Homochiral crystallization of helical coordination chains bridged by achiral ligands: can it be controlled by the ligand structure?†
Yong-Tao
Wang
a,
Ming-Liang
Tong
a,
Hai-Hua
Fan
b,
He-Zhou
Wang
b and
Xiao-Ming
Chen
*ab
aSchool of Chemistry & Chemical Engineering, Sun Yat-Sen University, Guangzhou, 510275, China. E-mail: cescxm@zsu.edu.cn; Fax: (+86)20-8411-2245; Tel: (+86)20-8411-2074
bState Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-Sen University, Guangzhou, 510275, China
First published on 4th January 2005
Abstract
Homochiral/heterochiral crystallizations of helical chiral polymer chains bridged by achiral poly-pyridyl ligands dependent on the structures of the bridging ligands and independent on the solvent are described, implying a possible strategy to design achiral crystals of helical chains using chiral bridging ligands.
Chiral coordination polymers with different networks,1–3 which have potential functions, such as enantioselective separations4 and catalysis,5 could be obtained by using chiral ligands,1a,6 and achiral ligands.7 The use of achiral ligands is more attractive since usually such ligands are easier to generate. Unfortunately, the construction of chiral coordination polymers using achiral ligands is currently a challenging research field, especially that of helical chains.8 So far our understanding of chiral coordination polymer chains constructed by achiral bridging ligands is very limited,9 only several asymmetric and achiral pyridyl-bridged polymers10 and chiral coordination chains constructed by some other asymmetric bridging ligands were recently reported.11 In fact, the construction of non-centrosymmetrical solids based on 1D chains is a formidable task owing to the lack of control in the two other dimensions.8 On the other hand, it should be anticipated that the structures of asymmetric bridging ligands have significant effect on recognition and crystallization of the chiral chains into solids. Therefore, we synthesized two new poly-pyridyl ligands, 2-[5-(pyridin-3-yl)-1,3,4-oxadiazol-2-yl]pyridine (L1) and 2-[5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl]pyridine (L2) (Scheme 1).† Both L1 and L2 have two sites (chelate and monodentate) for metal ligation and may extend into polymeric structures with different bridging geometries.
 | ||
| Scheme 1 The asymmetrical bridging ligands and the synthesis of their Cd(II) complexes. | ||
To generate polymer chains, L1 and L2 were used to react with CdI2 (ratio 1 ∶ 1) in MeOH, furnishing [CdI2(L1)]∞ (1) and [CdI2(L2)]∞ (2), respectively (Scheme 1). Solvent effect on the crystallization was also evaluated by reactions of CdI2 with L1 and L2 in N,N-dimethylformamide (DMF), which led to 1 and {[CdI2(L2)(H2O)]·2DMF}∞ (3), respectively. Interestingly, 1, 2 and 3 crystallize in the space groups C2/c, P21, and P212121, respectively.‡
Single-crystal X-ray analysis reveals that centrosymmetrical 1 consists of one Cd(II) atom and one L1 ligand in an asymmetric unit (Fig. 1a), and the metal atom is coordinated in square-pyramidal geometry [Cd–N 2.424(4)–2.434(4) Å, Cd–I 2.706(1)–2.738(1) Å, N–Cd–N 68.4(1)–151.8(1)°, N–Cd–I 92.5(1)–130.6(1)°, I–Cd–I 123.64(2)°]. Such chains helically extended along the 21 axis in the b direction have the both left- and right-handedness, having a pitch of 7.412(1) Å. A left-handed helix is represented in Fig. 2a. Adjacent right- and left-handed chains (1 ∶ 1) are packed in the lattice (see Fig. S1†), exhibiting significant π–π stacking interactions (offset face-to-face distance 3.64 Å) between 3-pyridyl rings from neighbour chains in a zipper-like fashion. It is notable that 1 could be isolated in both MeOH and DMF solutions, which implies 1 has the optimized molecular packing fashion.
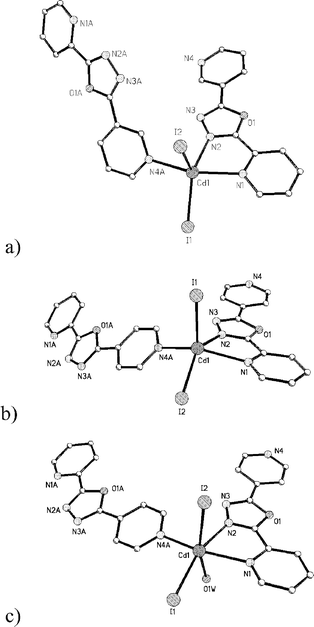 | ||
| Fig. 1 The coordination arrangements of the Cd(II) atoms in 1 (a), 2 (b) and 3 (c). | ||
 | ||
| Fig. 2 Perspective views of the chiral chains in 1 (a), 2 (b) and 3 (c). | ||
Each metal atom in 2 is coordinated in a square-pyramidal geometry [Cd–N 2.398(11)–2.469(11) Å, Cd–I 2.717(3)–2.745(2) Å, N–Cd–N 68.4(4)–143.5(4)°, N–Cd–I 90.6(3)–140.8(2)°, I–Cd–I 118.63(6)°] (Fig. 1b), very similar to that in 1. Interestingly, 2 crystallizes in the chiral space group with each (P) right-handed helical chain related by a 21-screw axis in the b direction, as shown in Fig. 2b. The pitch of the helix in 2 (14.116(1) Å) is almost twice than that of 1, attributable to the structural difference between L1 and L2. Consequently, the packing mode of 2 is different, neighbouring chains in 2 do not have significant π–π stacking interaction, and they are homochiral (Fig. S2†).
In 3, the metal atom exhibits a distorted octahedral geometry [Cd–N 2.360(5)–2.474(5) Å, Cd–I 2.790(1)–2.878(1) Å, Cd–O 2.463(5) Å, N–Cd–N 68.9(2)–155.5(2)°, N–Cd–I 86.4(1)–160.9(1)°, I–Cd–I 109.15(2)°, N–Cd–O 75.0(2)–83.1(2)°, O–Cd–I 88.7(1)–161.2(1)°], being additionally ligated by one aqua ligand (Fig. 1c). Although the coordination geometry has been drastically changed, similar to those in 2, homochiral, (P) right-handed helices exist in 3 (Fig. 2c) and are related by the 21-screw axes in the b direction. The pitch of 3 is only longer by 0.608 Å than that of 2, which is attributed to the increase of coordination number in 3. Different from those in 2, neighbouring chains in 3 have interchain π–π stacking interactions (offset face-to-face, distances between 2-pyridyl and 4-pyridyl rings 3.64 Å), being different from that in 1 (Fig. S3†). Also interestingly, two unusual C–H⋯I hydrogen bonds (C–H⋯I 4.03 and 4.08 Å, C–H⋯I 168 and 165°) (Fig. S4†) between chains are found in 3, which dominate the molecular packing of 3. Therefore, we may be able to conclude that the structure of the bridging ligand is critically important, compared to the solvent and coordination geometry. This observation represents a new strategy different from the literature reports that the existence of solvent, change of solvent or temperature can alter heterochiral/homochiral crystallization.7d,10c,12,13
Quasi-Kurtz powder second harmonic generation (SHG) measurements14 show that 2 and 3 display modest powder SHG efficiencies approximately 0.4 and 0.5 times than that potassium dihydrogen phosphate (KDP), respectively. Unfortunately, we were unable to clarify whether the bulk materials were homochiral.
In summary, this work represents a successful example of ligand-controlled homochiral crystallization of coordination polymer chains via structural variation of achiral, asymmetric bridging ligands. This implies that upon appropriate design of analogous asymmetric bridging ligands, homochiral crystallizations could be achieved and may lead to potential applications. Further study on the related coordination polymers is in progress.
This work was supported by the National Natural Science Foundation of China (No. 20131020) and the Scientific and Technological Bureau of Guangdong Province (No. 04205405).
Notes and references
- (a) B. Kesanli and W. Lin, Coord. Chem. Rev., 2003, 246, 305 CrossRef CAS; (b) N. G. Pschirer, D. M. Ciurtin, M. D. Smith, U. H. F. Bunz and H.-C. zur Loye, Angew. Chem., Int. Ed., 2002, 41, 583 CrossRef CAS; (c) Z.-R. Qu, H. Zhao, Y.-P. Wang, X.-S. Wang, Q. Ye, Y.-H. Li, R.-G. Xiong, B. F. Abrahams, Z.-G. Liu, Z.-L. Xue and X.-Z. You, Chem.–Eur. J., 2004, 10, 53 CrossRef CAS.
- (a) C. Piguet, G. Bernardinelli and G. Hopfgartner, Chem. Rev., 1997, 97, 2005 CrossRef CAS; (b) M. Albrecht, Chem. Rev., 2001, 101, 3457 CrossRef CAS; (c) P. A. Maggard, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2001, 123, 7742 CrossRef CAS; (d) A. D. Cutland-Van Noord, J. W. Kampf and V. L. Pecoraro, Angew. Chem., Int. Ed., 2002, 41, 4667 CrossRef CAS.
- (a) B. Moulton and M. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (b) M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS; (c) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1461 CrossRef.
- R.-G. Xiong, X.-Z. You, B. F. Abrahams, Z.-L. Xue and C.-M. Che, Angew. Chem., Int. Ed., 2001, 40, 4422 CrossRef CAS.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kimoon, Nature, 2000, 404, 982 CrossRef CAS.
- (a) A. Akasaka, K. Biradha, S. Sakamoto, K. Yamaguchi and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 3269 CrossRef CAS; (b) E. V. Anokhina and A. J. Jacobson, J. Am. Chem. Soc., 2004, 126, 3044 CrossRef CAS.
- (a) E. Q. Gao, S. Q. Bai, Z. M. Wang and C. H. Yan, J. Am. Chem. Soc., 2003, 125, 4984 CrossRef CAS; (b) L. Han, M. C. Hong, R. H. Wang, J. H. Luo, Z. Z. Lin and D. Q. Yuan, Chem. Commun., 2003, 2580 RSC; (c) U. Siemeling, I. Scheppelmann, B. Neumann, A. Stammler, H.-G. Stammler and J. Frelek, Chem. Commun., 2003, 2236 RSC; (d) T. Ezuhara, K. Endo and Y. Aoyama, J. Am. Chem. Soc., 1999, 121, 3279 CrossRef CAS.
- O. R. Evans and W. B. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS.
- X.-M. Chen and G.-F. Liu, Chem.–Eur. J., 2002, 8, 4811 CrossRef CAS.
- (a) M.-L. Tong, X.-M. Chen, B.-H. Ye and S. W. Ng, Inorg. Chem., 1998, 37, 5278 CrossRef CAS; (b) K. Uemura, S. Kitagawa, K. Fukui and K. Saito, J. Am. Chem. Soc., 2004, 126, 3817 CrossRef CAS; (c) A. N. Khlobystov, M. T. Brett, A. J. Blake, N. R. Champness, P. M. W. Gill, D. P. O'Neill, S. J. Teat, C. Wilson and M. Schröder, J. Am. Chem. Soc., 2003, 125, 6753 CrossRef CAS; (d) I. S. Lee, D. M. Shin and Y. K. Chung, Chem.–Eur. J., 2004, 10, 3158 CrossRef CAS; (e) D. A. Beauchamp and S. J. Loeb, Chem. Commun., 2002, 2484 RSC.
- (a) Y. Cui, H. L. Ngo and W. Lin, Chem. Commun., 2003, 1388 RSC; (b) A. Jouaiti, M. W. Hosseini and N. Kyritsakas, Chem. Commun., 2002, 1898 RSC; (c) M. Kondo, M. Miyazawa, Y. Irie, R. Shinagawa, T. Horiba, A. Nakamura, T. Naito, K. Maeda, S. Utsuno and F. Uchidac, Chem. Commun., 2002, 2156 RSC; (d) R.-H. Wang, L.-J. Xu, X.-S. Li, Y.-M. Li, Q. Shi, Z.-Y. Zhou, M.-C. Hong and A. S. C. Chan, Eur. J. Inorg. Chem., 2004, 1595 CrossRef CAS.
- J. Jacques, A. Collet and S. H. Wilen, Enantiomers, Racemates and Resolutions, Krieger Publishing Company, Malabar, FL, 1994 Search PubMed.
- L. Pérez-García and D. B. Amabilino, Chem. Soc. Rev., 2002, 31, 342 RSC.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis for L1, L2, 1–3, and additional plots for 1–3. See http://www.rsc.org/suppdata/dt/b4/b416412a/ |
| ‡ Crystal data for 1 (C12H8CdI2N4O): monoclinic, space group C2/c, M = 590.42, a = 33.006(7), b = 7.412(3), c = 13.816(3) Å, β = 111.460(3)°, V = 3146(1) Å3, Z = 8, µ = 5.314 mm−1, R1 = 0.0360, wR2 = 0.0947. Crystal data for 2 (C12H8CdI2N4O): monoclinic, space group P21, M = 590.42, a = 7.057(7), b = 14.116(1), c = 8.743(9) Å, β = 112.36(2)°, V = 805.5(14) Å3, Z = 2, µ = 5.188 mm−1, R1 = 0.0342, wR2 = 0.0890, Flack x = −0.03(6). Crystal data for 3 (C18H24CdI2N6O4): orthorhombic, space group P212121, M = 754.63, a = 8.295(1), b = 14.724(2), c = 21.236(2) Å, V = 2593.8(5) Å3, Z = 4, µ = 3.26 mm−1, R1 = 0.0453, wR2 = 0.0983, Flack x = 0.02(3). CCDC reference numbers 253093–253095. See http://www.rsc.org/suppdata/dt/b4/b416412a/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
