A new Co(II)-metalloviologen-based electrochromic material integrated in thin multilayer films†
Dirk G.
Kurth
*ab,
Jesús
Pitarch López
a and
Wen-Fei
Dong
a
aMax Planck Institute of Colloids and Interfaces, D-14424, Potsdam, Germany. E-mail: kurth@mpikg-golm.mpg.de; Fax: +49 (0)331 567 9202; Tel: +49 (0)331 567 9211
bNational Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
First published on 22nd March 2005
Abstract
A metallosupramolecular coordination polyelectrolyte prepared by the reaction of cobalt(II) with a novel bisterpyridine ligand has been assembled as the active component in electrochromic films by sequential deposition using electrostatic layer-by-layer self-assembly.
The design and fabrication of molecular materials have received a lot of attention in the last decades and it has been shown that control of the electronic and/or photonic properties of such materials can be achieved by means of accurate synthesis of tailored molecules.1 However, a fundamental step towards the realization of molecular-based devices relies on the ordered placement of the active components into a suitable device architecture. In this context, self-assembly processes are gaining importance and thin-film technologies are considered likely to play a major role in future applications.2
Our research is focused on the synthesis of molecular modules which upon metal ion coordination result in metallosupramolecular coordination polyelectrolytes (MEPE's) with electronic, optical and magnetic properties of possible technological interest. Typically, homotopic bisterpyridine ligands linked by more or less rigid spacers have been employed for this purpose. The resulting metallosupramolecular assemblies are usually positive charged and can be incorporated into thin multilayer films by using electrostatic layer-by-layer self-assembly. Recently we have shown that this simple methodology allows the facile fabrication of devices based on metallosupramolecular materials.3
The term metalloviologen was firstly coined by Constable by analogy with the well-known viologens (N,N′-dialkylated-4,4′-bipyridines) to describe N-monoalkylated-4,4′-bipyridines which are coordinated to a transition metal ion.4 Although the electrochemical properties of metalloviologens have been shown to be similar to those of their viologen analogues (facile ligand reduction) it is surprising that very little attention has been drawn to this kind of material, considering that they also show properties based on the transition metal ions (optic, magnetic, reactive).5 This fact contrasts with the notable popularity of viologens.6 For instance, if one thinks about molecular-based switching and display devices, metalloviologens are promising candidates for electrochromic materials since, as viologens, they can be reduced to give rise to coloured radical species but, moreover, the colour of the “bleached” state can be dictated by the metallic centre.
This work presents the synthesis of a new homoditopic bisterpyridine ligand which, after transition metal coordination, incorporates the metalloviologen moiety in its structure. The coordination polyelectrolyte obtained by addition of cobalt(II) ions is used to fabricate thin multilayers using layer-by-layer self-assembly and acts as an active electrochromic component. This is the first example of a metalloviologen being incorporated in an operating device.
Inspired by Constable's work on the regioselective alkylation of 4′-(4‴-pyridine)-2,2′:6′,2″-terpyridine (pyterpy), the synthesis of I was afforded by the reaction of 2 equivalents of pyterpy with one equivalent of α,α′-dibromo-p-xylol in refluxing acetonitrile.‡4 A suspension of IBr2 in a mixture of water ∶ ethanol reacts rapidly with cobalt(II) to yield a deep red solution, from which [Co(I)](Br)2(NO3)2] (CoMEPE) is isolated after slow evaporation of the solvent. Mutilayer build-up is achieved by repeated immersion of a poly(ethylenimine) (PEI) modified quartz substrate in solutions containing poly(styerenesulfonate) (PSS) and CoMEPE, and is followed by UV-vis spectroscopy. Absorption bands at 200 and 220 nm in the spectrum indicate the presence of PSS while π–π* transitions at 288 and 353 nm and weak d–d transitions around 500 nm prove the presence of the cobalt(II) coordination polyelectrolyte (see Fig. 1). The linear increase of the absorption maxima as a function of the number of adsorbed layers confirms the regular and linear growth of the film.
 | ||
| Fig. 1 UV-vis spectra of (PSS–CoMEPE)n multilayers with n = 1–9 on a PEI modified quartz substrate (inset: absorption of individual band maxima as a function of the number of adsorbed layers). | ||
The electrochemical behaviour of a (PSS–CoMEPE)40 film growth on ITO is investigated by cyclic voltammetry (Fig. 2). In the scanned potential range (between 0.2 and −1.1 V vs. Ag/AgCl/KCl 3 M) two peaks at potentials of −0.78 (C1) and −1.02 V (C2) during the cathodic sweep and two peaks at potentials of −0.54 (A1) and −0.80 V (A2) during the anodic sweep are detected. The anodic and cathodic current peaks are proportional to the square root of the scan rate (measured between 10 and 100 mV s−1, see supporting information†). This behaviour is consistent with electron self-exchange reactions within the electrochemical sites in the film, in which a semi-infinite electrochemical charge diffusion condition prevails.7 The first redox couple (C1/A1) is assigned to the reversible one-electron reduction of the metal centre Co(II)/Co(I) whereas the second redox couple (C2/A2) is attributed to the reversible one-electron reduction of the ligand, which was found to result in the formation of a coloured species. The film presents originally a red colouration, typical of octahedral cobalt(II) terpyridine complexes, and after full reduction turns green. Initially, the UV-vis spectrum of the multilayer film shows a weak absorption band centred at 525 nm, whereas after the reduction of the ligand at −1.1 V a strong and broad absorption band emerges centred at 643 nm (see supporting information†).
 | ||
| Fig. 2 Cyclic voltammogram (vs. Ag/AgCl/KCl 3 M, Pt wire as counter electrode, 0.1 M NaNO3 supporting electrolyte) of a (PSS–CoMEPE)40 multilayer film on an ITO electrode. | ||
In order to evaluate the electrochromic response of the film, a double-potential step chronoamperometric experiment was carried out by simultaneously recording the absorbance of the film. Fig. 3 shows the absorbance of the film at 643 nm as function of time. Reduction at −1.1 V results in a rapid increase of the absorbance (green colouration) whereas after stepping the potential back to 0.0 V the initial state (red colouration) is recovered. The response time is of the order of seconds in both colouration and “bleaching” processes. The optical contrast is visually noticeable and the optical density of the multilayer film is around 0.16. An average thickness of 114 nm for the (PSS–CoMEPE)40 film is estimated from AFM measurements.
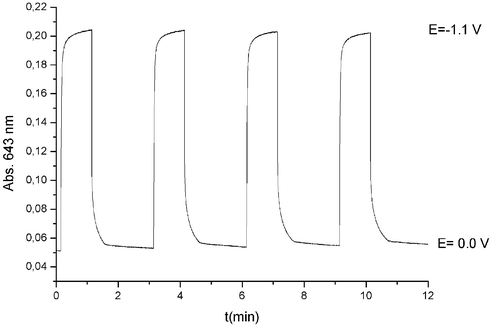 | ||
| Fig. 3 Absorbance at 643 nm of a (PSS–CoMEPE)40 coated ITO electrode during subsequent double-potential steps between 0.0 and −1.1 V vs. Ag/AgCl/KCl 3 M. | ||
In summary, a novel bisterpyridine ligand has been synthesized which after addition of cobalt(II) yields a metalloviologen-based coordination polyelectrolyte. This material can be incorporated as a functional component in electrochromic thin films making use of the electrostatic layer-by-layer self-assembly. This is a facile and promising approach for the development of display-type applications where different colours are desired in different redox states, since tuning of the colouration may be achieved easily by selecting the appropriate transition metal ion.8 Furthermore, the use of coordination compounds as active components opens the possibility for additional functions (magnetic, catalytic) of the films. Long term stability studies, exploration of different metal ions to tune the colour and incorporation of a second electrochromic component, i.e. polyoxometallates,9 to increase the optical density are underway and will be reported elsewhere.
Financial support of this investigation by the Deutsche Forschungsgemeinschaft is greatly appreciated. This work is dedicated to the memory of our colleague and friend Dr. Henning Krass.
Notes and references
- H. Hofmeier and U. S. Schubert, Chem. Soc. Rev., 2004, 33, 373 RSC; P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419 RSC; J. Yamada, H. Akutsu, H. Nishikawa and K. Kikuchi, Chem. Rev., 2004, 104, 5057 CrossRef CAS; V. Balzani and A. Juris, Coord. Chem. Rev., 2001, 211, 97 CrossRef CAS.
- P. T. Hammond, Adv. Mater., 2004, 16, 1271 CrossRef CAS.
- M. Schütte, D. G. Kurth, M. R. Linford, H. Cölfen and H. Möhwald, Angew. Chem., Int. Ed., 1998, 110, 3058 CrossRef; H. Krass, E. A. Plummer, J. M. Haider, P. R. Barker, N. W. Alcock, Z. Pikramenou, M. J. Hannon and D. G. Kurth, Angew. Chem., Int. Ed., 2001, 40, 3862 CrossRef CAS; D. G. Kurth, M. Schütte and J. Wen, Colloids Surf. A, 2002, 198–200, 633 CrossRef CAS; Y. Bodenthin, U. Pietsch, H. Möhwald and D. G. Kurth, J. Am. Chem. Soc., 2005, 127, 3110 CrossRef CAS.
- E. C. Constable and A. M. W. Cargill Thompson, J. Chem. Soc., Dalton Trans., 1992, 20, 2947 RSC; E. C. Constable, C. E. Housecroft, M. Neuburger, D. Phillips, P. R. Raithby, E. Schofield, E. Sparr, D. A. Tocher, M. Zehnder and Y. Zimmermann, J. Chem. Soc., Dalton Trans., 2000, 13, 2219 RSC.
- W. Goodall and J. A. G. Williams, J. Chem. Soc., Dalton Trans., 2000, 17, 2893 RSC.
- P. M. S. Monk, The Viologens, Wiley, Chichester, UK, 1998 Search PubMed.
- R. S. Murray, in Electroanalytical Chemistry, ed. A. J. Bard, Marcel Dekker, New York, 1984, vol. 13 Search PubMed; D. M. DeLongchamp, M. Kastantin and P. T. Hammond, Chem. Mater., 2003, 15, 1575 Search PubMed.
- A. A. Argun, P.-H. Aubert, B. C. Thompson, I. Schwendeman, C. L. Gaupp, J. Hwang, N. J. Pinto, D. B. Tanner, A. G. MacDiarmid and J. R. Reynolds, Chem. Mater., 2004, 16, 4401 CrossRef CAS.
- S. Liu, D. G. Kurth, H. Möhwald and D. Volkmer, Adv. Mater., 2002, 14, 225 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: multilayer film preparation procedure, dependence of the peak currents on the scan rate and UV-vis spectra of the oxidized and reduced films. See http://www.rsc.org/suppdata/cc/b5/b500994d/ |
| ‡ Synthesis: IBr2·3 H2O: To a warm solution of pyterpy (161 mg, 0.52 mmol) in anhydrous acetonitrile (40 ml), α,α′-dibromo-p-xylol (70 mg, 0.26 mmol) was added as a solid. The solution was refluxed and after 30 min a yellowish precipitate started to form. Reflux was continued for 12 h. The mixture was hot filtered and the collected yellow precipitate washed with dichloromethane and dried in air (130 mg, 57%). C48H32N8Br2·3 H2O (934.85) calc. 61.67% C, 4.10% H, 11.99% N; found 61.85% C, 4.51% H, 11.96% N. The obtained powder is insoluble in water or common organic solvents at room temperature but dissolves in boiling technical ethanol; addition of an excess of ammonium hexaflurophosphate in water to the ethanolic solution yields a white precipitate, which is soluble in deuterated acetonitrile. I(PF6)21H NMR (CD3CN, ppm) δ: 8.68 (2H, d, Hc), 8.64 (2H, s, H′3), 8.52 (2H, d, H6), 8.49 (2H, d, H3), 8.29 (2H, d, Hb), 7.81 (2H, t, H4), 7.40 (2H, s, Hp), 7.30 (2H, t, H5), 5.63 (2H, s –CH2–). CoMEPE: To a suspension of IBr2·3 H2O (158 mg, 0.169 mmol) in ethanol (30 ml) a solution of Co(NO3)2·6 H2O (24.57 mg, 0.084 mmol) was added in water (20 ml). The resulting red solution was stirred at room temperature for 1 h. After filtration, the solvent was allowed to evaporate slowly to yield a red powder (∼70 mg, 35%). C48H32N10 O6Br2Co·8 H2O (1207.81) calc. 47.73% C, 4.00% H, 11.60% N; found 48.12% C, 3.89% H, 11.05% N. UV-vis (H2O ∶ EtOH) λmax: 277 (44298), 343 (13468), 534 (1413) nm (dm3 mol−1 cm−1). |
| This journal is © The Royal Society of Chemistry 2005 |

