Connecting cyclophosphazenes via ring N-centres with covalent linkers
Mark A.
Benson
and
Alexander
Steiner
*
Department of Chemistry, University of Liverpool, Crown Street, Liverpool, UK L69 7ZD. E-mail: a.steiner@liv.ac.uk
First published on 21st September 2005
Abstract
Two cyclotriphosphazene rings can be covalently linked via ring N-centres by a 2-butene-1,4-diyl unit and vice versa a cyclotriphosphazene molecule is able to bridge two cinnamyl groups via two ring N-centres yielding in either case dicationic assemblies which offers a route to novel polycations.
Polyphosphazenes have played an important role in the development of inorganic polymers due to the ease of introducing a wide variety of substituents onto P-centres.1 Additionally, cyclophosphazenes can be used as building blocks for macromolecular and polymeric species. This is achieved by connecting two or more cyclophosphazene units via P-centres using di- or poly-functional linkers.2 However, owing to the multitude of potential anchoring sites, linking cyclophosphazenes via P-centres is a rather unselective route.3
Herein we show that two cyclophosphazene molecules can be connected via ring N-centres with covalent linkers resulting in distinct products. So far only non-covalent linkages between phosphazenes have been described, in which ring N-centres play a role. These include hydrogen bonding4 and metal coordination.5 Nonetheless, it has been reported that cyclotriphosphazenes carrying electron-donating substituents are quaternised by methyl iodide at ring N-sites.6 We assumed that equivalent reactions with bifunctional electrophiles provide a convenient route to link phosphazenes in covalent fashion.
Both hexakis(iso-butylamino) cyclotriphosphazene, 1a,4 and dichloro tetrakis(cyclohexylamino) cyclotriphosphazene, 1b,7 were treated with 1,4-dibromo-2-butene yielding compounds 2a and 2b, which contain dications comprising two cyclophosphazenes linked by a 2-butene-1,4-diyl unit (Scheme 1). 2a is obtained after heating a freshly ground mixture of 1a and 1,4-dibromo-2-butene in a 2 ∶ 1 molar ratio to 90 °C for 1 h. The 31P NMR spectrum of 2a in chloroform displays an AX2 signal {δ 12.9 (t), 16.8 (d), 2JP–P = 45.3 Hz}. Single crystals for X-ray structure analysis† were obtained from slow evaporation of a methanol solution giving crystals of the solvate 2a·2CH3OH.
 | ||
| Scheme 1 | ||
The dication in 2a (Fig. 1) exhibits inversion symmetry, thus both phosphazene rings are aligned in parallel; their mean planes are displaced by 2.15 Å. The phosphazene ring is slightly puckered, adopting a half-chair conformation. The quaternisation of one ring N-atom has a notable effect on the P–N bonds in the ring: the bonds associated with the quaternised N atom measure 1.688(5) and 1.689(5) Å. This is rather long for cyclophosphazene bonds, which are on average 1.60 Å in the parent phosphazene 1a.4 Fairly long bonds were also found in cyclophosphazenes featuring protonated ring N-centres (∼ 1.67 Å).8 On the other hand, the remaining ring P–N bonds in 2a range between 1.577(5) and 1.591(5) Å and are slightly shorter than those found in 1a.4 The supramolecular structure of 2a·2CH3OH consists of a two-dimensional assembly held together by an extensive network of hydrogen bonds (Fig. 2). All six NH groups and two ring N-atoms of each phosphazene unit are involved in H-bonding either towards bromide ions or methanol molecules.
 | ||
| Fig. 1 Crystal structures of the dications in 2a (top) and 2b (bottom). H-atoms are omitted; the bridging 2-butene-1,4-diyl unit is highlighted in orange; blue: N, purple: P, green: Cl. | ||
 | ||
| Fig. 2 Supramolecular structure of 2a·2CH3OH in the solid state. H-bonds are drawn as dashed lines, H-atoms and Bui-groups are omitted; red: O; green: Br. | ||
The dichloro derivative 2b was obtained after refluxing a solution of 1b and 1,4-dibromo-2-butene (2 ∶ 1 ratio) in thf for 12 h. The 31P NMR spectrum of 2b shows an AX2 signal pattern {δ 13.3 (d), 18.9 (t), 2JP–P = 45.5 Hz}. The solvate 2b·2CHCl3 crystallised from chloroform solution containing three molecules in the asymmetric unit. The X-ray crystal structure† reveals that the ring N-centre opposite the PCl2 unit is quaternised exclusively (Fig. 1). Evidently, the electron withdrawing effect of the PCl2 unit prevents quaternisation of the adjacent ring N-centres.
It is interesting to note that heating a 1 ∶ 1 mixture of 1a and 1,4-dibromo-2-butene to 90 °C produces exclusively 2a. There is no trace of the supposed monocationic species 3a apparent in the 31P NMR. However, reacting 1a in the presence of 10 equivalents of 1,4-dibromo-2-butene in refluxing chloroform leads to a product mixture that contains 2a and 3a in a 1 ∶ 2 molar ratio as inidcated by 31P NMR which exhibits a set of AX2 signals for each species {3a resonates at δ 13.1 (t) and 16.0 (d), 2JP–P
= 43.3 Hz}. The cations of 2a and 3a were identified by electrospray mass spectrometry. We are currently investigating the mechanism leading to the preferential formation of 2a over 3a. The dicationic species 2a is stable towards mild nucleophiles such as water, alcohols and dilute mineral acids. However, in the presence of alkali metal hydroxides and alkoxides the bridging 2-butene-1,4-diyl unit slowly (t½
∼ 24 h) cleaves off the phosphazene rings yielding 1a.
In order to probe whether not only 2-butene-1,4-diyl, but also the cyclotriphosphazenes, can act as linkers, 1a was mixed with excess cinnamyl bromide and the mixture was heated to 90 °C yielding the mono- and the di-quaternised species 4 and 5, respectively (Scheme 2). The product ratio of 4 and 5 is 3 ∶ 1 after 2 h, however, 5 is the main product after heating for 24 h accompanied by only traces of 4. Solutions of both 4 and 5 in chloroform display AX2 signals in the 31P NMR spectrum {4: δ 12.7 (t), 17.6 (d), 2JP–P = 40.5 Hz; 5: δ 15.6 (d), 32.1 (t), 2JP–P = 29.3 Hz}. While 4 shows 31P NMR shifts similar to 2a and 3a, the triplet signal of 5 appears at lower field owing to the direct neighbourhood of two quaternised N-centres. Correspondingly, the P–P coupling constant in 5 is lower, since coupling occurs across a quaternised N-atom.
 | ||
| Scheme 2 | ||
Single crystals of 5 for X-ray structure analysis† were obtained from hexane/thf. The crystal structure confirms that the phosphazene ring binds two cinnamyl groups via two ring N-atoms (Fig. 3). There are two dications in the asymmetric unit, one of which contains a disordered phosphazene core. In the following only the bonding parameters of the non-disordered dication are discussed. The quaternisation of the two ring N-centres results in elongation of the adjacent P–N bonds. The P–N bonds between quaternised N-atoms and the two chemically equivalent P-atoms are longer (av. 1.684 Å) than the P–N bonds associated with the quaternised N-atom and the chemically unique P-atom (av. 1.635 Å). In contrast, the P–N bonds involving the unreacted N-atom are short (av. 1.573 Å). 5 forms an extensive network of hydrogen bonds in the solid state. The supramolecular structure consists of a double sheet arrangement of dications, which are connected by hydrogen bonding across bromide ions (Fig. 4). Again, all NH-units engage in hydrogen bonding. The phenyl groups of the cinnamyl groups are facing towards the inside of the double sheet arrangement.
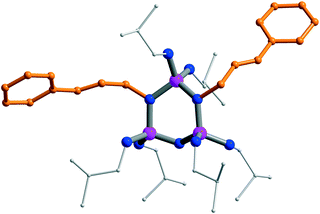 | ||
| Fig. 3 Crystal structure of the dication in 5. H-atoms are omitted; cinnamyl groups are highlighted in orange; blue: N, purple: P. | ||
 | ||
| Fig. 4 Fraction of the supramolecular structure of 5 in the solid state. H-bonds drawn as dashed lines, H-atoms and Bui groups omitted. | ||
Polycations find applications in many areas including polyelectrolytes, membranes, drug delivery and there is an on-going search for new and improved systems.9 The potential to link phosphazenes with alkylene groups and to use phosphazenes as linkers via ring N-sites offers a route towards novel polycations consisting of alternating arrangements of phosphazenes and alkylene groups.
We greatly acknowledge EPSRC for financial support.
Notes and references
- J. E. Marck, H. R. Allcock and R. West, Inorganic Polymers, Second Edition, Oxford University Press, New York, 2005 Search PubMed; V. Chandrasekhar, Inorganic and Organometallic Polymers, Springer, Heidelberg, 2005 Search PubMed.
- See for example: J.-F. Labarre, G. Guerch, F. Sournies, R. Lahana, R. Enjalbert and J. Galy, J. Mol. Struct., 1984, 116, 75 Search PubMed; D. B. Davies, T. A. Clayton, R. E. Eaton, R. A. Shaw, A. Egan, M. B. Hursthouse, G. D. Sykara, I. Porwolik-Czomperlik, M. Siwy and K. Brandt, J. Am. Chem. Soc., 2000, 122, 12447 CrossRef CAS; S. J. Coles, D. B. Davies, R. J. Eaton, M. B. Hursthouse, A. Kilic, R. A. Shaw and A. Uslu, Eur. J. Org. Chem., 2004, 1881 CrossRef CAS; S. Bilge, A. Natsagdorj, S. Demiriz, N. Caylak, Z. Kilic and T. Hokelek, Helv. Chim. Acta, 2004, 87, 2088 CrossRef CAS; A. J. Elias, R. L. Kirchmeier and J. M. Shreeve, Inorg. Chem., 1994, 33, 2727 CrossRef CAS; H. R. Allcock, W. R. Laredo, E. C. Kellam III and R. V. Morford, Macromolecules, 2001, 34, 787 CrossRef CAS; C. W. Allen, E. D. Brown and S. D. Worley, Inorg. Chem., 2000, 39, 810 CrossRef CAS; V. Chandrasekhar, A. Athimoolam, S. G. Srivatsan, P. S. Sundaram, S. Verma, A. Steiner, S. Zacchini and R. Butcher, Inorg. Chem., 2002, 41, 5162 CrossRef CAS.
- G. Guerch, J.-F. Labarre, R. Lahana, R. Roques and F. Sournies, J. Mol. Struct., 1983, 99, 275 CrossRef CAS.
- J. F. Bickley, R. Bonar-Law, G. T. Lawson, P. I. Richards, F. Rivals, A. Steiner and S. Zacchini, Dalton Trans., 2003, 1235 RSC.
- P. I. Richards and A. Steiner, Inorg. Chem., 2004, 43, 2810 CrossRef CAS; V. Chandrasekhar, V. Krishnan, A. Steiner and J. F. Bickley, Inorg. Chem., 2004, 43, 166 CrossRef CAS.
- H. R. Allcock, M. L. Levin and P. E. Austin, Inorg. Chem., 1986, 25, 2281 CrossRef CAS; N. L. Paddock, T. N. Ranganathan and J. N. Wingfield, J. Chem. Soc., Dalton Trans., 1972, 1578 RSC.
- V. Chandrasekhar, K. Vivekanandan, S. Nagendran, G. T. Senthil Andavan, N. R. Weathers, J. C. Yarbrough and A. W. Cordes, Inorg. Chem., 1998, 37, 6192 CrossRef CAS.
- See for example: H. R. Allcock, E. C. Bissell and E. T. Shawl, Inorg. Chem., 1973, 12, 2963 Search PubMed; H. R. Allcock, A. G. Scopelianos, R. R. White and N. M. Tollefson, J. Am. Chem. Soc., 1983, 105, 1316 CrossRef CAS; M. Bloy, M. Kretschmann, S. Scholz, M. Teichert and U. Diefenbach, Z. Allg. Anorg. Chem., 2000, 626, 1946 CrossRef CAS.
- See for example: D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581 Search PubMed; Z. Wang, A. Lough and I. Manners, Macromolecules, 2002, 35, 7669 CrossRef CAS; I. F. J. Vankelecom, Chem. Rev., 2002, 102, 3779 CrossRef CAS; A. W. Kleij, R. van de Coevering, R. J. M. Klein Gebbink, A.-M. Noordman, A. L. Spek and G. van Koten, Chem.–Eur. J., 2001, 7, 181 CrossRef CAS; C. Larr, B. Donnadieu, A. M. Caminade and J. P. Majoral, J. Am. Chem. Soc., 1998, 120, 4029 CrossRef CAS.
Footnote |
| † Crystallographic data were recorded on a Bruker Apex diffractometer using MoKα-radiation (λ = 0.71073 Å), T = 100 K, structures were refined by full-matrix least squares against F2 using all data (SHELXTL). Apart from disordered atoms, non-hydrogen atoms were refined anisotropically and hydrogen atoms were fixed geometrically. 2a·2CH3OH: C54H134Br2N18O2P6, M = 1413.43, P21/c, a = 12.8506(8), b = 16.7507(10), c = 19.3666(12) Å, β = 108.3600(10)°, V = 3956.6(4) Å3, Z = 2, μ(Mo-Kα) = 1.192, 5127 independent reflections, R1 [I > 2σ(I)] = 0.060, wR2 (all data) = 0.174; 2b·2CHCl3: C54H104Br2Cl10N14P6, M = 1649.65, P-1, a = 15.580(3), b = 26.870(5), c = 28.283(6) Å, α = 90.25(3), β = 92.69(3), γ = 94.36(3)°, V = 11792(4) Å3, Z = 6, μ(Mo-Kα) = 1.537, 23661 independent reflections, R1 [I > 2σ(I)] = 0.078, wR2 (all data) = 0.230; 5: C42H78Br2N9P3, M = 961.86, C2/c, a = 49.929(6), b = 15.2205(17), c = 30.940(3) Å, β = 118.633(2)°, V = 20637(4) Å3, Z = 16, μ(Mo-Kα) = 1.701, 10705 independent reflections, R1 [I > 2σ(I)] = 0.106, wR2 (all data) = 0.310. All three crystal structures show disorder of one or more R-groups. In addition, 2b contains disordered chloroform molecules and in 5 one bromide ion and one P(NHR)2 unit in one of the two unique dications are disordered. Disordered atoms were split on two positions and refined isotropically using similar-distance and similar-U restraints. Crystals of both 2b and 5 were of small size and diffracted to only low resolution. In both cases the datasets were truncated at 2θ = 40°. CCDC 280844–280846. See http://dx.doi.org/10.1039/b510898e for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |

