Diastereoselective metal-catalyzed [4 + 2 + 2] carbocyclization reactions utilizing a rhodium N-heterocyclic carbene (NHC) complex: the first example of a rhodium NHC-catalyzed [m + n + o] carbocyclization†
P. Andrew
Evans
*,
Erich W.
Baum
,
Aleem N.
Fazal
and
Maren
Pink‡
Department of Chemistry, Indiana University, Bloomington, IN 47405, USA. E-mail: paevans@indiana.edu; Fax: +1 (812) 855-8300
First published on 19th November 2004
Abstract
The air and moisture stable rhodium N-heterocyclic carbene (NHC) complex, RhCl(IMes)(COD) (IMes = N,N′-bis(2,4,6-trimethylphenyl)imidazole-2-ylidine; COD = 1,5-cyclooctadiene), facilitates a diastereoselective metal-catalyzed [4 + 2 + 2] carbocyclization of 1,6-enynes in the presence of silver triflate and 1,3-butadiene.
We recently reported a series of intermolecular rhodium-catalyzed [4 + 2 + 2] carbocyclization reactions of heteroatom-tethered 1,6-enynes with 1,3-butadiene, using silver triflate modified Wilkinson's catalyst for the construction of bicyclic cyclooctanoids.1–3 In the course of these studies, we demonstrated that this reaction was highly efficient and diastereoselective with the C-2 napthyl substituted 1,6-enyne 1 (R = Np). Surprisingly, the extension of this methodology to other C-2 substituted 1,6-enynes, particularly alkyl derivatives, afforded only trace amounts of the product thus indicating that the original catalyst was highly substrate dependent. Herein, we now report a highly efficient and diastereoselective metal-catalyzed [4 + 2 + 2] carbocyclization reaction utilizing the recently reported rhodium N-heterocyclic carbene (NHC) complex, RhCl(IMes)(COD),4–6 which provides a general solution to this problem over a broad range of C-2 substituted 1,6-enynes. Moreover, this represents the first example, as far as we can determine, of a rhodium NHC-catalyzed [m + n + o] type carbocyclization.7
 | (1) |
In the course of extending the scope of the diastereoselective rhodium-catalyzed [4 + 2 + 2] carbocyclization with C-2 substituted 1,6-enynes, we determined the original silver triflate modified Wilkinson's catalyst to be substrate specific. For example, treatment of the enyne 1a (R = Me) with this catalyst in the presence of 1,3-butadiene, afforded only trace amounts of the carbocycle 2a (eqn. (1); <5%). Table 1 summarizes the examination of various precatalysts and ligands in an attempt to develop a more general reaction. Bidentate phosphine ligands were initially explored (Entries 1–3) which, with the exception of ethane-1,2-diylbis(diphenylphosphine) (dppe) (Entry 1), provided analogous results to Wilkinson's catalyst. Various monodentate trialkyl phosphines were also examined, as exemplified by tri-n-butylphosphine and tricyclohexylphosphine (Entries 4 and 5), which afforded the desired product as a single diastereoisomer, with improved yield. Based on the improved efficiency demonstrated with the electron-rich monodentate ligands, we speculated that strong σ-donation might be a crucial component for catalytic turnover in this particular reaction.
| Entry | Precatalyst b,c | Ligand | Ratio of 2 ∶ 3d | Yield (%)e |
|---|---|---|---|---|
| a All reactions (0.25 mmol) were carried out in toluene (0.08 M) at 110 °C. b 10 mol% of monomer and 5 mol% of dimer were employed. c 2 equivalents of AgOTf relative to the metal were utilized. d Ratios determined by 400 MHz 1H NMR on crude reaction mixtures. e GLC yields (COE = cyclooctene). | ||||
| 1 | [RhCl(COE)2]2 | dppe | >19 ∶ 1 | 34 |
| 2 | ″ | dppp | — | <5 |
| 3 | ″ | dppb | — | <5 |
| 4 | ″ | P(nBu)3 | >19 ∶ 1 | 45 |
| 5 | ″ | P(cHex)3 | >19 ∶ 1 | 30 |
| 6 | RhCl(IMes)(COD) | — | >19 : 1 | 75 |
N-heterocyclic carbene ligands are known phosphine surrogates, which fulfill the criterion for strong σ-donation. Indeed, since the first independent reports of N-heterocylic carbene complexes by Wanzlick8 and Öfele9 and the isolation of the first crystalline NHC by Arduengo,10 these carbenes have gained considerable prominence as important ligands in organometallic chemistry. This may be attributed to the fact that these singlet carbenes behave in an analogous manner to amines, ethers, and phosphines as classical 2e− donors,11 in which the NHC's serve as better donors than some of the best phosphines. Furthermore, NHC's are often more resistant to dissociation from the metal center in many systems.12 As a result, NHC–metal complexes have been successfully utilized as catalysts or precatalysts for a variety of transition metal-catalyzed transformations.11,13 Despite the myriad of transformations utilizing NHC–metal complexes, there are relatively few examples of their use in [m + n + o] carbocyclization reactions.14
| (2) |
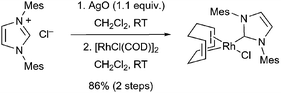
The rhodium–NHC complex, RhCl(IMes)(COD), was prepared in good yield via transmetallation of [RhCl(COD)]2 with a silver carbene complex (eqn. (2)).12c,15 The intermediate Ag–NHC complex, which was prepared in situ through the treatment of the imidazolium salt with silver oxide, was added directly into a solution of [RhCl(COD)]2 in methylene chloride to afford the desired metal complex. The air and moisture stable complex was then purified by column chromatography to remove any residual salts and [RhCl(COD)]2. While spectroscopic data for this complex have been reported,4 a definitive structure was not forthcoming. We were able to obtain crystals suitable for X-ray crystallographic analysis, and thereby prove the structure of this complex (Fig. 1).§
 | ||
| Fig. 1 Thermal ellipsoid plot of RhCl(IMes)(COD) (50% probability thermal ellipsoids). | ||
Gratifyingly, the utilization of the rhodium–NHC complex, RhCl(IMes)(COD), provided a remarkable improvement in the overall efficiency of the reaction, in which the cyclooctanoid 2a was obtained in 75% yield, as a single diastereoisomer (Entry 6). In light of this result, we decided to examine the substrate scope of this catalyst for a variety of C-2 substituted 1,6-enynes, since it demonstrated considerable promise as a potential solution to the [4 + 2 + 2] reaction and also represents a novel catalyst for carbocyclization reactions.
Table 2 summarizes the reactions of various enynes 1a–l with silver triflate modified RhCl(IMes)(COD) in the presence of excess 1,3-butadiene. In all cases the rhodium-catalyzed [4 + 2 + 2] carbocyclization reaction proceeds with excellent diastereocontrol affording the desired carbocycles 2a–l in high yield.¶ This catalytic system works efficiently with various linear and branched alkyl and benzyl substituents at the C-2 position (Entries 1-5). Interestingly, the reaction is also tolerant of a free hydroxyl group, albeit resulting in lower yield (Entry 6). Presumably, the free hydroxyl group's ability to coordinate the metal center hinders the efficiency of the reaction. In line with this reasoning, protection of the hydroxyl substituent allowed for improved efficiencies (Entry 7–8). Likewise, the catalytic system is tolerant of functional groups such as ethers, esters, other olefins, and aryl groups (Entries 9–12).
| Entry | 1,6-Enyne 1a R = | Ratio of 2 ∶ 3b | Yield (%)c | |
|---|---|---|---|---|
| a All reactions (0.25 mmol) were carried out using 10 mol% of the catalyst and 20 mol% AgOTf in toluene (0.08 M) at 110 °C. b Ratios determined by 400 MHz 1H NMR on crude reaction mixtures. c Isolated yields. | ||||
| 1 | Me | a | ≥19 ∶ 1 | 84 |
| 2 | Bn | b | ≥19 ∶ 1 | 79 |
| 3 | Ph(CH2)2 | c | ≥19 ∶ 1 | 83 |
| 4 | i Pr | d | ≥19 ∶ 1 | 84 |
| 5 | cHex | e | ≥19 ∶ 1 | 75 |
| 6 | HOCH2 | f | ≥19 ∶ 1 | 55 |
| 7 | BnOCH2 | g | ≥19 ∶ 1 | 77 |
| 8 | TBSOCH2 | h | ≥19 ∶ 1 | 83 |
| 9 | CO2Me | i | ≥19 ∶ 1 | 71 |
| 10 | CH2=CH | j | ≥19 ∶ 1 | 81 |
| 11 | CH2=CHCH2 | k | ≥19 ∶ 1 | 79 |
| 12 | Ph | l | ≥19 ∶ 1 | 89 |
We envisioned that the application of this methodology to substituted alkenes would further highlight the synthetic utility of this catalyst through the introduction of an additional stereogenic center. Moreover, alkene substitution is often poorly tolerated, given that these substrates are prone to enyne cycloisomerization.16,17 Treatment of the enyne 4 under the standard [4 + 2 + 2] carbocyclization conditions, using the RhCl(IMes)(COD) catalyst, furnished the carbocycle 5 in 70% yield, with ≥19 ∶ 1 diastereoselectivity (eqn. (3)).

| (3) |
In conclusion, we have demonstrated that the air and moisture stable complex RhCl(IMes)(COD) is a suitable catalyst for the diastereoselective [4 + 2 + 2] carbocyclization and thus provides the first example of a rhodium–NHC-catalyzed carbocyclization. Additionally, the utilization of the carbene catalysts allows for the construction of three contiguous stereocenters in a single transformation from a substituted 1,6-enyne.
We sincerely thank the National Science Foundation (CHE-0316689) for generous financial support. We also thank Johnson and Johnson for a Focused Giving Award and Pfizer Pharmaceuticals for the Creativity in Organic Chemistry Award (PAE). Roche Pharmaceuticals is kindly acknowledged for an Excellence in Chemistry Award (EWB) and Pfizer GRD for a Fellowship Supporting Diversity in Organic Chemistry (ANF).
Notes and references
- (a) P. A. Evans, J. E. Robinson, E. W. Baum and A. N. Fazal, J. Am. Chem. Soc., 2002, 124, 8782 CrossRef CAS; (b) P. A. Evans, J. E. Robinson, E. W. Baum and A. N. Fazal, J. Am. Chem. Soc., 2003, 125, 14648 CrossRef CAS.
- For another example of a rhodium-catalyzed [4 + 2 + 2] carbocyclizations, see: S. R. Gilbertson and B. DeBeof, J. Am. Chem. Soc., 2002, 124, 8784 Search PubMed.
- For related metal-catalyzed [4 + 2 + 2] carbocyclizations reactions, see: Fe; (a) A. Greco, A. Carbonaro and G. Dall'Asta, J. Org. Chem., 1970, 35, 271 CrossRef CAS; Co; (b) A. Carbonaro, F. Cambisi and G. Dall'Asta, J. Org. Chem., 1971, 36, 1443 CrossRef CAS; (c) M. Lautens, W. Tam, J. C. Lautens, L. G. Edwards, C. M. Crudden and A. C. Smith, J. Am. Chem. Soc., 1995, 117, 6863 CrossRef CAS; Ru; (d) J. A. Varela, L. Castedo and C. Saá, Org. Lett., 2003, 5, 2841 CrossRef CAS.
- A. M. Seayad, K. Selvakumar, M. Ahmed and M. Bellar, Tetrahedron Lett., 2003, 44, 1679 CrossRef CAS.
- For a saturated carbene version of this metal complex, see: K. Denk, P. Sirsch and W. A. Herrmann, J. Organomet. Chem., 2002, 649, 219 Search PubMed.
- For other Rh–IMes complexes, see: (a) A. C. Chen, L. Ren, A. Decken and C. M. Crudden, Organometallics, 2000, 19, 3459 CrossRef CAS; (b) G. A. Grasa, Z. Moore, K. L. Martin, E. D. Stevens, S. P. Nolan, V. Paquet and H. Lebel, J. Organomet. Chem., 2002, 658, 126 CrossRef CAS.
- For an example of a rhodium–NHC-catalyzed hydrosilylation/cyclization of 1,6-enynes, see: K. H. Park, S. Y. Kim, S. U. Son and Y. K. Chung, Eur. J. Org. Chem., 2003, 4341 Search PubMed.
- H.-W. Wanzlick and H.-J. Schönherr, Angew. Chem., Int. Ed. Engl., 1968, 7, 141 CrossRef CAS.
- K. Öfele, J. Organomet. Chem., 1968, 12, P42 CrossRef.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef.
- For recent review, see: W. A. Herrmann, Angew. Chem., Int. Ed.., 2002, 41, 1290 Search PubMed.
- (a) J. Huang, H.-J. Schanz, E. D. Stevens and S. P. Nolan, Organometallics, 1999, 18, 2370 CrossRef CAS; (b) J. Huang, E. D. Stevens and S. P. Nolan, Organometallics, 2000, 19, 1194 CrossRef CAS; (c) A. R. Chianese, X. Li, M. C. Janzen, J. W. Faller and R. H. Crabtree, Organometallics, 2003, 22, 1663 CrossRef CAS.
- (a) A. C. Hillier and S. P. Nolan, Platinum Met. Rev., 2002, 46, 50 CAS; (b) T. M. Trnka and R. H. Grubbs, Acc. Chem. Res., 2001, 34, 18 CrossRef CAS.
- (a) For an example of Ni–NHC catalyzed [2 + 2 + 2] cycloaddition, see: J. Louie, J. E. Gibby, M. V. Farnworth and T. N. Tekavec, J. Am. Chem. Soc., 2002, 124, 15188 Search PubMed; (b) For an example of Co–NHC catalyzed Pauson Khand reaction, see: S. E. Gibson, C. Johnstone, J. A. Loch, J. W. Steed and A. Strevenazzi, Organometallics, 2003, 22, 5374 Search PubMed.
- H. M. J. Wang and I. J. B. Lin, Organometallics, 1998, 17, 972 CrossRef CAS.
- For an example of an ene-cycloisomerization and the consequence of olefin geometry, see: P. Cao, B. Wang and X. Zhang, J. Am. Chem. Soc., 2000, 122, 6490 Search PubMed.
- P. A. Evans and E. W. Baum, J. Am. Chem. Soc., 2004, 126, 11150 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental procedures, X-ray crystallographic analysis of the 3,5-dinitrobenzoates of 2f and 5, and spectral data (IR, 1H and 13C NMR) including high resolution MS for 1a–l, 2a–l and 5. See http://www.rsc.org/suppdata/cc/b4/b413438a/ |
| ‡ To whom all correspondence regarding the X-ray crystallography should be addressed: E-mail: mpink@indiana.edu. |
| § Crystal structure data for RhCl(IMes)(COD), C29H36ClN2Rh, yellow block, tetragonal, I41/a, a = 32.464(2) Å c = 9.9286(7) Å V = 10463.7(10) Å3, Z = 16, T= 113(2) K, ρcalc = 1.399 Mg m−3, μ = 0.774 mm−1, GOF = 1.033, R(F) = 0.0276 and wR(F2) = 0.0635 for 6359 observed reflections I > 2σ(I), 4.28° ≤ 2θ ≤ 60.16°. CCDC 249419. See http://www.rsc.org/suppdata/cc/b4/b413438a/ for crystallographic data in .cif or other electronic format. |
| ¶ The relative configuration of the major diastereoisomer 2f was confirmed by X-ray crystallographic analysis after conversion to the corresponding 3,5-dinitrobenzoate. The relative configuration was then relayed to that of deprotected 2g–h. The alkyl substituted product 2a, was confirmed via reduction of the tosylate of 2f. |
| This journal is © The Royal Society of Chemistry 2005 |
