Direct injection analysis of bisphenol A in serum by combination of isotope imprinting with liquid chromatography-mass spectrometry
Haruyo Sambeab, Kaori Hoshinab, Ken Hosoyac and Jun Haginaka*b
aLaboratory of Intellectual Fundamentals for Environmental Studies, National Institute for Environmental Studies, 16-2, Onogawa, Tukuba, Ibaraki 305-8506, Japan
bFaculty of Pharmaceutical Sciences, Mukogawa Women's University, 11-68, Koshien Kyuban-cho, Nishinomiya, Hyogo 663-8179, Japan. E-mail: haginaka@mwu.mukogawa-u.ac.jp; Fax: +81 798 45 9949; Tel: +81 798 41 2792
cDepartment of Polymer Science and Engineering, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan
First published on 1st December 2004
Abstract
A restricted access media-molecularly imprinted polymer was prepared using bisphenol A (BPA)-d16 as a template molecule, and was successfully applied to direct injection analysis of BPA in serum combined with column-switching LC-MS.
Molecularly imprinted polymers (MIPs)1–3 have been used for selective on/off-line extraction of target analytes, while restricted access media (RAM)4–6 have been applied for direct injection assays of drugs and their metabolites in biological fluids. Recently, we prepared RAM-MIPs, which have characteristics of both RAM and MIP, and showed their usefulness for selective extraction of an analyte from complex matrices such as serum.7–9 However, when a MIP was applied to the ultra-trace assay of an analyte, leakage of a template molecule remaining in the MIP prevented the accurate and precise assay of the analyte.8–11 Therefore, it is necessary to prepare a MIP for an analyte using its structurally related analogue as a template molecule. However, the MIP is inferior to that prepared using the analyte as a template molecule in the molecular recognition ability. The MIP prepared using a template molecule, whose shape and functionality are more similar to those of an analyte, would give higher molecular recognition ability for the analyte. From this view point, it is interesting to prepare a MIP for an analyte using its isotopologue form as a template molecule.
In this study, we prepared the RAM-MIP for bisphenol A (BPA)-d16 and evaluated its molecular recognition ability for BPA, BPA-d6, BPA-d16 and other structurally related analogues by LC. Furthermore, we applied the RAM-MIP for BPA-d16 to direct injection analysis of BPA in serum combined with column-switching LC-MS.
The RAM-MIP for BPA-d16 was prepared by a multi-step swelling and polymerization method, and hydrophilic surface modification techniques as reported previously.7–9 In a previous study,12 we prepared the MIP for BPA using ethylene glycol dimethacrylate (EDMA) as a cross-linker, toluene as a porogen and methacrylic acid, 2-diethylaminoethyl methacrylate or 4-vinylpyridine (4-VPY) as a functional monomer or without use of a functional monomer. Among the MIPs prepared, BPA-imprinted 4-VPY-co-EDMA polymers showed the highest molecular recognition ability for BPA. Thus, we similarly prepared the MIP for BPA-d16 using 4-VPY as a functional monomer. Next, a mixture of glycerol monomethacrylate (GMMA) and glycerol dimethacrylate (GDMA) as hydrophilic monomers was added directly to the MIP after 4 h from the start of polymerization. Then further polymerization was carried out for 20 h. After washing, the uniformly sized RAM-MIP for BPA-d16 (particle size, ca. 6 µm) was obtained. RAM-MIP1 was prepared using 4 mmol BPA-d16, 9 mmol 4-VPY, 5 ml EDMA, 5 ml toluene, 0.5 ml GDMA and 0.5 ml GMMA, while RAM-MIP2 was prepared in the same manner as RAM-MIP1 except that 7 ml EDMA and 3 ml toluene were used. RAM-MIPs 1 and 2 were packed into a stainless-steel column (4.0 mm id × 10 mm) using a slurry packing procedure.
Table 1 shows the retention and selectivity factors of BPA, BPA-d6, BPA-d16 and other structurally related analogues on RAM-MIPs 1 and 2 for BPA-d16. The selectivity factor (S), which is the ratio of the retention factor (k) on the RAM-MIP to that on a non-imprinted polymer, kimprinted/knon-imprinted, was used to evaluate the molecular recognition ability of the RAM-MIPs. As expected, the RAM-MIPs had excellent retentivity and selectivity for BPA, BPA-d6 and BPA-d16. This is due to that the shape and functionality of BPA and its deuterated forms are almost the same with one another. Thus, two phenolic groups of BPA could interact with pyridyl groups of BPA-d16-imprinted 4-VPY-co-EDMA polymers in hydro-organic eluents as reported previously.12,13 Small differences in k values among BPA and its deuterated forms are ascribable to hydrogen/deuterium isotope effects: that is, BPA and its deuterated forms give different retentions on a reversed-phase LC column.14 With regard to the molecular recognition of other structurally related analogues, the selectivity of the RAM-MIP for 4,4′-methylenediphenol and p-t-buthylphenol is lower than that for BPA and its deuterated forms, while it showed almost no selectivity for phenol. These results suggest that two methyl groups of BPA could interact with BPA-d16-imprinted 4-VPY-co-EDMA polymers in addition to the two phenolic groups. Retentivity and selectivity of RAM-MIP2 were lower than those of RAM-MIP1, as shown in Table 1. Fewer recognition sites in RAM-MIP2 than in RAM-MIP1 could result in less retention and therefore less selectivity as reported previously.7
| Solute | RAM-MIP1 | RAM-MIP2 | ||
|---|---|---|---|---|
| kb | Sc | kb | Sc | |
| a HPLC conditions: column size, 4.0 mm id × 10 mm; column temperature, 40 °C; eluent, water–acetonitrile (60 ∶ 40, v/v); flow rate, 1.0 ml min−1; detection, 210 nm; loaded amount, 100 ng.b k is the retention factor.c S is the selectivity factor, kimprinted/knon-imprinted. | ||||
| BPA | 20.6 | 10.2 | 15.0 | 6.78 |
| BPA-d6 | 20.2 | 10.2 | 14.6 | 6.81 |
| BPA-d16 | 19.3 | 10.1 | 13.9 | 6.70 |
| 4,4′-Methylenediphenol | 6.92 | 3.02 | 4.87 | 2.63 |
| p-t-Buthylphenol | 3.33 | 2.06 | 3.52 | 1.80 |
| Phenol | 0.88 | 1.19 | 0.87 | 1.20 |
In previous studies,7,8 bovine serum albumin (BSA) was completely recovered from RAM-MIPs using a mixture of acetonitrile and sodium phosphate buffer as an eluent. However, non-volatile sodium phosphate buffer can not be used as an eluent for MS detection. Thus, we examined the recovery of BSA from RAM-MIPs using volatile eluents. Table 2 shows the recovery of BSA from RAM-MIPs 1 and 2 using a mixture of acetonitrile and sodium phosphate buffer (pH 3.2) (A), sodium phosphate buffer (pH 6.9) (B), acetic acid (C), ammonium formate (D) or ammonium acetate (E). With eluents A, B and C, the recovery of BSA from RAM-MIP1 was complete, while with eluents D and E, the recovery was incomplete. On the other hand, RAM-MIP2 showed the complete recovery of BSA with all eluents tested. This could be ascribable to smaller macropore volumes of RAM-MIP2 than RAM-MIP1.7 These results indicate that RAM-MIP2 could be applied to direct injection analysis of BPA in serum by LC-MS.
| Eluent | Recovery (%)b | |
|---|---|---|
| RAM-MIP1 | RAM-MIP2 | |
| a HPLC conditions: column size, 4.0 mm id × 10 mm; column temperature, ambient; flow rate, 1.0 ml min−1; detection, 280 nm; loaded amount, 0.08 mg; eluent A, 20 mM sodium phosphate buffer (pH 3.2)–acetonitrile (80 ∶ 20, v/v); eluent B, 20 mM sodium phosphate buffer (pH 6.9)–acetonitrile (80 ∶ 20, v/v); eluent C, water–acetonitrile (80 ∶ 20, v/v) containing 0.5% acetic acid; eluent D, water–acetonitrile (80 ∶ 20, v/v) containing 5 mM ammonium formate; eluent E, water–acetonitrile (80 ∶ 20, v/v) containing 5 mM ammonium acetate.b Average ± SD (n = 10). | ||
| A | 99.3 ± 1.1 | 100.0 ± 0.7 |
| B | 92.6 ± 5.4 | 101.0 ± 1.0 |
| C | 99.0 ± 1.3 | 99.3 ± 1.4 |
| D | 80.8 ± 16.4 | 98.6 ± 1.2 |
| E | 86.0 ± 13.2 | 100.7 ± 1.1 |
Next, we applied RAM-MIP2 to direct injection analysis of BPA in serum combined with column-switching LC-MS, where RAM-MIP2 and C18 columns were used as pretreatment and analytical columns, respectively, and BPA was detected by selective ion monitoring. Fig. 1(a) and (b) show chromatograms of control serum spiked with BPA and control serum, respectively, monitored at m/z 227. BPA was separated from the ordinary components of serum and selectively detected. Fig. 1(c) shows a chromatogram obtained with 20 µl injections of water. BPA-d16, which leaked out of RAM-MIP2, was detected at m/z 241. The above results indicate that BPA and BPA-d16 are completely separable by MS detection. Thus, leakage of a template molecule could be overcome by isotope imprinting and MS detection.
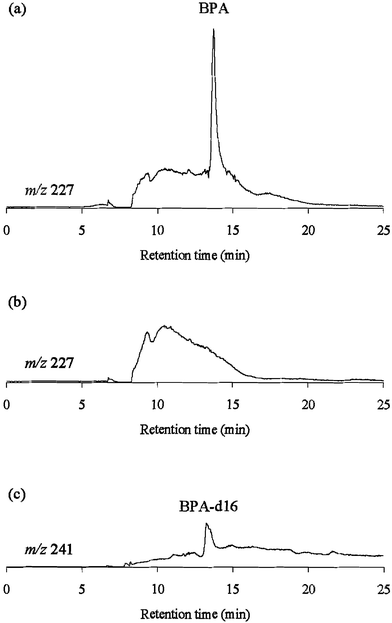 | ||
| Fig. 1 Chromatograms of control serum spiked with BPA (a) and control serum (b), monitored at m/z 227, and of 20 µl injections of water (c), monitored at m/z 241 by column-switching LC-MS using RAM-MIP2 as a pretreatment column. Pretreatment LC conditions: column, RAM-MIP2 (4.0 mm id × 10 mm); eluent, water–acetonitrile (95 ∶ 5, v/v) containing 5 mM ammonium acetate at 1.0 ml min−1 for 5 min; column temperature, 40 °C; concentration of BPA, 200 ng ml−1; injection volume, 20 µl. Analytical LC conditions: column, Migthysil RP-18 (2.0 mm id × 150 mm); eluent, water–acetonitrile (60 ∶ 40, v/v); flow rate, 0.2 ml min−1; column temperature, 40 °C. MS conditions: ionization mode, electrospray (negative); data collection, selected ion recording (m/z 227 or 241). | ||
In conclusion, we attained highly selective analysis of BPA in serum by combination of isotope imprinting with MS detection. The RAM-MIP for BPA-d16 should be useful for direct injection analysis of BPA in serum. Furthermore, the RAM-MIP, which can exclude humic acids,15,16 could be applicable for environmental analysis of BPA. The combined use of isotope imprinting and MS detection seems useful for MIP-based extraction of a target analyte in the case of its ultra-trace analysis. Further studies are on-going in our laboratories.
Acknowledgements
This research was partly supported by the Nanotechnology Project of the Ministry of Environment, Japan.References
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812 CrossRef CAS
.
- A. G. Mayes and K. Mosbach, Trends Anal. Chem., 1997, 16, 321 CrossRef CAS
.
- L. I. Andersson, J. Chromatogr. B, 2000, 739, 163 CrossRef CAS
.
- J. Haginaka, Trends Anal. Chem., 1991, 10, 17 CrossRef CAS
.
- D. J. Anderson, Anal. Chem., 1993, 65, 434R
.
- K.-S. Boos and C.-H. Grim, Trends Anal. Chem., 1999, 18, 175 CrossRef CAS
.
- J. Haginaka, H. Takehira, K. Hosoya and N. Tanaka, J. Chromatogr. A, 1999, 849, 331 CrossRef CAS
.
- J. Haginaka and H. Sanbe, Anal. Chem., 2000, 72, 5206 CrossRef CAS
.
- H. Sanbe and J. Haginaka, Analyst, 2003, 128, 593 RSC
.
- L. I. Andersson, A. Paprica and T. Arvidsson, Chromatographia, 1997, 46, 57 CAS
.
- J. Matsui, K. Fujiwara and T. Takeuchi, Anal. Chem., 2000, 72, 1810 CrossRef CAS
.
- J. Haginaka and H. Sanbe, J. Pharm. Biomed. Anal., 2002, 30, 1835
.
- J. Haginaka and H. Sanbe, Chem. Lett., 1999, 757 CrossRef CAS
.
- M. Turowski, N. Yamakawa, J. Meller, K. Kimata, T. Ikegami, K. Hosoya, N. Tanaka and E. R. Thornton, J. Am. Chem. Soc., 2003, 125, 13836 CrossRef CAS
.
- R. Koeber, C. Fleischer, F. Lanza, K-S. Boos, B. Sellergren and D. Barcelo, Anal. Chem., 2001, 73, 2437 CrossRef CAS
.
- T. Kubo, K. Hosoya, Y. Watabe, T. Ikegami, N. Tanaka, T. Sano and K. Kaya, J. Chromatogr. A, 2003, 987, 389 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2005 |
