Synthesis of multivalent aminoglycoside mimics via the Ugi multicomponent reaction†
Bernhard
Westermann
* and
Simon
Dörner
Leibniz Institute of Plant Biochemistry, Department of Bioorganic Chemistry, Weinberg 3, 06120, Halle (Saale), Germany. E-mail: bwesterm@ipb-halle.de; Fax: +49 345 5582 1309; Tel: +49 345 5582 1340
First published on 4th March 2005
Abstract
The synthesis of multivalent neoglycoconjugates with 2,6-diamino-2,6-dideoxyglucose is accomplished by a flexible Ugi multicomponent approach leading to mono-, di- and tri-valent carbohydrate clusters.
Aminoglycosides such as neomycin B (1) or kanamycin B (2) are valuable antibiotics, that target prokaryotic RNA. It is believed that the β-hydroxyamine motif of these natural products interacts with the phosphodiester group (a) and the Hoogsteen site of RNA-residues (b), respectively (Fig. 1).1 Unfortunately, resistance mechanisms and the toxicity have limited the applicability of this class of antibiotics severely. In order to overcome these drawbacks the groups of Wong and Tor fashioned new aminoglycoside surrogates by dimerisation of suitable aminoglycoside elements.2 They enhanced the binding affinity between aminoglycosides and RNA by targeting multiple binding sites. Therefore, dimerized aminoglycosides can be considered as multivalent carbohydrate mimics. Through cooperative effects binding of these ligands can be improved quite drastically in comparison to their monovalent congeners.
 | ||
| Fig. 1 Binding modes of aminoglycosides to RNA. | ||
In our approach presented in this communication we achieve aminoglycoside multivalency by using the Ugi multicomponent reaction (Ugi-MCR).3 In addition we want to show that this approach leads to products exhibiting a great structural diversity. Based on previous findings by Wong et al. we incorporate 2,6-diamino-2,6-dideoxyglucopyranose moieties (ring I of neomycin B) which offer the appropriate binding motif as well as the possibility to achieve polycationic species under physiological conditions.4
The Ugi reaction involves the combination of an amine, aldehyde, carboxylic acid and isocyanide in an one-pot process (Fig. 2).
 | ||
| Fig. 2 The Ugi multicomponent reaction (U-4CR). | ||
For the access to suitable 2,6-diamino-2,6-dideoxyglucose containing starting materials (amine 5, carboxylic acid 6, aldehyde 7) we used the bromide 4 whose straightforward synthesis by degradation of moderately priced neomycin B is described by Hunziker et al.5 We found that subsequent substitution of bromine by sodium azide under phase transfer conditions6 followed by reduction furnishes amine 5 in very good yield. Based on the result of Kunz and Ugi7 who employed glycosylamines in Ugi reactions we expected that 5 should be equally useful for that purpose. Starting from 4 Königs–Knorr reaction allows for the introduction of an O-allyl side chain which can be transformed into carboxylic acid 6 or aldehyde 7 by ruthenium catalyzed periodate cleavage or ozonolysis, respectively (Scheme 1). In all multicomponent reactions described in this communication isocyanide 3 has been used. It was synthesized starting from 6-aminohexanoic acid and offers superior reactivity compared to other isocyanides such as acetyl isocyanide. Furthermore, it can be utilized for later derivatisation.
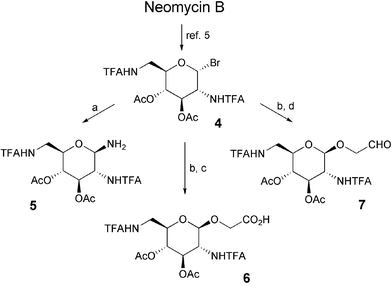 | ||
| Scheme 1 Synthesis of Ugi-MCR building blocks with a 2,6-diamino-2,6-dideoxyglucose core unit. (a) i. NaN3, Bu4NHSO4, DCM–NaHCO3–H2O, 86%, ii. H2–PtO2, MeOH, 94%; (b) i. AllOH, AgOTf, MS 4 Å, DCM, 40%; (c) NaIO4, RuCl3·xH2O, CCl4–CH3CN–H2O, 70%; (d) O3, DCM, −78°C, then Me2S, 99%. | ||
In preliminary experiments, the Ugi-MCR of amine 5, acetone, isocyanide 3 and N-trifluoroacetyl-protected γ-aminobutyric acid 15 provided monovalent product 16 in 64% yield.
To establish and optimize the reaction conditions, the corresponding glucose-derivatives 8, 11 and 13 have been used (Scheme 2). It was found that carrying out the reaction in a 0.2 molar methanolic solution at room temperature was best with precondensation of the amine and the carbonyl compound leading to the di- and trivalent products 17 and 21 in 83 and 64% yield, respectively. After optimization the glucose based building blocks were successfully exchanged by protected amino sugars. Employing under the same conditions amine 12 and carboxylic acid 9 which both contain a 2-deoxy-2-acetaminoglucose (GlcNAc) core unit, the yield of the divalent compound 18 was 58%. Molecules 19 and 20 exhibiting N-trifluoroacetyl-protected sugar units could be isolated in 44–46% yield. In analogy to test compound 21 trivalent aminoglycoside mimics 22 and 23 can be synthesized by replacing acetone with appropriate aldehyde building blocks and are isolated as diastereomeric mixtures in 49% and 89% yield. In all experiments the Ugi-product was the major reaction product formed. Noteworthy amounts of the corresponding Passerini-products were not detected.
 | ||
| Scheme 2 Synthesis of multivalent aminoglycoside mimics. | ||
In summary, we have developed a very straightforward and short synthesis to structurally diverse aminoglycoside mimics. By using the Ugi-MCR approach, different aminosugar units can be coupled in a very defined way. The ability of the unprotected products to bind to RNA targets has been evaluated by surface plasmon resonance spectroscopy and will be reported in due course.‡
S. D. appreciates the support by the Studienstiftung des deutschen Volkes (Ph D grant). The authors would like to thank Dr Thomas Weimar for lively discussions.
Notes and references
- M. Hendrix, P. B. Alper, E. S. Priestley and C.-H. Wong, Angew. Chem., Int. Ed., 1997, 36, 95 CrossRef CAS.
- S. J. Sucheck, A. L. Wong, K. M. Koeller, D. D. Boehr, K. Draker, P. Sears, G. D. Wright and C.-H. Wong, J. Am. Chem. Soc., 2000, 122, 5230 CrossRef CAS; H. Wang and Y. Tor, Bioorg. Med. Chem. Lett., 1997, 7, 1951 CrossRef CAS.
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169; O. Lockhoff, Angew. Chem., Int. Ed., 2000, 39, 3436; D. P. Sutherlin, T. M. Stark, R. Hughes and R. W. Armstrong, J. Org. Chem., 1996, 61, 8350 CrossRef CAS; C.-Y. Tsai, W. K. C. Park, G. Weitz-Schmidt, B. Ernst and C.-H. Wong, Bioorg. Med. Chem. Lett., 1998, 8, 2333 CrossRef CAS.
- C.-H. Wong, M. Hendrix, D. D. Manning, C. Rosenbohm and W. A. Greenberg, J. Am. Chem. Soc., 1998, 120, 8319 CrossRef CAS.
- R. Tona, R. Bertolini and J. Hunziker, Org. Lett., 2000, 2, 1693 CrossRef CAS.
- H. Kunz and H. Waldmann, Angew. Chem., Int. Ed., 1985, 24, 883 CrossRef; H. Kunz, H. Waldmann and J. März, Liebigs Ann. Chem., 1989, 45 CrossRef CAS; H. Waldmann, J. März and H. Kunz, Carbohydr. Res., 1990, 196, 75 CrossRef CAS.
- H. Kunz and W. Pfrengle, J. Am. Chem. Soc., 1988, 110, 651 CrossRef CAS; H. Kunz, W. Pfrengle and W. Sager, Tetrahedron Lett., 1989, 30, 4109 CrossRef CAS; H. Kunz and W. Pfrengle, Tetrahedron, 1988, 44, 5487 CrossRef CAS; H. Kunz, W. Pfrengle, K. Rück and W. Sager, Synthesis, 1991, 1039 CrossRef; H. Bradley, G. Fitzpatrick, W. K. Glass, H. Kunz and P. V. Murphy, Org. Lett., 2001, 3, 2629 CrossRef CAS; M. Goebel and I. Ugi, Synthesis, 1991, 1095 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: spectroscopic data and copies of NMR spectra. See http://www.rsc.org/suppdata/cc/b5/b501028d/ |
| ‡ Typical experimental procedure: to a solution of amine 5 (0.6 mmol) in methanol (3 ml) is added acetone (0.6 mmol). The mixture is stirred for 1 h followed by addition of carboxylic acid 6 (0.6 mmol) and isocyanide 3 (0.6 mmol). After stirring for 48 h the solvent is evaporated and the crude product is purified on silica (eluent: ethyl acetate–hexanes) to give product 20 in 46% yield. |
| This journal is © The Royal Society of Chemistry 2005 |
