Slow relaxation of magnetisation in an octanuclear cobalt(II) phosphonate cage complex†
Stuart J. Langleya, Madeleine Helliwella, Roberta Sessoli*b, Patrick Rosac, Wolfgang Wernsdorfer*d and Richard E. P. Winpenny*a
aDepartment of Chemistry, The University of Manchester, Oxford Road, Manchester, UK M13 9PL. E-mail: richard.winpenny@man.ac.uk
bLaboratorio di Magnetismo Molecolare, Dipartimento di Chimica, Università degli Studi di Firenze & INSTM, Via della Lastruccia n. 3, Sesto Fiorentino, 50019, Italy
cGroupe de Sciences Moléculaires, Institut de Chimie de la Matière Condensée de Bordeaux – CNRS UPR 9048, 87 avenue du Docteur Schweitzer, Pessac Cedex, 33608, France
dLaboratoire Louis Néel – CNRS, BP 166, 25 Avenue des Martyrs, Grenoble Cedex 9, 38042, France
First published on 23rd September 2005
Abstract
The synthesis, structural and magnetic characterisation of a new polymetallic cobalt complex are reported; the magnetic behaviour is unusual.
While single molecule magnets (SMMs) have been studied for ten years, the vast majority still feature manganese(III) as the metal ion present.1 SMMs have also been found for FeIII,2 NiII,3 FeII4 and an isolated case for VIII,5 and there are increasing studies of heavier metals.6 Cobalt(II), which has a large single ion anisotropy due to the orbitally degenerate ground state, may potentially be a good candidate for making SMMs, and there are two claims in the literature of cobalt-based SMMs: a tetranuclear cage made by Hendrickson and co-workers,7 and a hexanuclear cage reported by Murrie et al.8 However both examples demonstrate the difficulty in proving that cobalt cages are SMMs as neither shows all the features found in more clear-cut SMMs. Here we report a new {Co8} cage, which again shows features due to slow relaxation of magnetization, and discuss the behavior of these cobalt(II) SMMs.
We have reported several cobalt(II) phosphonate cage complexes,9,10 made from reaction of a cobalt salt with a pyridone ligand and phosphonic acid in presence of a suitable base. If the base is an alkali metal hydroxide, the alkali metal ion is found in the final compound,10 therefore we have extended the studies to amines as the base. The compound discussed here is made from hydrated cobalt nitrate reacted with two equivalents of 6-chloro-2-hydroxypyridine (Hchp), one-sixth of an equivalent of PhPO3H2 and two and one-third equivalents of triethylamine in MeCN.‡ The solution was filtered, evaporated to dryness and extracted with CH2Cl2; crystals of [Et3NH] [Co8(chp)10(O3PPh)2(NO3)3(Hchp)2] 1 (Fig. 1) were grown by layering with ether.§
 | ||
| Fig. 1 Structure of 1 in the crystal. | ||
The structure is irregular. Two P-atoms from phosphonate and four Co atoms lie on the vertices of a central trigonal prism. The final four Co atoms lie above the triangular faces of the prism. The phosphonates both show the 3.111 mode (Harris notation12). The structure also contains two chelating nitrates and a 2.11 bridging nitrate. Two of the Co sites (Co4 and Co6) within the trigonal prism are five-coordinate, bound to 2 N and 3 O-donors, with geometries derived from a trigonal pyramid. The remaining six Co sites are six-coordinate, but fall into three pairs. Co1 and Co8 are bound to 2 N and 4 O-donors, Co2 and Co7 are bound to 1 N and 5 O-donors and Co3 and Co5 are bound to 6 O-donors. The degree of distortion also varies as Co1, Co8, Co2 and Co7 are bound to both donors of two chelating groups. All O-atoms bound to Co3 and Co5 are from ligands that are singly attached to these metal centers.
The anions of 1 are not isolated, but are linked via H-bonding which involves the NEt3H cations (Fig. 2). The interaction involves three groups: the terminal O-atoms from chelating nitrates attached to Co2 and Co7 and the cation. The N⋯O and O⋯O distances within the resulting triangle are 2.95 and 2.78 Å respectively. The result is to form 1D-chains of {Co8} cages running parallel to the a-axis of the crystal. The interactions between these chains are much less significant, with the closest contacts occurring between chlorides in chp ligands in one strand and aromatic H-atoms in the next; the Cl⋯H distances are in excess of 2.7 Å. There are also disordered molecules of CH2Cl2 in the crystal lattice, lying in the vicinity of the H-bond between anions.
![The H-bonded link of two anions of 1 in the crystal, via the [HNEt3] cation.](/image/article/2005/CC/b510106a/b510106a-f2.gif) | ||
| Fig. 2 The H-bonded link of two anions of 1 in the crystal, via the [HNEt3] cation. | ||
The variable temperature magnetic behaviour¶ of a powder sample of 1 (Fig. 3) shows a decline in χmT as temperature decreases, from a room temperature value of ca. 20 emu K mol−1 to below 12 emu K mol−1 at 30 K. There is then a small maximum as temperature falls further (ca. 11.9 emu K mol−1 at 12 K), before a further rapid fall at the lowest temperature studied. Spin–orbit coupling of CoII could be causing the decline at higher temperatures and the maximum is probably due to competing anti-ferromagnetic exchange interactions leading to a ground state which is magnetic. It is impossible to assign a “spin” ground state to such a compound. The magnetization of the sample shows no sign of saturating up to a field of 6 T (inset of Fig. 3). The temperature dependence of the a.c. susceptibility, shown in Fig. 4, is more intriguing. Between 4 and 6 K a frequency dependent maximum is found in both the in-phase, χ′, and out-of-phase susceptibility, χ″, this last one however is about one-tenth the height of the equivalent maximum in χ′. In well-established SMMs, this ratio is normally 1 ∶ 2. The observed behaviour suggests that only a fraction of the magnetization is relaxing slowly, as the χ′ continues to increase on lowering the temperature, while a comparison of χ′ and χ″ values suggests that a moderate distribution of relaxation times is present. The relaxation time extracted from the temperature of the maximum χ″ (ω) as τ(Tmax) = ω−1 suggests an Arrhenius law, τ = τ0exp(Δ/kBT), with Δ = 84(±2) K and τ0 = 1.8(±3) × 10−12 s. We repeated the measurement on a second sample of 1 and found a similar energy barrier (80 K), but with a pre-exponential factor of 2.1 × 10−11 s. This would be a very high energy barrier for an SMM.
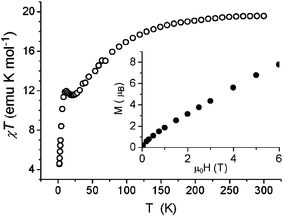 | ||
| Fig. 3 A plot of the molar χT against T for 1. In the inset the molar magnetization against field measured at T = 2.0 K. | ||
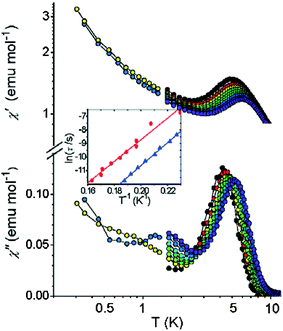 | ||
| Fig. 4 Real (top) and imaginary (bottom) components of the a.c. susceptibility of 1 in zero static field and frequencies ranging from 250 Hz (black) to 20 kHz (violet). For the sake of clarity data below 1.2 K are shown only for 1.1 kHz and 7.6 kHz. In the inset the Arrhenius plot of the temperature dependence of the relaxation time for the sample of the main picture (blue triangles) and for a second sample (red circles). The lines are the linear fit giving the parameters discussed in the text. | ||
Both χ′ and χ″ increase on lowering the temperature below the main peak. The investigation was therefore extended down to 300 mK. The data, reported in Fig. 4 for two frequencies (more frequencies available in Supplementary Information) show a nonzero χ″ that increases on lowering the temperature, except for a small peak around 1.5 K only resolvable at the highest frequencies. At the same time χ′ increases steadily on lowering the temperature but shows a dispersion in frequency that is substantially temperature independent (see Fig. S2).
In order to better characterize the dynamic properties of the material the a.c. susceptibility has been measured under applied static field (data available in Supplementary Information, Fig. S1). Interestingly the effect of the field is different on the two peaks in χ″: while χ′ decreases in both cases, as is expected for saturation effects, χ″ decreases for the high temperature peak but increases for the lower one. In SMMs the application of a moderate static field is well known to suppress tunneling in zero field, thus shifting the χ″ peak to higher temperatures. This effect seems only to be active on the low temperature peak.
If the energy barrier were as high as 80 K we should see hysteresis in a magnetization against field plot. Studies on single crystals of 1 have been performed using a micro-SQUID array. Hysteresis is observed at 4 K and lower temperatures as shown in Fig. 5; however the temperature dependence of the width of the hysteresis is unusual. The hysteresis widens as the temperature falls, as is usually observed. However at temperatures lower than 0.5 K the hysteresis narrows and the profile changes towards a “butterfly” shape; as a result at 40 mK there is a very abrupt change in magnetization at approximately zero-field. This behavior is in agreement with the presence of two relaxation mechanisms in the sample studied. For some part of the sample slow relaxation is observed, with a portion behaving as an SMM with a considerable energy barrier that hampers the reversal of magnetization. However most of the sample shows a fast relaxation process, especially near zero-field. The superposition of these two types of magnetization loops would provide the observed butterfly shape.
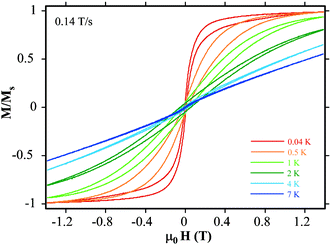 | ||
| Fig. 5 Magnetization curves recorded on a single crystal of 1 with a μ-SQUID magnetometer at different temperatures (sweep rate 0.14 T/s). | ||
The key question is why a relatively small fraction (around 10%) of the chemical species present in the crystal relaxes slowly, with no significant effect of a d.c. field, while others relax quickly. A possible explanation is that the magnetization dynamics reflects the presence of significant inter-cluster interactions through H-bonds. They seem to provide a 1D structure of weakly interacting SMMs and in this case tunneling in zero field is expected to be suppressed as the up and down orientations of the magnetic moments of one cluster are no longer degenerate. Moreover the exchange interactions can contribute to enhancing the barrier height.13 Relatively long segments of H-bonded clusters are thus expected to relax slowly as tunneling is not efficient. The sample dependent value of τ0 could also be rationalized within this picture, as τ0 for slow relaxing 1D systems has been recently found to be proportional to the length of the segments.14 Less strongly interacting clusters could show fast relaxation and their behavior, at least at the time scale of the a.c. experiments, is not simply paramagnetic, but rather that of species relaxing with a widely distributed temperature independent rate. This distribution seems to peak well above 10 kHz, the highest frequency used in the low temperature investigation, and therefore is in agreement with the absence of coercivity for the majority of the sample.
The observed wide distribution of the tunneling rate could come from the different probability of co-tunneling of very short segments comprising different numbers of interacting {Co8} units, the tunnel probability scaling exponentially with the number of units,15 as well as from a distribution of distortion of the environment around non interacting {Co8} clusters. It has already been shown that the dynamic properties of Co-based SMMs are extremely sensitive to the environment modification induced, for instance, by loss of solvation molecules.8
The co-existence of two very different mechanisms of magnetic relaxation deserves to be further investigated because any mechanism that can modify the relaxation of the magnetization in such a significant way is of potential interest for fast switching of the magnetization.
This work was supported by the EU Research and Training Network “QuEMolNa” (MRTN-CT-2003-504880), and by the EPSRC (UK). S. J. L. thanks EST, FP6-504204: MOLMAG for supporting a study period in Florence. M. A. Novak is gratefully acknowledged for his assistance in the a.c. probe construction.
Notes and references
- For example: (a) R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS; (b) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (c) C. Boskovic, W. Wernsdorfer, K. Folting, J. C. Huffman, D. N. Hendrickson and G. Christou, Inorg. Chem., 2002, 41, 5107 CrossRef CAS; (d) E. K. Brechin, L. F. Jones, D. Collison and S. J. Teat, Chem. Commun., 2002, 2974–2975 RSC.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS.
- (a) H. Andres, R. Basler, A. J. Blake, E. K. Brechin, C. Cadiou, G. Chaboussant, C. M. Grant, H.-U. Güdel, S. G. Harris, M. Murrie, S. Parsons, C. Paulsen, F. Semadini, V. Villar, W. Wernsdorfer and R. E. P. Winpenny, Chem. Eur. J., 2002, 8, 4867 CrossRef CAS; (b) M. Murrie, H. Stöeckli-Evans and H. U. Güdel, Angew. Chem., Int. Ed., 2001, 40, 1957 CrossRef CAS; (c) R. S. Edwards, S. Maccagnano, E. C. Yang, S. Hill, W. Wernsdorfer, D. Hendrickson and G. Christou, J. Appl. Phys., 2003, 93, 7807 CrossRef CAS.
- H. Oshio, N. Hoshino and T. Ito, J. Am. Chem. Soc., 2000, 122, 12602–12603 CrossRef CAS.
- Z. Sun, D. N. Hendrickson, C. M. Grant, S. L. Castro and G. Christou, Chem. Commun., 1998, 721–722 RSC.
- For example: N. Ishikawa, M. Sugita and W. Wernsdorfer, J. Am. Chem. Soc., 2005, 127, 3650–3651 Search PubMed.
- E.-C. Yang, D. N. Hendrickson, W. Wernsdorfer, M. Nakano, L. N. Zakharov, R. D. Sommer, A. L. Rheingold, M. Ledezma-Gairaud and G. Christou, J. Appl. Phys., 2002, 91, 7382–7384 CrossRef CAS.
- M. Murrie, S. J. Teat, H. Stöeckli-Evans and H. U. Güdel, Angew. Chem., Int. Ed., 2003, 42, 4653–4656 CrossRef CAS.
- E. K. Brechin, R. A. Coxall, A. Parkin, S. Parsons, P. A. Tasker and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2001, 40, 2700–2703 CrossRef CAS.
- S. Langley, M. Helliwell, J. Raftery, E. I. Tolis and R. E. P. Winpenny, Chem. Commun., 2004, 142–143 RSC.
- SHELX-PC Package. Bruker Analytical X-ray Systems: Madison, WI, 1998.
- Harris notation describes the binding mode as [X.Y1Y2Y3…Yn], where X is the overall number of metals bound by the whole ligand, and each value of Y refers to the number of metal atoms attached to the different donor atoms. See: R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker and R. E. P. Winpenny, Dalton Trans., 2000, 2349–2356 Search PubMed.
- M. Ferbinteanu, H. Miyasaka, W. Wernsdorfer, K. Nakata, K. Sugiura, M. Yamashita, C. Coulon and R. Clerac, J. Am. Chem. Soc., 2005, 127, 3090 CrossRef CAS.
- L. Bogani, A. Caneschi, M. Fedi, D. Gatteschi, M. Massi, M. A. Novak, M. G. Pini, A. Rettori, R. Sessoli and A. Vindigni, Phys. Rev. Lett., 2004, 92 Search PubMed , art. no. 207204.
- A. Vindigni, A. Rettori, L. Bogani, A. Caneschi, D. Gatteschi, R. Sessoli and M. A. Novak, Appl. Phys. Lett. Search PubMed (submitted). Available as a pdf file from http://it.arXiv.org/find/cond-mat/1/vindigni/0/1/0/past/3/0.
Footnotes |
| † Electronic supplementary information (ESI) available: further magnetic measurements recorded on 1. See http://dx.doi.org/10.1039/b510106a |
| ‡ 1. Co(NO3)2·6H2O (0.58 g, 2 mmol) was dissolved in MeCN (25 ml). To this was added Hchp (0.52 g, 4 mmol), PhPO3H2 (0.052 g, 0.33 mmol) and NEt3 (0.64 ml, 4.66 mmol) resulting in a deep purple solution, which was stirred for 4 h. The solution was filtered and solvent removed to give a purple oil. Recrystallisation from CH2Cl2/Et2O gave purple crystals. Yield: 50%. Elemental analysis (%) calculated for C78H64Co8N16O27P2Cl12: C 35.8, H 2.5, N 8.6; found C 35.7, H 2.5, N 8.6%. |
| § Crystal data for C82H69Cl20Co8N16O27P2, 1: monoclinic, P21/n, a = 20.551(2), b = 21.853(2), c = 25.909(3) Å, β = 104.703(2)°, V = 11255(2) Å3, M = 2952.91, R1 = 0.0554. Data collection, structure solution and refinement used SHELXL.11 Full details have been deposited. CCDC 210158. See http://dx.doi.org/10.1039/b510106a for crystallographic data in CIF or other electronic format. |
| ¶ The magnetic properties of polycrystalline samples of 1 and 2 were investigated using a Cryogenic M600 SQUID magnetometer. The a.c. susceptibility has been measured with home made probes combined either with the MAGLAB platform, or with an OXFORD 3He Heliox cryostat for temperatures above and below 1.5 K, respectively. Supplementary data include further magnetic measurements recorded on 1. |
| This journal is © The Royal Society of Chemistry 2005 |
