Cysteine methyl ester modified glassy carbon spheres for removal of toxic heavy metals from aqueous media†
Gregory G.
Wildgoose
a,
Henry C.
Leventis
a,
Andrew O.
Simm
a,
John H.
Jones
b and
Richard G.
Compton
*a
aPhysical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QX. E-mail: richard.compton@chem.ox.ac.uk; Fax: +44 (0)1865 275410; Tel: +44 (0)1865 275413
bChemical Research Laboratory, University of Oxford, Mansfield Road, Oxford, UK OX1 3TA
First published on 22nd June 2005
Abstract
Glassy carbon spherical powder (10–20 µm diameter) modified with cysteine methyl ester is found to be an inexpensive, novel material for the rapid removal of large quantities of toxic heavy metal ions such as Cd(II), Cu(II) and As(III) from aqueous media, with wide ranging potential applications such as third world drinking water filtration or environmental cleanup.
The accumulation of toxic heavy metals in the environment is increasing due to human activity.1 In particular the impact of mining operations2,3 and heavy industry4,5 in the developing world is leading to high concentrations of toxic heavy metals such as Cd(II), Pb(II), Hg(II), and Cu(II) accumulating in rivers and lakes. Inadequate filtration and treatment of these waters for human consumption, particularly in poorer countries, leads to many people in these affected regions suffering adverse health effects. The most obvious example of this is the widespread poisoning of millions of people a year in Argentina, China, Mexico, Taiwan, India and in particular Bangladesh,6 where more than 60% of the groundwater contains naturally occurring arsenic concentrations greatly in excess of the World Health Organisation's (WHO) guidelines of 10 ppb.7
The potentially damaging impact of toxic heavy metal pollution on the environment from mining activities was illustrated as recently as 1998 when, in Andalucia, south western Spain, 5 million m3 of acidic waste water containing high concentrations of Pb(II), Cu(II), Zn(II), Cd(II) and other metals spilled from a mining reservoir polluting up to 185,000 acres of warm marshland in the Coto de Do'ana National Park, home to some 250 species of migratory birds and a World Heritage Site.3
There is fierce competition within the scientific community to develop a facile, rapid, and inexpensive method to remove toxic heavy metals from aqueous media for drinking water filtration and/or environmental cleanup. In particular, many attempts have been made to develop materials to remove As(III) and As(V) from drinking water. For example, the recent work of Al Rmalli et al. used the powdered form of dried water hyacinth roots to reduce the concentration of As to below the WHO guidelines within one hour of exposure.6 In our previous work we attached a biohomopolymer, poly-L-cysteine, to modified graphite powder (to form PCcarbon) and demonstrated that this was an excellent but expensive material for the removal of large amounts of Cd(II) from aqueous media.8 We now develop an alternative, inexpensive material by covalently attaching monomeric L-cysteine methyl ester to the surface of glassy carbon (GC) spheres (10–20 µm in diameter) as shown in Scheme 1 and referred to as CysOMe-GC henceforth. This material is then shown to be highly effective in removing mM and trace (ppb) concentrations of Cd(II), Cu(II) and most importantly As(III) from “real” matrix samples of river water and mineral water.
 | ||
| Scheme 1 The covalent modification of glassy carbon spherical powder (GC) with L-cysteine methyl ester. | ||
After adding the CysOMe-GC powder to contaminated samples for varying lengths of time, the uptake of Cd(II), Cu(II) and As(III) by CysOMe-GC was monitored by filtering off the modified carbon powder and measuring the remaining metal ion concentrations in the sample using sensitive voltammetric techniques described in the ESI and elsewhere.9–11 In every case a control experiment using unmodified GC powder was carried out. The uptake of metal ions by blank GC powder was found to be immeasurably small.
First we investigate the efficacy of CysOMe-GC powder. 10 mg of CysOMe-GC was stirred with 25 cm3 of ca. 1 mM As(III) in pure water, corresponding to an environmentally disastrous spillage, and the uptake analysed at various time intervals. Figure ESI.1 in the supplementary information details the resulting uptake. After 1 hour a mere 10 mg of CysOMe-GC removed ca. 28% of As(III) from the sample. This corresponds to an uptake of 58 mg of As(III) per gram CysOMe-GC, a far greater uptake than other materials reported to date,6 thereby demonstrating that CysOMe-GC is an excellent material for the removal of high concentrations of As(III), for example in environmental cleanup.
Experiments investigating the removal of trace amounts of As(III) by CysOMe-GC in pure water are detailed in the ESI. Having confirmed that CysOMe-GC was effective for the trace removal of As(III) we next investigated whether CysOMe-GC could be used in a real sample of Bangladeshi well water to remove As(III). A 25 cm3 sample of Bangladeshi well water containing 120 ppb of As(III), more than twice the Bangladeshi legal limit of 50 ppb,12 was stirred with 200 mg of CysOMe-GC and the uptake analysed as before. Fig. 1 shows the concentration profile of As(III) in the sample with time. Within 10 minutes of exposure to CysOMe-GC the As(III) concentration has fallen below the Bangladeshi legal limit of 50 ppb.
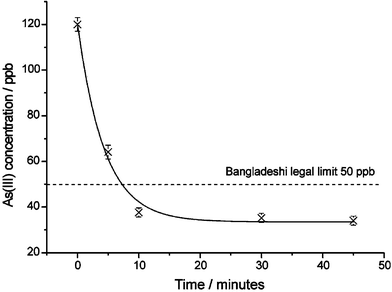 | ||
| Fig. 1 The As(III) concentration profile remaining in Bangladeshi well water after exposure to CysOMe-GC for varying times. | ||
Previously PCcarbon has been shown to be effective for Cd(II) removal,8 and so we investigate the efficacy of CysOMe-GC powder for Cd(II) and Cu(II) removal from real samples of river water and mineral water. For example, many rivers in the Russian Federation are thought to be heavily polluted by Cd(II), particularly the river Neva flowing through St Petersburg (formerly Leningrad), a heavily industrialised city.13 Again, 10 mg of CysOMe-GC was added to 10 cm3 of: a) river water (River Cherwell, Oxford) spiked to ca. 1.5 mM Cd(II) to simulate a disastrous environmental spillage, b) Evian mineral water spiked to 50 ppb Cd(II), ten times the WHO and U.S. Environmental Protection Agency (USEPA) limit of 5 ppb,14–16 and c) river water (River Cherwell, Oxford) which was found to contain ca. 30 µM Cu(II) which is just above the USEPA limit for treated drinking water of 1.3 mg L−1 or 20.1 µM and was therefore used without spiking the Cu(II) concentration. Figs. 2a–c demonstrate that a mere 10 mg of CysOMe-GC powder rapidly reduced the Cd(II) concentration in river water from mM to µM levels, the level of Cu(II) found in untreated River Cherwell water was reduced below the USEPA limit within 10 minutes and to almost zero after 20 minutes, and the level of Cd(II) in mineral water was reduced to almost half the USEPA limit within ten minutes!
 | ||
| Fig. 2 The concentration profiles of: a) Cd(II) remaining in River Cherwell water sample, b) Cd(II) remaining in Evian mineral water sample and c) Cu(II) remaining in River Cherwell water sample after exposure to CysOMe-GC for varying times. | ||
We postulate that the very high uptake of metal ions from aqueous media by CysOMe-GC is due to the high surface concentration of carboxyl groups and some element of porosity of the GC powder, allowing significantly more L-cysteine methyl ester to be coupled to the surface and accessible parts of the carbon ball interior than other solid state supports. It is envisaged that cysteine modified carbon nanotubes (CNTs) could offer enhanced metal sequestration properties due to their larger active surface area, however these materials could not be used for drinking water filtration at present as their removal is not facile and the toxicity of CNTs to humans is at present not fully investigated. It is worth noting that cysteine itself in aqueous solutions has been shown to complex various metal ions. Of particular interest is the mechanism by which cysteine complexes As(III) in the form of the arsenite ion and some details of the mechanism are presented in reference 17. However the thermodynamics, kinetics and mechanism of metal ion chelation, in particular As(III) in the form of arsenite, by CysOMe on the surface of GC spheres is currently under investigation and will be published as a full paper shortly. In conclusion we have demonstrated that CysOMe-GC powder is a powerful “catch-all” material for the removal of heavy metal ions such as Cd(II), Cu(II) and As(III). Furthermore CysOMe-GC is inexpensive and likely easy to produce in bulk quantities, thus proffering the opportunity to utilise this material in third world drinking water filtration in order to alleviate the poisoning and suffering of millions of people worldwide. Patent protection for this material and its applications has been applied for.
Notes and references
- C. Locatelli, Electroanalysis, 2004, 16, 1478 CrossRef CAS.
- V. M. Sol, S. W. M. Peters and H. Aiking, Toxic Waste Storage Sites in EU Countries: A Preliminary Investigation, 1999, http://hdl.handle.net/1871/1698 Search PubMed.
- V. Short, Toxic Waste Devastates Marshlands in Southern Spain, 1998, http://www.wsws.org/news/1998/may1998/span-m13.shtml Search PubMed.
- A. Ahmad and M. Alam, Indian J. Chem. Technol., 2004, 11, 555 Search PubMed.
- Y.-X. Chen, H. Liu, G.-W. Zhu, H.-L. Chen and G.-M. Tian, J. Environ. Sci., 2004, 16, 34 Search PubMed.
- S. W. Al Rmalli, C. F. Harrington, M. Ayub and P. I. Haris, J. Environ. Monit., 2005, 7, 279 RSC.
- WHO Arsenic Compounds, Environmental Health Criteria 224, World Health Organisation, Geneva, 2001 Search PubMed.
- G. G. Wildgoose, H. C. Leventis, I. J. Davies, A. Crossley, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, J. Mater. Chem., 2005, 15, 2375 RSC.
- J. Kruusma, L. Nei, J. L. Hardcastle, R. G. Compton, E. Lust and H. Keis, Electroanalysis, 2004, 16, 399 CrossRef CAS.
- C. E. Banks, O. V. Klymenko and R. G. Compton, Phys. Chem. Chem. Phys., 2003, 5, 1652 RSC.
- A. O. Simm, C. E. Banks and R. G. Compton, Electroanalysis, 2005, 17, 335 CrossRef CAS.
- H. M. Anawar, J. Akai, K. M. G. Mostofa, S. Safiullah and S. M. Tareq, Environ. Int., 2002, 27, 597 CrossRef CAS.
- Mikael Sillfors, Water Management in St. Petersburg, www.valt.helsinki.fi//projects/enviro/articles/PetL.pdf Search PubMed.
- USEPA. 1984. Ambient Water Quality Criteria for Cadmium – 1984. EPA Report No 440/5-84-032. NTIS PB85-224031 Search PubMed.
- WHO. 1992a. Environmental Health Criteria No 134 – Cadmium. World Health Organisation, Geneva Search PubMed.
- WHO. 1992b. Environmental Health Criteria No 135 – Cadmium – Environmental Aspects. World Health Organisation, Geneva Search PubMed.
- N. A. Rey, O. W. Howarth and E. C. Pereira-Maia, J. Inorg. Biochem., 2004, 98, 1151 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental details and additional experimental data. See http://dx.doi.org/10.1039/b506461a |
| This journal is © The Royal Society of Chemistry 2005 |
