M corresponds to the late 1700s A.D.
From 23 cm (ca. 1800 A.D.) to 12 cm (1928 ± 11 A.D.) in 01CM-1, both Sb and Pb concentrations and the corresponding mean Sb/Sc (0.87 ± 0.13) and Pb/Sc (93 ± 18) ratios continued to increase (Fig. 3 and 5). The mean anthropogenic Sb/Pb ratio of 0.009 ± 0.002, however, was lower than formerly but the mean 206Pb/207Pb ratio remained the same at 1.173 ± 0.002. Similarly, from 33 cm (late 1700s A.D.) to 18 cm (1910 ± 8 A.D.) in 04-1-M, Sb and Pb concentrations increased (Fig. 4) while the corresponding mean Sb/Sc (0.98 ± 0.24) and Pb/Sc (89 ± 16) ratios, mean anthropogenic Sb/Pb ratio of 0.011 ± 0.003 (Fig. 7) and mean 206Pb/207Pb ratio of 1.175 ± 0.002 (Fig. 4) were similar to those in 01CM-1. While these trends are consistent with increasing input of both Sb and Pb, the lower anthropogenic Sb/Pb ratio is indicative of relatively greater inputs of Pb, including, as further suggested by the specific value of the observed 206Pb/207Pb ratio, contributions from indigenous Pb ore sources (e.g. at Leadhills and Wanlockhead), which have lower Sb/Pb (0.0056) and 206Pb/207Pb (1.170) ratios than those of coal (0.0036; 1.181). The mining and smelting of indigenous Pb ores, however, which for Leadhills and Wanlockhead was greatest from 1850 to 1920 until closure in the 1930s,52 went into general decline in the UK after the 19th century.53,54
 | ||
| Fig. 7 Profiles of Sb/Sc, Pb/Sc and anthropogenic Sb/Pb ratios in the 210Pb-dated Flanders Moss core 04-1-M. | ||
Over the time periods 1928 ± 11 A.D. to 1969 ± 8 A.D. in 01CM-1 (12 to 5 cm) and 1910 ± 8 A.D. to 1968 ± 2 A.D. in 04-1-M (18 to 12 cm), the main features for the two cores were similar: maximum concentrations in Sb and Pb (at 1948 ± 10; 1955 ± 3 A.D.), maximum Sb/Sc ratios (4.89; 2.54), elevated Pb/Sc ratios (reaching 288; 125), increased mean anthropogenic Sb/Pb ratios (0.014 ± 0.003; 0.020 ± 0.001), and decreasing 206Pb/207Pb ratios (to 1.145 at 1967 ± 9 A.D.; 1.158 for 1943 ± 4 to 1968 ± 2 A.D.) (Fig. 3, 4, 5, 7). The increasing influence of imported Australian Pb (206Pb/207Pb ratio = 1.04), used along with Pb from other sources in the manufacture of alkylPb additives for petrol from the 1930s and then emitted to the atmosphere as inorganic Pb compounds in vehicle exhaust emissions, can be seen in the decline in 206Pb/207Pb ratios. The intermediate values observed for 206Pb/207Pb ratios, however, indicate that there were significant contributions from other sources of Pb, such as coal combustion. Farmer et al.9,34 have previously demonstrated how varying emission contributions from coal combustion (206Pb/207Pb = 1.181) and the mining, smelting and subsequent use of indigenous (1.170) and Australian (1.04) Pb could give rise to the observed trends in atmospheric 206Pb/207Pb during the 20th century. Consumption of coal in the UK peaked in ca. 1950–1960 A.D. and was always greater than 150 Mt y−1 from ca. 1900 to 1970 A.D.34 For 1930 in Scotland,34 the calculated estimated contributions to anthropogenic Pb in the atmosphere from Scottish coal (46%), Pb smelting in Scotland (35%) and sources in the rest of the UK (19%, with a directly measured 206Pb/207Pb ratio55 of 1.145) gave rise to a calculated 206Pb/207Pb ratio of 1.170, the same as that observed in both Flanders Moss peat cores here (Fig. 3 and 4). Using these percentage contributions and the measured Sb/Pb values for coal (0.036) and the indigenous Pb ore (0.0056), along with an estimated value for the rest of the UK of 0.026 (based on a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for coal
1 ratio for coal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) smelter Pb emissions, cf. Table 4 in Farmer et al.34), yields an estimated anthropogenic Sb/Pb ratio of 0.023, which is close to the maximum values of 0.018 and 0.020 observed in 01CM-1 and 04-1-M, respectively. This is further confirmation of the influence of coal combustion on Sb emissions during the first 70 years of the 20th century.
smelter Pb emissions, cf. Table 4 in Farmer et al.34), yields an estimated anthropogenic Sb/Pb ratio of 0.023, which is close to the maximum values of 0.018 and 0.020 observed in 01CM-1 and 04-1-M, respectively. This is further confirmation of the influence of coal combustion on Sb emissions during the first 70 years of the 20th century.
From the late 1960s to the early 1980s A.D. (i.e. from 5 to 3 cm and 12 to 9 cm in 01CM-1 and 04-1-M, respectively), both Sb and Pb concentrations and the corresponding Sb/Sc (1.67 ± 0.32; 1.15) and Pb/Sc (142 ± 25) ratios, with the exception of Pb/Sc (179) for 04-1-M, decreased (Fig. 3, 4, 5, 7). The corresponding mean anthropogenic Sb/Pb ratios also decreased (to 0.012 ± 0.001; 0.008) quite markedly (Fig. 5 and 7), as did the 206Pb/207Pb ratios (to 1.134) (Fig. 3 and 4), indicative of the decreasing influence of coal combustion and the increasing influence of vehicle exhaust emissions of Pb.
From 1982 to 1990 A.D. (i.e. 9 to 6 cm) in 04-1-M, Sb and Pb concentrations continued to fall (Fig. 4) while the Sb/Sc ratio increased to 2.23 and the Pb/Sc ratio, at 184, was similar to the previous value of 179 (Fig. 7). The anthropogenic Sb/Pb ratio of 0.012 (Fig. 7), however, was higher, while the 206Pb/207Pb ratio attained its minimum value of 1.130 (Fig. 4). This evidence suggests a new source of Sb during the period of maximum influence of petrol Pb on the 206Pb/207Pb ratio. Although UK Pb emissions from car exhausts are known to have fallen, in response to reductions in the maximum permitted concentration of Pb in petrol, from ca. 8500 t y−1 in the early 1970s A.D. to ca. 7000 t y−1 by the early 1980s A.D., the final reduction to 0.15 g l−1 and the introduction of unleaded petrol produced a sharp fall to ca. 2900 t in 1986 A.D. and ca. 2200 t by 1990 A.D.26 In 01CM-1, dates for the three sections from 0-3 cm were not available due to the loss of material from the top of this core and a sister Cuttle and Malcolm core collected in 2001.20 Nevertheless, the results for 01CM-1 were in broad agreement with those of 01-4-M, viz. decreasing Sb and Pb concentrations, increasing Sb/Sc (2.18 ± 0.71) and Pb/Sc (269 ± 25), an anthropogenic Sb/Pb ratio of 0.008 ± 0.003 and a minimum mean 206Pb/207Pb value of 1.134 ± 0.001 (Fig. 3 and 5).
The suggestion of new recent sources of Sb while inputs of Pb have been declining is strengthened by the data for the top 6 cm (1990–2004 A.D.) of 01-4-M (Fig. 4 and 7): (i) Sb concentrations and Sb/Sc ratios (2.35 ± 0.04) levelling out while Pb concentrations and Pb/Sc ratios (81 ± 14) decline, (ii) a significant increase in the corresponding mean anthropogenic Sb/Pb ratio to 0.030 ± 0.006, and (iii) an increase in the 206Pb/207Pb ratio to 1.139 ± 0.002. Thus while Pb emissions from car exhausts fell steeply, for example to 800 t by 1997 A.D.,26 as the use of unleaded petrol became increasingly predominant and then complete with the ban on leaded petrol from 2000 A.D., the input of Sb from comparatively new recent sources (i.e. not coal combustion, which has been declining) has probably contributed to the increase in the Sb/Pb ratio. Such recent sources could include the release of Sb from automotive brake linings, where it has been used instead of asbestos to improve heat resistance, and from the degradation or combustion of a variety of other products, including plastics, to which it is added (in the form of Sb2O3) as a flame retardant.25
Historical trends in depositional fluxes and inventories of Sb and Pb
The calculated inferred atmospheric depositional fluxes of anthropogenic Sb and Pb since the mid-19th century are plotted, along with the anthropogenic Sb/Pb ratios and the measured 206Pb/207Pb ratios, versus210Pb-derived calendar dates for 04-1-M in Fig. 8. The maxima of ∼0.42 and ∼21 mg m−2 y−1 for the anthropogenic Sb and Pb fluxes from the mid-1920s to the mid-1950s are evident. The influence of contributions from coal combustion emissions explains both the enhancement in the associated anthropogenic Sb/Pb ratio over the late 19th century to ∼0.20 at that time and, to a certain extent, the limitation of the post-19th century decline in 206Pb/207Pb that stemmed from the introduction and use of Australian Pb in alkylPb additives in petrol. MacKenzie et al.7 have reported peaks in depositional fluxes of Sb and Pb at ca. 1940 A.D. in a peat core from South Drumboy Hill, approximately 18 km southwest of Glasgow although, interestingly, the magnitudes of the maximum fluxes there were greater, at ∼1.5 mg m−2 y−1 and ∼50 mg m−2 y−1, respectively. This could reflect geographical factors related to the proximity to industry etc., but it should also be borne in mind, however, that significant variations in apparent depositional fluxes can be found in the analysis of cores from even the same bog as a consequence of topographical and plant compositional features that affect the efficiency of particle trapping and retention.4,16 Indeed, we have previously observed a range of calculated maximum Pb depositional fluxes from 20–60 mg m−2 y−1 for Flanders Moss.19,20 Further to the northwest in Scotland, at the remote rural Loch Laxford peat bog, Shotyk et al.24,25 found that maximum, albeit much lower, anthropogenic Sb and Pb concentrations of 0.8 mg kg−1 and 27 mg kg−1, respectively, occurred during the same time period at ca. 1930 A.D.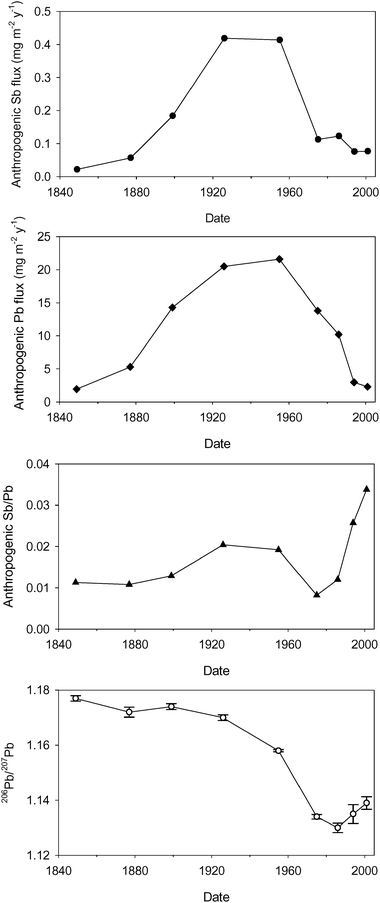 | ||
| Fig. 8 Calculated atmospheric depositional fluxes of anthropogenic Sb and Pb, and the anthropogenic Sb/Pb and measured 206Pb/207Pb ratios for the Flanders Moss core 04-1-M versus210Pb-derived dates since 1840 A.D. | ||
After 1960 A.D., the rapid decline in coal combustion emissions at the same time as the increase in vehicle exhaust emissions is reflected in not only the fall in the 206Pb/207Pb ratio but also in the steeper decline in anthropogenic Sb fluxes (to ∼0.1 mg m−2 y−1) relative to those of Pb, with a concomitant decline in the corresponding Sb/Pb ratio to its minimum value of 0.008 in the mid-1970s A.D. (Fig. 8). Thereafter, the Sb fluxes stayed relatively constant while those of Pb, due to the phasing out of leaded petrol, continued to fall. The 206Pb/207Pb ratio, after reaching a minimum in the mid-1980s A.D., increased to the present because of the increasing relative importance of other sources of Pb (e.g. waste incineration) relative to petrol. The increase in the anthropogenic Sb/Pb ratio, however, probably began slightly earlier, in the mid-1970s A.D., increasing to 0.034 by the early 2000s A.D.
The corresponding values of anthropogenic Sb/Pb ratios and measured 206Pb/207Pb ratios for core 01CM-1 (210Pb-dated by extrapolation from sister core 01CM-2, but only up to the 1970s A.D.) are generally in good agreement with those for 04-1-M (Fig. 9). Likewise there is broad agreement with values obtained independently for preserved herbarium and freshly collected Sphagnum moss samples of known age collected across Scotland.9,56 The mean anthropogenic Sb/Pb ratios for the moss samples over various time periods from the mid-19th century were 0.010 ± 0.005 for 1855–1904 A.D. (n = 9), 0.028 ± 0.013 for 1909–1969 A.D. (n = 16), 0.028 ± 0.019 for 1970–1988 A.D. (n = 10) and 0.107 ± 0.065 for 1991–2000 A.D. (n = 11),56i.e. there was a near 3-fold increase from the lower values of the second half of the 19th century for most of the 20th century and then, in the final decade of the 20th century, a 10-fold increase, somewhat greater than that observed in the peat cores. Interestingly, a freshly collected Sphagnum moss sample at Wanlockhead in 2000 A.D. had an anthropogenic Sb/Pb ratio of 0.0023, in accord with the much lower Sb/Pb ratios observed in former Pb mining/smelting areas.
 | ||
| Fig. 9 Anthropogenic Sb/Pb and measured 206Pb/207Pb ratios versus210Pb-derived dates since 1840 for Flanders Moss cores 04-1-M (open symbols) and 01CM-1 (shaded symbols). | ||
The total anthropogenic Sb and Pb inventories for core 04-1-M (i.e. from the late 1700s to 2004 A.D.) were 0.035 g m−2 and 2.10 g m−2, respectively, of which 3.1% and 1.7% were deposited post-1990 A.D., 7.5% and 13.2% from 1968 to 1990 A.D., 70.2% and 58.3% from 1910 to 1968 A.D., and 19.2% and 26.7% pre-1910 A.D. For the 01CM-1 core, the total anthropogenic Sb and Pb inventories (i.e. from ca. 500 B.C. to ca. mid-1980s, given that the very top of the core was lost during collection) were 0.051 g m−2 and 3.77 g m−2, respectively, of which 0.0485 g m−2 and 3.59 g m−2 were deposited post-1800 A.D. Of the post-1800 inventories of anthropogenic Sb and Pb, 10.4% and 12.9% were deposited from 1969 A.D. to the mid-1980s, 83.6% and 78.4% from 1907 to 1969 A.D., and 6.0% and 8.7% pre-1907 A.D. Compared with the data for 04-1-M, the higher percentages for the ca. 1910–1970 A.D. period in 01CM-1 may partially be attributable to the loss of surface material from the latter and the inclusion of late 1700s data for the former. It should be remembered, however, that the 210Pb dating for 01CM-1 is extrapolated from that of a sister core 01CM-2 and this, with its associated uncertainty, may contribute to some differences in the vertical (temporal) distribution (%) of anthropogenic elemental inventories between the two cores.
For 01CM-1, the pre-1800 A.D. inventories of 0.0023 g Sb m−2 and 0.17 g Pb m−2 constitute just 4.6% of the total anthropogenic Sb and Pb inventories for the core. For the period embracing the Sb peak (86–96 cm) corresponding to 210/40 B.C. to 20/220 A.D. in pre-Roman/Roman times, the anthropogenic Sb and Pb inventories were 0.000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 g m−2 and 0.019 g m−2, respectively. If the mid-points of the 14C-derived dates are taken (i.e. 125 B.C. to 120 A.D.), the corresponding average depositional fluxes over this period would be 0.9 μg Sb m−2 y−1 and 0.078 mg Pb m−2 y−1. These correspond to only ∼0.2% and ∼0.4% of the maximum fluxes of anthropogenic Sb and Pb during the 20th century but are ∼2.6 and ∼7.8 times those estimated by Shotyk et al.24 to be the natural background rate of deposition of Sb and Pb for Switzerland. Over the entirety of the Pb peak in the 01CM-1 core at that time (i.e. 71–102 cm), the anthropogenic Sb and Pb inventories were 0.000
22 g m−2 and 0.019 g m−2, respectively. If the mid-points of the 14C-derived dates are taken (i.e. 125 B.C. to 120 A.D.), the corresponding average depositional fluxes over this period would be 0.9 μg Sb m−2 y−1 and 0.078 mg Pb m−2 y−1. These correspond to only ∼0.2% and ∼0.4% of the maximum fluxes of anthropogenic Sb and Pb during the 20th century but are ∼2.6 and ∼7.8 times those estimated by Shotyk et al.24 to be the natural background rate of deposition of Sb and Pb for Switzerland. Over the entirety of the Pb peak in the 01CM-1 core at that time (i.e. 71–102 cm), the anthropogenic Sb and Pb inventories were 0.000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 g m−2 and 0.045 g m−2.
29 g m−2 and 0.045 g m−2.
The extent of maximum enrichment of Sb and Pb relative to “natural background” can be calculated, using measured Sb/Sc and Pb/Sc ratios and corresponding values for the Upper Continental Crust, via the following expressions:
| Sb EF = ([Sb]/[Sc])MAX/([Sb]/[Sc])UCC and Pb EF = ([Pb]/[Sc])MAX/([Pb]/[Sc])UCC |
Conclusions
The general agreement in the major trends in the concentration profiles of Sb and Pb over the past 2500 years in dated cores from Flanders Moss ombrotrophic peat bog suggests common sources of these two elements and supports the view that Sb, like Pb, is immobile in peat. The derived atmospheric deposition fluxes of anthropogenic Sb and Pb were greatest in the industrial era, peaking during 1920–1960 A.D. Perturbations in the anthropogenic Sb/Pb ratio since 1800 are attributable to temporal variations in the relative importance of atmospheric emissions from different sources such as Pb ore mining and smelting, coal combustion and, in recent decades, automobile-related use of compounds of Pb (in leaded petrol) and of Sb (in brake linings). In the Roman/pre-Roman period, some two millennia ago, the mining and smelting of British Pb ores appears to have been responsible for the small, but clearly discernible, peaks in Sb and Pb concentration in the Flanders Moss peat core. Overall, the 50- to 100-fold maximum enhancement of both Sb and Pb in the atmosphere during the 20th century, when compared with ancient natural levels, suggests that the toxicological threat from Sb may well have been underestimated in the past and warrants further study, especially in the light of continuing input of Sb from newer sources such as waste incineration of plastics.Acknowledgements
We gratefully acknowledge permission from Scottish Natural Heritage to collect peat cores from Flanders Moss, and the assistance of L. J. Eades, M. Halter and C. Yafa at the University of Edinburgh in various aspects of field work, sample preparation and elemental/isotopic analysis. We also acknowledge the SUERC AMS staff for their assistance in making the 14C measurements and C. Donnelly for gamma spectrometric analysis. J.M.C. thanks the Natural Environment Research Council for funding her PhD studentship.References
- A. B. MacKenzie, J. G. Farmer and C. L. Sugden, Sci. Total Environ., 1997, 203, 115 CrossRef CAS.
- M. A. Vile, R. K. Wieder and M. Novak, Biogeochemistry, 1999, 45, 35 CrossRef CAS.
- W. Shotyk, M. E. Goodsite, F. Roos-Barraclough, N. Givelet, G. Le Roux, D. Weiss, A. K. Cheburkin, K. Knudsen, J. Heinemeier, W. O. van der Knaap, S. A. Norton and C. Lohse, Geochim. Cosmochim. Acta, 2005, 69, 1 CrossRef CAS.
- S. A. Norton, G. C. Evans and J. S. Kahl, Water, Air, Soil Pollut., 1997, 100, 271 CrossRef CAS.
- W. Shotyk, A. K. Cheburkin, P. G. Appleby, A. Fankhauser and J. D. Kramers, Water, Air, Soil Pollut., 1997, 100, 297 CrossRef CAS.
- W. Shotyk, D. Weiss, P. G. Appleby, A. K. Cheburkin, R. Frei, M. Gloor, J. D. Kramers, S. Reese and W. O. van der Knaap, Science, 1998, 281, 1635 CrossRef CAS.
- A. B. MacKenzie, E. M. Logan, G. T. Cook and I. D. Pulford, Sci. Total Environ., 1998, 223, 25 CrossRef CAS.
- D. Weiss, W. Shotyk, P. G. Appleby, J. D. Kramers and A. K. Cheburkin, Environ. Sci. Technol., 1999, 33, 1340 CrossRef CAS.
- J. G. Farmer, L. J. Eades, H. Atkins and D. F. Chamberlain, Environ. Sci. Technol., 2002, 36, 152 CrossRef CAS.
- D. Weiss, W. Shotyk, E. A. Boyle, J. D. Kramers, P. G. Appleby and A. K. Cheburkin, Sci. Total Environ., 2002, 292, 7 CrossRef CAS.
- A. Martinez-Cortizas, E. García-Rodeja, X. Pontevedra Pombal, J. C. Nóvoa Muñoz, D. Weiss and A. Cheburkin, Sci. Total Environ., 2002, 292, 33 CrossRef CAS.
- L. J. Eades, J. G. Farmer, A. B. MacKenzie, A. Kirika and A. E. Bailey-Watts, Sci. Total Environ., 2002, 292, 55 CrossRef CAS.
- J. Klaminder, I. Renberg and R. Bindler, Global Biogeochem. Cycles, 2003, 17 Search PubMed , art. no. 1019.
- M. Novák, S. Emmanuel, M. A. Vile, Y. Erel, A. Véron, T. Pačes, R. Wieder, M. Vaněček, M. Štěpánova, E. Břízová and J. Hovorka, Environ. Sci. Technol., 2003, 37, 437 CrossRef CAS.
- W. Shotyk, M. E. Goodsite, F. Roos-Barraclough, R. Frei, J. Heinemeier, G. Asmund, C. Lohse and T. S. Hansen, Geochim. Cosmochim. Acta, 2003, 67, 3991 CrossRef CAS.
- R. Bindler, M. Klarqvist, J. Klaminder and J. Forster, Global Biogeochem. Cycles, 2004, 18 Search PubMed , art. no. GB3020.
- G. Le Roux, D. Weiss, J. Grattan, N. Givelet, M. Krachler, A. Cheburkin, N. Rausch, B. Kober and W. Shotyk, J. Environ. Monit., 2004, 6, 502 RSC.
- J. G. Farmer, M. C. Graham, J. R. Bacon, S. M. Dunn, S. I. Vinogradoff and A. B. MacKenzie, Sci. Total Environ., 2005, 346, 121 CrossRef CAS.
- J. G. Farmer, A. B. MacKenzie, C. L. Sugden, P. J. Edgar and L. J. Eades, Water, Air, Soil Pollut., 1997, 100, 253 CrossRef CAS.
- J. G. Farmer, M. C. Graham, C. Yafa, J. M. Cloy, A. J. Freeman and A. B. MacKenzie, Global Planet. Change Search PubMed , in press.
- W. Shotyk, A. K. Cheburkin, P. G. Appleby, A. Fankhauser and J. D. Kramers, Earth Planet. Sci. Lett., 1996, 145, E1 CrossRef.
- A. B. MacKenzie, E. M. Logan, G. T. Cook and I. D. Pulford, Sci. Total Environ., 1998, 222, 157 CrossRef CAS.
- W. Shotyk, M. Krachler, A. Martinez-Cortizas, A. K. Cheburkin and H. Emons, Earth Planet. Sci. Lett., 2002, 199, 21 CrossRef CAS.
- W. Shotyk, M. Krachler and B. Chen, Global Biogeochem. Cycles, 2004, 18 Search PubMed , art. no. GB1016.
- W. Shotyk, M. Krachler and B. Chen, in Biogeochemistry, Availability, and Transport of Metals in the Environment, Metal Ions in Biological Systems, ed. A. Sigel, H. Sigel and R. K. O. Sigel, M. Dekker, New York, 2005, vol. 44, pp. 177–203 Search PubMed.
- Digest of Environmental Statistics, Department for Environment, Food and Rural Affairs (DEFRA), 2002, http://www.defra.gov.uk/environment/statistics/des/index.htm Search PubMed.
- J. G. Farmer, L. J. Eades, M. C. Graham and J. R. Bacon, J. Environ. Monit., 2000, 2, 49 RSC.
- S. I. Vinogradoff, M. C. Graham, G. J. P. Thornton, S. M. Dunn, J. R. Bacon and J. G. Farmer, J. Environ. Monit., 2005, 7, 431 RSC.
- R. Lindsay, Bogs: The Ecology, Classification and Conservation of Ombrotrophic Mires, Scottish Natural Heritage, Edinburgh, 1995 Search PubMed.
- S. Brooks and R. E. Stoneman, Conserving Bogs: The Management Handbook, The Stationery Office, Edinburgh, 1997 Search PubMed.
- S. P. Cuttle and D. C. Malcolm, Plant Soil, 1979, 51, 297 Search PubMed.
- C. Yafa, J. G. Farmer, M. C. Graham, J. R. Bacon, C. Barbante, W. R. L. Cairns, R. Bindler, I. Renberg, A. Cheburkin, H. Emons, M. J. Handley, S. A. Norton, M. Krachler, W. Shotyk, X. D. Li, A. Martinez-Cortizas, I. D. Pulford, V. MacIver, J. Schweyer, E. Steinnes, T. E. Sjøbakk, D. Weiss, A. Dolgopolova and M. Kylander, J. Environ. Monit., 2004, 6, 493 RSC.
- C. Yafa and J. G. Farmer, Anal. Chim. Acta Search PubMed , in press.
- J. G. Farmer, L. J. Eades and M. C. Graham, Environ. Geochem. Health, 1999, 21, 257 Search PubMed.
- C. Barbante, W. Shotyk, H. Biester, A. Cheburkin, H. Emons, J. G. Farmer, E. Hoffman, A. Martinez-Cortizas, J. Matschullat, S. Norton, J. Schweyer and E. Steinnes, in Proceedings of the 11th International Conference on Heavy Metals in the Environment, ed. J. O. Nriagu, Ann Arbor, MI, USA, 2000, Contribution No. 1106 (CD-ROM) Search PubMed.
- M. Krachler, H. Emons, C. Barbante, G. Cozzi, P. Cescon and W. Shotyk, Anal. Chim. Acta, 2002, 458, 387 CrossRef CAS.
- P. G. Appleby, W. Shotyk and A. Fankhauser, Water, Air, Soil Pollut., 1997, 100, 223 CrossRef CAS.
- G. T. Cook, A. J. Dugmore and J. S. Shore, Radiocarbon, 1998, 40, 21 CAS.
- S. Moorbath, Philos. Trans. R. Soc. London, Ser. A, 1962, 254, 295 CrossRef CAS.
- C. L. Sugden, J. G. Farmer and A. B. MacKenzie, Environ. Geochem. Health, 1993, 15, 59 CrossRef CAS.
- M. Rohl, Archaeometry, 1996, 38, 165 Search PubMed.
- W. Shotyk, D. Weiss, J. D. Kramers, R. Frei, A. K. Cheburkin, M. Gloor and S. Reese, Geochim. Cosmochim. Acta, 2001, 65, 2337 CrossRef CAS.
- C. Reimann and P. de Caritat, Environ. Sci. Technol., 2000, 34, 5084 CrossRef CAS.
- K. H. Wedepohl, Geochim. Cosmochim. Acta, 1995, 59, 1217 CrossRef CAS.
- R. F. Tylecote, A History of Metallurgy, Metals Society, London, 1976 Search PubMed.
- R. F. Tylecote, The Prehistory of Metallurgy in the British Isles, The Institute of Metals, London, 1986 Search PubMed.
- J. O. Nriagu, Lead and Lead Poisoning in Antiquity, John Wiley and Sons, New York, 1983 Search PubMed.
- G. Ritchie and A. Ritchie, Scotland Archaeology and Early History, Edinburgh University Press, Edinburgh, 1991 Search PubMed.
- Z. Stos-Gale, N. H. Gale, J. Houghton and R. Speakman, Archaeometry, 1995, 37, 407 CrossRef CAS.
- F. Monna, C. Petit, J.-P. Guillaumet, J. Jouffroy-Bapicot, C. Blanchot, J. Dominik, R. Losno, H. Richard, J. Lèvêque and C. Chateau, Environ. Sci. Technol., 2004, 38, 665 CrossRef CAS.
- M.-L. Bränvall, R. Bindler, I. Renberg, O. Emteryd, J. Bartnicki and K. Billström, Environ. Sci. Technol., 1999, 33, 4391 CrossRef.
- G. V. Wilson, Special Reports on the Mineral Resources of Great Britain, Memoirs of the Geological Survey, Scotland, 1921, Vol. XVII Search PubMed.
- W. Y. Elliott, E. S. May, J. W. F. Rowe, A. Skelton and D. H. Wallace, International Control in the Non-Ferrous Metals, McMillan, New York, 1937 Search PubMed.
- J. Day and R. F. Tylecote, The Industrial Revolution in Metals, The Institute of Metals, London, 1991 Search PubMed.
- J. R. Bacon, K. C. Jones, S. P. McGrath and A. E. Johnston, Environ. Sci. Technol., 1996, 30, 2511 CrossRef CAS.
- M. Halter and J. G. Farmer, in preparation.
Footnote |
| † This work was presented at the First International Workshop on Antimony in the Environment, Heidelberg, Germany, 16th to 19th May 2005. |
| This journal is © The Royal Society of Chemistry 2005 |
