Biofunctionalization of fluorescent-magnetic-bifunctional nanospheres and their applications†
Guo-Ping
Wang
a,
Er-Qun
Song
a,
Hai-Yan
Xie
b,
Zhi-Ling
Zhang
a,
Zhi-Quan
Tian
a,
Chao
Zuo
a,
Dai-Wen
Pang
*a,
Dao-Cheng
Wu
c and
Yun-Bo
Shi
d
aCollege of Chemistry and Molecular Sciences, State Key Laboratory of Virology, Wuhan University, Wuhan, 430072, P. R. China. E-mail: dwpang@whu.edu.cn; Fax: 86-27-68754067; Tel: 86-27-68756759
bSchool of Life Science and Technology, Beijing Institute of Technology, Beijing, P. R. China
cSchool of Life Science and Technology, Xi'an Jiaotong University, P. R. China
dLaboratory of Gene Regulation and Development, National Institute of Child Health and Human Development (NICHD), National Institute of Health (NIH), Building 18T Room 106, 18 Library Dr MSC 5431, Bethesda, MD 20892-5431, USA
First published on 8th August 2005
Abstract
Hydrazide-containing bifunctional nanospheres were covalently coupled on the surface with IgG, avidin, and biotin, to generate novel fluorescent-magnetic-biotargeting trifunctional nanospheres, which can be used in a number of biomedical applications, including visual sorting and manipulation of apoptotic cells as demonstrated here.
Nanospheres are becoming the materials of choice for a rapidly increasing number of pharmaceutical applications and in biomedical research. Quantum dots, nanometer-sized semiconductor crystals, are a new class of fluorescent labeling reagents,1 which can be attached to biomolecules such as immunoglobulin G (IgG)2 and streptavidin,3etc. Magnetic microspheres coated with a specific ligand can be used to isolate cells,4 cell organelles,5 and biologically active molecules such as nucleic acids6 and proteins.7 Combining the fluorescent and magnetic properties into a single nanosphere would greatly increase its application potential in biomedical and biopharmaceutical fields. Recently, several methods have been developed by using QDs and magnetic nanoparticles8,9 but the resulting nanospheres have little use in biomedical fields because of the lack of biomolecules on their surface. We have previously developed a new strategy to fabricate bifunctional nanospheres with both fluorescence and magnetism8,10 with higher structural stability by co-embedding quantum dots and nano-γ-Fe2O3 into poly(styrene/acrylamide) copolymer nanospheres, and used it to generate folic acid-containing trifunctional nanospheres,10 which show capacity for the capture and separation of specific cancer cells. We describe here new methods for the biofunctionalization of bifunctional nanospheres (100–150 nm in diameter) with immunoglobulin G (IgG), avidin, and biotin to generate trifunctional nanospheres with a wide range of potential biomedical applications.
Goat anti-rabbit IgG (0.4 mL, 5 mg/mL, Sigma) was oxidized to create active aldehydes in its Fc fragment with sodium meta-periodate (0.1 mL, 50 mmol/L in 0.1 M pH 6.8 PBS, Sigma) in an amber vial for 30 min at room temperature with constant shaking.11 The reaction was stopped and unreacted sodium meta-periodate was removed by passing the mixture through a desalting column (PD10, Amersham Biosciences). Hydrazide-containing bifunctional nanospheres embedded with orange-red quantum dots were completely resuspended by sonication for a few min after a third wash with PBS. The suspension of hydrazide-containing bifunctional nanospheres (0.5 mL, 20 mg/mL in PBS ) and the oxidized antibody (0.5 mL, ca. 4 mg/mL in 0.1 M pH 6.8 PBS) were mixed and incubated with constant shaking for at least 6 h at room temperature (Scheme 1A), and subsequently washed ten times with PBS. As a result, fluorescent-magnetic-biotargeting trifunctional IgG-nanospheres were obtained.
 | ||
| Scheme 1 (A) Fabrication of a trifunctional IgG-nanosphere. IgG was first oxidized to create active aldehydes in its Fc fragment. The aldehyde-containing IgG was then conjugated to hydrazide groups on the surface of a bifunctional nanosphere. The same approach was also used to produce a trifunctional avidin-nanosphere by using avidin to substitute IgG. (B) Fabrication of a trifunctional biotin-nanosphere. Sulfo-NHS-LC-LC-biotin reacted directly with hydrazide on the surface of the bifunctional nanosphere. | ||
To characterize the bioactivity of goat anti-rabbit IgG on their surface, the trifunctional nanospheres (0.2 mL, in 0.1 M pH 7.2 PBS) were incubated with rabbit anti-human IgG-FITC (10 µL, 3.0 g/L, Beijing Zhongshan Golden Bridge Biotech. Co. Ltd.) for 30 min at 4 °C with gentle shaking, followed by washing ten times with PBS to remove the unbound rabbit anti-human IgG-FITC. Microscopic analysis of the FITC fluorescence clearly demonstrated the binding of rabbit anti-human IgG to the goat anti-rabbit IgG on the surface of the nanospheres (Figs. 1A and 1B), indicating that the activity of the goat anti-rabbit IgG was preserved during the coupling process. On the other hand, no FITC fluorescence was detected on the nanospheres when rabbit anti-human IgG-FITC was incubated with the nanospheres coupled with non-oxidized goat anti-rabbit IgG antibody (Fig. 1C), or when bifunctional nanospheres were incubated with rabbit anti-human IgG-FITC (Fig. 1D). These results demonstrated that the trifunctional nanospheres specifically recognized rabbit anti-human IgG through their surface goat anti-rabbit IgG and that covalent coupling was needed to generate such trifunctional nanospheres.
 | ||
| Fig. 1 The trifunctional anti-rabbit IgG-nanospheres bind to rabbit anti-human IgG-FITC. The trifunctional nanospheres were mixed with rabbit anti-human IgG-FITC. After washing off unbound rabbit anti-human IgG-FITC, the nanospheres were irradiated for about 20 min with a Hg-lamp to excite the complex of trifunctional IgG-nanospheres–rabbit anti-human IgG-FITC (A). The strong green colour indicates the binding of rabbit anti-human IgG-FITC. After FITC was photobleached, the complex returned to the orange-red colour of the nanospheres themselves (B). Control experiments: No green fluorescence (from FITC) except the orange-red fluorescence of the nanospheres themselves was seen when the nanospheres were coupled with IgG without oxidation (thus no coupling of anti-rabbit IgG to the nanosphere) (C) or when the bifunctional nanospheres were used in the binding reaction with rabbit anti-human IgG-FITC directly (D). | ||
To investigate the versatility of biofunctionalization of our bifunctional nanospheres, we generated two additional types of trifunctional nanospheres. First, we coupled avidin to the bifunctional nanospheres as described above for goat anti-rabbit IgG. When these avidin-nanospheres were incubated for 1 h with biotin-FITC in PBS, followed by thorough washing with PBS to remove excess biotin-FITC, biotin capture by the avidin on the surface of the nanospheres was confirmed by fluorescence microscopy, while no capture occurred when bifunctional nanospheres without avidin coupling were incubated with biotin-FITC, or when biotin-FITC was incubated with the nanospheres having incubated with non-oxidized avidin (see Supplementary Information, Fig. SI-1), again demonstrating the specificity and bioactivity of the trifunctional nanospheres.
Second, we coupled biotin to the bifunctional nanospheres. Sulfo-NHS-LC-LC-biotin (4.8 mg, Pierce) was directly added to the suspension of hydrazide-containing bifunctional nanospheres (0.5 mL, 20 mg/mL in PBS) embedded with green quantum dots, followed by a 3 h reaction with shaking at room temperature and subsequent washing for ten times with PBS to produce trifunctional nanospheres with surface biotin (Scheme 1B). To assess the bioactivity of biotin on their surface, streptavidin-phycoerythrin (10 µL, Sigma) was added to trifunctional biotin-nanospheres (0.2 mL) and incubated for 1 h at room temperature with gentle shaking, followed by thorough washing with PBS. Analysis of phycoerythrin fluorescence demonstrated streptavidin-binding to the surface of the biotin-nanospheres (see Supplementary Information Figs. SI-2A and SI-2B), while the binding did not take place with nanospheres coupled with unmodified biotin (see Fig. SI-2C) or between bifunctional nanospheres without biotin coupling and streptavidin-phycoerythrin (see Fig. SI-2D).
To validate the potential uses of the trifunctional nanospheres in biomedical fields, we investigated their suitability in visual gathering and isolation of apoptotic Hela cells. Apoptosis, or programmed cell death, is a normal component of the development and homostasis of multicellular organisms. Excessive apoptosis occurs in disease states including AIDS, chronic hepatitis, and transplant rejection.12 On the other hand, tumor growth is often associated with insufficient apoptosis. In vivo imaging of apoptosis induction would be extremely useful in monitoring the effects of chemotherapeutic drugs, antihormonal therapeutics, or antiangiogenic therapies.13 Hence, development of methods for sorting and manipulation of apoptotic cells is very important for the investigation of pathological processes involving apoptosis. For this purpose, we made use of the covalent complex annexin V-biotin, where the annexin V group can recognize the phosphatidylserine on the surface of early apoptotic cells while the biotin group can bind to the avidin on our avidin-conjugated nanospheres, to link the nanospheres to the apoptotic cells for visualization and isolation of apoptotic cells.
Apoptotic Hela cells (1 × 106) induced by UV-irradiation (8 min, 20 W) were first collected by centrifugation at approximately 1000 g for 5–10 min at room temperature. Then, the cells were washed three times with cold 1 × PBS and incubated with annexin V-biotin (0.1 µg/100 µL) for 15 min at room temperature. The cells were subsequently washed again, mixed with trifunctional avidin-nanospheres (0.2 mL, 20 mg/mL), and incubated for 30 min at room temperature. Apoptotic cells were isolated with a magnet (Scheme 2) and visualized by fluorescence microscopy based on the intrinsic fluorescence of the nanospheres (Fig. 2).
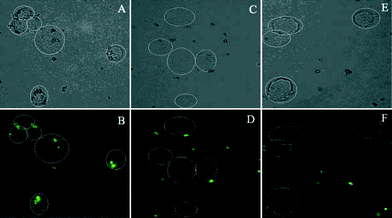 | ||
| Fig. 2 Microscopic images of apoptotic Hela cells captured by trifunctional avidin-nanospheres in the presence of annexin V-biotin (A, B). Control experiments: (C, D) Hela cells without UV treatment incubated first with annexin V-biotin and then trifunctional avidin-nanospheres; (E, F) apoptotic Hela cells incubated first with annexin V-biotin and then with bifunctional nanospheres without coupled avidin. (A) Bright field, apoptotic Hela cells with nanospheres on their surface shown in the white circle region. (B) Fluorescence image: fluorescence came from green quantum dots. (C, D, E, F) No nanospheres (green) were found on cells when Hela cells without UV irradiation or bifunctional nanospheres without coupled avidin were used. | ||
 | ||
| Scheme 2 Visual gathering and isolation of apoptotic Hela cells. | ||
In summary, the combination of fluorescent and magnetic properties with specific bioselective surface functional groups should greatly extend the potential bioapplications of the nanospheres. We have shown here that it is possible to generate such nanospheres and demonstrated that they can be used to develop novel methods for isolation and/or detection of apoptotic cells. Clearly, they can be used in many other applications. For example, the trifunctional nanospheres with anti-rabbit IgG on the surface could potentially be used for detection of any molecules bound by rabbit antibodies and for sorting/isolation of cells/organelles recognized by specific rabbit antibodies by using both the magnetic and fluorescent properties of the particles (thus improving the purity of the isolated/sorted materials due to double isolation/sorting procedures), etc. In addition, it is easy with an essentially identical approach to couple antibodies against specific proteins or targeting agents to the surface of the bifunctional nanospheres for detection and/or isolation. Furthermore, one can couple two different functional groups, e.g., antibody and biotin or avidin, to improve/enhance the use of nanospheres in such and other applications such as MR-imaging, isolation and sorting of different normal or cancer cells for molecular and genetic analyses, clinical diagnosis, or even simply for studying cell surface interactions and signalling processes, etc. Finally, we can envisage the synthesis and application of bifunctional nanospheres embedded with different quantum dots (thus yielding different colours of fluorescence), incorporated with different biorecognition molecules in high throughput detection and isolation of multiple target materials. Thus, with the approaches described here or similar ones, we believe that biofunctionalization of bifunctional nanospheres opens a way for the fabrication of truly smart bioprobes and biosensors, thereby greatly extending the usefulness of nanospheres.
This work was supported by the National Science Fund for Distinguished Young Scholars (No. 20025311), the National Key Program for Nanoscience and Nanotechnology (No. 2003BA310A22), and the National Natural Science Foundation of China (Grant Nos. 30170268; 20299034; 30370404; 20305011), and also in part by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH. The authors wish to thank Xing-Hu Ji for technical support.
Notes and references
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013 CrossRef CAS; W. C. Chan and S. Nie, Science, 1998, 281, 2016 CrossRef CAS.
- X. Wu, H. Liu, J. Liu, K. N. Haley, J. A. Treadway, J. P. Larson, N. Ge, F. Peale and M. P. Bruchez, Nat. Biotechnol., 2003, 21, 41 CrossRef CAS.
- A. Mansson, M. Sundberg, M. Balaz, R. Bunk, I. A. Nicholls, P. Omling, S. Tagerud and L. Montelius, Biochem. Biophys. Res. Commun., 2004, 314, 529 CrossRef CAS.
- J. J. Gomm, P. J. Browne, R. C. Coope, Q. Y. Liu, L. Buluwela and R. C. Coombes, Anal. Biochem., 1995, 226, 91 CrossRef CAS.
- R. V. Stan, W. G. Roberts, D. Predescu, K. Ihida, L. Saucan, L. Ghitescu and G. E. Palade, Mol. Biol. Cell, 1997, 8, 595 CAS.
- K. Rudi, M. Kroken, O. J. Dahlberg, A. Deggerdal, K. S. Jakobsen and F. Larsen, Biotechniques, 1997, 22, 506 CAS.
- A. R. Ahmed, G. W. Olivier, G. Adams, M. E. Erskine, R. G. Kinsman, S. K. Branch, S. H. Moss, L. J. Notarianni and C. W. Pouton, Biochem. J., 1992, 286, 377 CAS; M. J. Turner, C. S. Abdul-Alim, R. A. Willis, T. L. Fisher, E. M. Lord and J. G. Frelinger, J. Immunol. Methods, 2001, 256, 107 CrossRef CAS.
- D. W. Pang, H. Y. Xie, Y. Liu, C. Zuo and Z. L. Zhang, “Method for the construction of fluorescent magnetic multifunctional nanoparticles”, Patent application No. 200310111290.9, Oct. 29, 2003.
- H. Gu, R. Zheng, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2004, 126, 5664 CrossRef CAS; H. Kim, M. Achermann, L. P. Balet, J. A. Hollingsworth and V. I. Klimov, J. Am. Chem. Soc., 2005, 127, 544 CrossRef CAS; D. K. Yi, S. T. Selvan, S. S. Lee, G. C. Papaefthymiou, D. Kundaliya and J. Y. Ying, J. Am. Chem. Soc., 2005, 127, 4990 CrossRef CAS.
- H. Y. Xie, C. Zuo, Y. Liu, Z. L. Zhang, D. W. Pang, X. L. Li, J. P. Gong, C. Dickinson and W. Zhou, Small, 2005, 1, 506 Search PubMed.
- C. A. C. Wolfe and D. S. Hage, Anal. Biochem., 1995, 231, 123 CrossRef CAS.
- F. G. Blankenberg, J. F. Tait and H. W. Strauss, Eur. J. Nucl. Med., 2000, 27, 359 CrossRef CAS; F. G. Blankenberg and H. W. Strauss, Apoptosis, 2001, 6, 117 Search PubMed.
- P. C. Brooks, A. M. Montgomery, M. Rosenfeld, R. A. Reisfeld, T. Hu, G. Klier and D. A. Cheresh, Cell, 1994, 79, 1157 CrossRef CAS; C. B. Thompson, Science, 1995, 267, 1456 CrossRef CAS; F. G. Blankenberg, L. Naumovski, J. F. Tait, A. M. Post and H. W. Strauss, J. Nucl. Med., 2001, 42, 309 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figs. SI-1 and SI-2. See http://dx.doi.org/10.1039/b508075d |
| This journal is © The Royal Society of Chemistry 2005 |
