Porous materials show superhydrophobic to superhydrophilic switching†
Neil J.
Shirtcliffe
*,
Glen
McHale
,
Michael I.
Newton
,
Carole C.
Perry
and
Paul
Roach
School of Biomedical and Natural Sciences, Nottingham Trent University, Clifton Lane, Nottingham, UK NG11 8NS. E-mail: neil.shirtcliffe@ntu.ac.uk; Fax: +44 (0)115 8486616; Tel: +44 (0)115 8486375
First published on 28th April 2005
Abstract
Switching between superhydrophobicity and superhydrophilicity in porous materials was predicted theoretically and demonstrated experimentally with the example of thermally induced contact angle change; tunability of this system was also demonstrated.
A surface that is both hydrophobic and rough can become superhydrophobic; the roughness magnifies the effect of the hydrophobicity and can lead to surfaces being so hydrophobic that water drops on them appear to be spherical. The same effect can be observed with other liquids on the surface of rough solids. If the roughness is slight liquid can follow the contours of the surface, but if it is very high water tends to bridge the tops of peaks of the roughness. Both cases can lead to increases in contact angle for small droplets of liquid deposited on the surface.1–4 The same principle applies for liquids other than water, but the transition is then a super-non-wetting to super-wetting one. This effect has been observed on natural and synthetic surfaces.5,6
Porous materials will either show bridging type non-wetting or will fill with liquid. When liquid sits on a flat, heterogeneous surface or bridges the peaks of a rough one, the Cassie–Baxter model, (eqn. 1)7 applies; the liquid drop suspends itself across surface protrusions and the contact angle can be derived from the sum of the cosines of the angles on each type of interface multiplied by their fractional area.
| cos θr = f cos θs + (1 − f) cos θx | (1) |
In eqn. 1, θr is the contact angle of the liquid on the rough surface, θs the contact angle of the liquid on a smooth surface having the same chemistry as the rough surface, f is the fraction of the base of the drop in contact with the solid and (1 − f) is the remaining fraction of the drop base. θx is the contact angle of a liquid on air in the case of suspended drops; this is 180°. The formula can be used for any heterogeneous surface with a flat water interface. Significantly on a porous surface filled with a liquid θx would become 0° for droplets of the same liquid added to the surface and these droplets would then tend to spread more than on a flat surface of the solid material. This difference in the meaning of θx, corresponding to whether gaps between surface protrusions are empty or filled, changes cos θx from −1 to 1, allowing eqn. 1 to be reduced to eqn. 2.
| cos θr = f cos θs ± (1 − f) | (2) |
Eqn. 2 applies for porous materials where the pores can either be empty, in which case the negative sign would be used, or filled with the same liquid as is present on the surface, when the positive sign would be used. If the material has a high volume fraction of pores f will be small so the second component of the equation will dominate and one would expect liquid to switch from very high (nearly 180°) to very low (nearly 0°) contact angles when the contact angle on a flat surface is varied by a small amount around the value of 90°; at this point sudden intrusion into the pores will occur. This is likely to be particularly effective on materials with interconnected pores as the liquid in the material will fill pores ahead of the advancing drop.
Enhancement of contact angle variation using roughness has been suggested as a measurement technique in previous work,8,9 but mesoporous materials may have even sharper transitions than rough surfaces as the pores can only be empty or filled, partial states are not energetically favoured.
The porous materials used in this report were produced using a phase separation method in which a hardening process freezes a phase separation that occurs concurrently with hardening of one of the phases.10 This method produces co-continuous materials consisting of a solid phase and a liquid phase. When the liquid was removed a porous structure remained. The system used was the condensation of an organo-triethoxysilane in a mixture of organic solvent and water. Sol-gel materials, such as this, show promise as superhydrophobic surfaces,11,12 particularly as hard, superhydrophobic coatings but also as bulk materials13 and are relatively cheap to produce.14 Reaction occurs through hydrolysis of the ethoxy groups and polymerisation of the silanol groups thus formed. Polymerisation causes a decrease in dipole moment, leading to hydrophobic phase separation. The dried material presents the organic group on its surface, causing the foams to be superhydrophobic. The structure of these materials has been reported in an earlier paper15 where we reported that the advancing contact angles on such gels heated to 300 °C were 155 ± 2°. If a flat surface of the organosilica is heated it becomes gradually less hydrophobic, but the foams undergo a more sudden change. The contact angle values are similar to those achieved by other researchers using similar materials.16–18
The central image in Fig. 1 is an electron micrograph of a typical material used showing the large pore fraction (measured previously as 75%) that generates the Cassie–Baxter type superhydrophobicity of these materials. The structure of the material did not significantly change on heating to 400 °C.
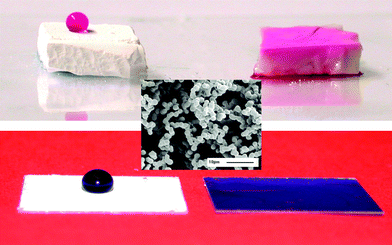 | ||
| Fig. 1 Top, phenolphthalein in water on MTEOS sol-gel foams heated to 390 °C (left) and 400 °C (right). Centre, SEM of sol-gel foam unheated. Bottom, foam films on glass cover slips with (left) drop of water with brilliant blue G and (right) imbibed. | ||
The top row of Fig. 1 shows two samples of methyltriethoxysilane (MTEOS) sol-gel foams that were heated in a furnace and then allowed to cool to room temperature before a drop of water was placed upon them. The dye-containing water sat on the top of the sample heated to 390 °C but was fully absorbed into that heated to 400 °C. This transition was effectively binary, either the drop penetrated or it did not. Varying the temperature of this transition will be considered later in this communication. A practical device could consist of a line of thin gel films with different transition temperatures on a baseplate that would be dipped into a coloured solution after heating. Hydrophilic gels would absorb the dye and become coloured while those not reaching their transition temperature would not.
Similar foam materials were prepared as adherent films, which would be more practical as a device. Examples of these are shown in the bottom row of Fig. 1 with a drop of dyed water on a hydrophobic and a hydrophilic foam film.
To investigate the mechanism behind the change in wettability of the material, infrared spectra were taken after heating gels to various temperatures.
The transmission infrared spectra shown in Fig. 2a were measured using a single KBr plate containing MTEOS foam. It was heated to different temperatures and infrared spectra measured. The spectra show the C–H stretching region, which is almost unchanged upon heating up to 400 °C, apart from a slight change in peak shape at around 2925 cm−1 between 200 and 240 °C. This indicates that methyl functionality was not lost during the hydrophobic to hydrophilic transition, which occurred at just below 400 °C for these samples.
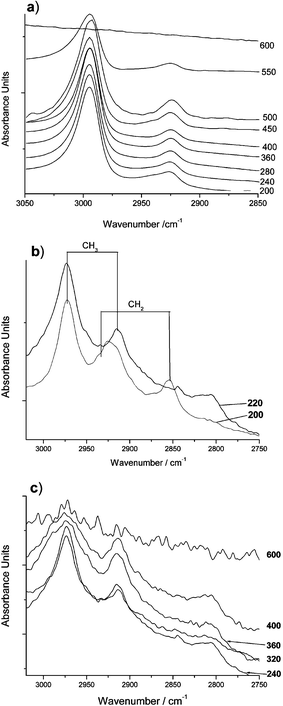 | ||
| Fig. 2 IR spectra of MTEOS sol-gel materials heated to various temperatures; a) transmission spectra, b) and c) diffuse reflection spectra. | ||
The same region of diffuse reflection spectra (measuring the surface region) of pure samples heated to similar temperatures are shown in Fig. 2 parts b and c. Part b shows spectra taken after heating to 180 and 220 °C, bands at 2930 and 2850 cm−1 corresponding to a CH2 stretch disappear on heating to 220 °C and a broad band appears at 2810 cm−1. The two CH2 bands probably arise from unreacted ethoxysilyl groups. On heating these would be expected to crosslink with hydroxy groups at low temperature releasing ethanol, which explains the loss of these signals by 220 °C. Above this temperature the only C–H functionality expected and observed is Si–CH3. Fig. 2c shows reflectance spectra taken upon further heating. The CH3 bands broaden, possibly due to changes in chemical environment and they lose some intensity. The band at 2810 cm−1, possibly also CH3 groups in a strained environment, did not appear to change once formed.
The infrared data suggest that the surface of the gel is initially covered with methyl and ethoxy groups. The ethoxy groups are lost at a low temperature long before the materials become hydrophilic. Slight broadening and loss of intensity was observed in C–H bands at the surface of the material where bulk measurements showed no changes. Changes in the shape of the Si–O region indicate crosslinking of the silica and relative loss of Si–C intensity in reflection IR backs up the C–H band data†. To become hydrophilic the surface must become more polar. This could occur by the formation of new groups or by a change in the relative abundances of apolar methyl groups and polar silica species. Methyl groups could be oxidatively cleaved or become hidden in the bulk of the material. The infrared data only show loss of methyl groups from the surface of the material, not where they go. Transmission infrared measurements show that bulk methyl concentration does not change until the temperature exceeds 550 °C. No C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality appeared at any time in the spectra, so there is no evidence that methyl groups are being oxidised†.
O functionality appeared at any time in the spectra, so there is no evidence that methyl groups are being oxidised†.
In order for the transition of the materials from superhydrophobic to superhydrophilic to be of use in a sensor it must be tuneable. If the transition temperature is controlled by the crosslinking of silica rotating organo-groups away from the surface, it should be affected by the density of silicon–organic bonds and the bulk of the organic groups; equally if it is due to the oxidative loss of the organic groups it should be affected by the reactivity of these groups. Sol-gel foams were prepared using varying proportions of phenyl triethoxysilane (PhTEOS) and TEOS to test this. Ph–Si is known to be more resistant to heating than CH3–Si19 and the phenyl group is also bulkier so an increase in switching temperature would be expected.
The temperatures at which switching occurred were increased when larger fractions of PhTEOS were used but decreased considerably when larger fractions of TEOS were used (Table 1). Similarly Rao and Haranath17 used MTEOS/TEOS mixtures and observed hydrophilicity at 280 °C. The transition temperatures of PhTEOS and MTEOS containing foams both decreased significantly on increasing the fraction of TEOS. This suggests that the hydrophilic–hydrophobic transition is due to crosslinking of the silica backbone causing redistribution of the organic groups from the surface into the bulk of the material. It is not surprising that the maximum temperature appears to be limited to 550–600 °C as this is where loss of the C–H groups became apparent in the infrared measurements.
| Gel composition | Transition/°C (±10 °C) |
|---|---|
| MTEOS | 400 |
| PhTEOS ∶ TEOS 1 ∶ 2 | 275 |
| PhTEOS ∶ TEOS 1 ∶ 1 | 550 |
| PhTEOS ∶ TEOS 2 ∶ 1 | 550 |
A range of foam materials were prepared as bulk and films. Heating caused a gradual increase in hydrophilicity of a flat surface, but a sudden change from superhydrophobicity to absorption in porous ones. This behaviour is described by a modification of Cassie and Baxter's formula where the pores of a porous surface can either be empty or they can be full of the wetting liquid. This situation would result in switching from very high to very low contact angle without intermediate states being possible. The transition from superhydrophobicity to hydrophilicity of these materials shows promise for detecting thermal history with the wetting, or measurement stage taking place at room temperature. Addition of dyes to the liquid or the foam allowed visualisation of the changes. Alternatively shorting of electrodes under the foam could be used to detect imbibition events.‡
Notes and references
- A. Adamson, Physical Chemistry of Surfaces, 5th Edn., Wiley: New York, 1990 Search PubMed.
- A. Otten and S. Herminghaus, Langmuir, 2004, 20(6), 2405–2408 CrossRef CAS.
- P. De Gennes, Rev. Mod. Phys., 1985, 57, 827–863 CrossRef CAS.
- R. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202(1), 1–8 CrossRef CAS.
- S. Shibuichi, T. Yamamoto, T. Onda and K. Tsujii, J. Phys. Chem., 1996, 100, 19512–19517 CrossRef CAS.
- D. Quéré, Physica A, 2002, 313(1–2), 32–46 CAS.
- G. McHale, N. Shirtcliffe and M. Newton, Analyst, 2004, 129(4), 284–287 RSC.
- R. Rosario, D. Gust, A. Garcia, M. Hayes, J. Taraci, T. Clement, J. Dailey and S. Picraux, J. Phys. Chem. B, 2004, 108(34), 12640–12642 CrossRef CAS.
- R. Takahashi, K. Nakanishi and N. Soga, J. Ceram. Soc. Jpn., 1998, 106(8), 772–777 CAS.
- K. Tadanaga, K. Kitamuro, A. Matsuda and T. Minami, J. Sol-Gel Sci. Technol., 2003, 26(1–3), 705–708 CrossRef CAS.
- K. Tadanaga, J. Morinaga and T. Minami, J. Sol-Gel Sci. Technol., 2000, 19, 211–214 CrossRef CAS.
- A. Rao and M. Kulkarni, Mater. Res. Bull., 2002, 37(9), 1667–1677 CrossRef CAS.
- L. Klein (ed.), Sol-Gel Technology For Thin Films, Fibers, Preforms, Electronics and Speciality Shapes, Noyes: New Jersey, 1998 Search PubMed.
- N. Shirtcliffe, G. McHale, M. Newton and C. Perry, Langmuir, 2003, 19(14), 5626–5631 CrossRef CAS.
- H. Erbil, A. Demirel, Y. Avci and O. Mert, Science, 2003, 299(5611), 1377–1380 CrossRef CAS.
- A. Rao and D. Haranath, Microporous Mesoporous Mater., 1999, 30(2–3), 267–273 CrossRef CAS.
- A. Roig, E. Mollins, S. Martinez, M. Moreno-Manas and A. Vallribera, Chem. Commun., 2004, 20, 2316–2317 RSC.
- M. Brook, Silicon in organic, organometallic and polymer chemistry, Wiley: New York, 2000 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: IR transmission and reflection spectra. See http://www.rsc.org/suppdata/cc/b5/b502896e/ |
| ‡ Sol-gel foams were prepared as described in previous work,7 except that dimethyl formamide (DMF, Acros 99%) was used as the co-solvent. DMF does not evaporate very rapidly under normal conditions, so solvent exchange was necessary, achieved by placing the samples into a large volume of methanol (Fisher 99%) and exchanging it each day for three days. Once dry the samples were placed in a Pyrex® beaker and heated to various temperatures to cross-link and eventually oxidise the materials.Phenyl terminated foams were prepared by mixing defined molar ratios of phenyl triethoxysilane (PhTEOS, Lancaster 97%) and tetraethoxysilane (TEOS, Aldrich 98%), ratios used were 2 ∶ 1, 1 ∶ 1 and 1 ∶ 2. DMF was used as co-solvent here and the molar amount of silicon was kept the same as before so as to maintain the molar ratios of silicon, water and acid.Infrared measurements were taken using a Nicolet Magna IR-750 with a Spectra-Tech diffuse reflection accessory and KBr plateholder. KBr plates were prepared using 2 mg of sol-gel foam in 200 mg of KBr. |
| This journal is © The Royal Society of Chemistry 2005 |
