Intracellular localization of a long alkyl chain tetraphenylporphyrin and chloride channel activation in Psammobatis extenta electrocytes
María
Prado Figueroa
a and
Julio
Santiago
*b
aInstituto de Investigaciones Bioquímicas, Universidad Nacional del Sur, C.C. 857, B-8000FWB Bahía Blanca, Argentina. E-mail: inprado@criba.edu.ar
bInstituto Peruano de Energía Nuclear, Av. Canadá 1470, Lima 41, Perú. E-mail: jsantiago@ipen.gob.pe
First published on 3rd November 2003
Abstract
The intracellular localization of a tetraphenylporphyrin bearing four long lipophilic alkyl chains in electrocytes from Psammobatis extenta (Rajidae) is described. In contrast to what is usually the case, this porphyrin derivative is localized in the electromotor nerves and the nuclear chromatin of the electrocytes. Both structures exhibited intense fluorescence, whereas, the mitochondria were only slightly fluorescent. These data are discussed in relation to electrocyte death in a weakly electric fish. Additionally, electron probe X-ray microanalysis suggests a migration of chloride and cationic ions, which might be implicated in chloride and cationic channel activation in the electrocyte.
The development of new generation photosensitizers to improve the efficiency of photodynamic therapy (PDT) is an area of intensive research. In PDT, the activation of a photosensitizer by light generates singlet molecular oxygen (1O2), a highly active form of oxygen that reacts with many biomolecules, including lipids, proteins, and nucleic acids.1 The biomolecules are chemically modified by the action of 1O2 and, therefore, cannot accomplish their function, which leads to cell death. This therapy is applied for treatment of cancer, as well as for bacterial and viral eradication.2–6 The fluorescence exhibited by photosensitizers means they can also be used for detection of tumors.7 The advantages of this method, as compared to other conventional cancer treatment modalities, are its low systemic toxicity and its ability to destroy tumors selectively. Nevertheless, the application of PDT remains restricted due to the limited penetration of light into tissues, the photosensitization of normal tissues, and the residual skin photosensitivity that is observed for several weeks following treatment.
The lipophilicity of a particular photosensitizer determines its localization and, therefore, the site and nature of the damage it gives rise to in cells;6,8,9 in general, lipophilic photosensitizers accumulate in the membrane of a cell and its organelles.5 On the other hand, hydrophilic, as well as aggregated states of photosensitizers, enter the cell by pynocitosis and are localized mainly in lysosomes and endosomes.10 There are many photosensitizers, mostly porphyrin or phthalocyanine derivatives, under clinical study that can be classified as lipophilic or hydrophilic.11 However, there are a few examples of neutral macrocycles with long alkyl chains which have been studied as potential photosensitizers for PDT. The efficiency and selectivity of tumor targeting has been shown to increase slightly upon increasing the length of the alkyl groups connected to a Zn(II)–phthalocyanine complex.12,13
In this work, we have investigated the uptake and intracellular localization of 5,10,15,20-tetrakis(4-n-dodecylphenyl)porphyrin (1),† and the elemental composition and morphological changes in electrocytes of Psammobatis extenta. This species belongs to the Rajidae, one of three groups of weakly electric fish. We chose electrocytes for this study because they are large cells with very few organelles. This facilitates the study of the intracellular localization of the photosensitizer. In addition, electric ray electrocytes have myoproteins.14,15
The electrocytes are highly polarized and multinuclear cells. They are semicircular in shape (Fig. 1) and have their concave face receiving innervations (IF) from electromotor neurons of the spinal cord. The other face, which is convex, is non-innervated (NIF) and shows a system of caveolae.14 The nuclei are localized at the posterior region of the cytoplasm.14
 | ||
| Fig. 1 Scanning electron micrograph showing three electrocytes (×100). | ||
Electric organ cryostat sections were performed along the antero-posterior axis; sections were incubated with a solution of 1 in a mixture of xylene, EtOH, and imidazole buffer‡ and observed with an epifluorescence microscope (Fig. 2). The phase-contrast micrograph [Fig. 2(a)] shows part of two electrocytes and the fluorescence micrograph [Fig. 2(b)] shows strong fluorescence in the nuclei, but only very slight fluorescence in the mitochondria. When the incubation was performed in CHCl3 solution only, the intracellular localization of 1 is the same, but the micrograph shows better contrast (Fig. 3).
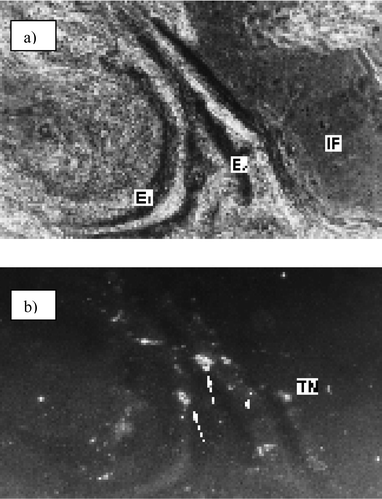 | ||
| Fig. 2 Localization of 1 in xylene–EtOH–imidazole buffer: (a) phase-contrast micrograph; (b) fluorescence micrograph (×100), fluorescence can be observed in the nuclei (arrow) and mitochondria (arrow-head). | ||
 | ||
| Fig. 3 Localization of 1 in the nuclei (black arrow), terminal nerves (white arrow), and mitochondria (arrow-head) of an electrocyte (×600). | ||
The photosensitizer also shows affinity for the terminal nerves (TN). The affinity of this lipophilic photosensitizer for the nerves is a result of this tissue being rich in fatty acids. The nuclei show an intense red fluorescence and the shape of this fluorescence is similar to the semicircular distribution of the chromatin of these cells (Fig. 3), suggesting that 1 interacts with DNA. In contrast, the mitochondria show only slight fluorescence. This localization is unusual for other photosensitizers of similar polarity. For example, a lipophilic phthalocyanine has been found to localize on the lysosomes.16
Immediately after the penetration of 1 (1.2 × 10−4 M in chloroform) into the electrocytes, they start to swell and the convex faces lose all their invaginations (Fig. 4). In order to understand the reason for the swelling of cells, microanalysis by energy-dispersive X-ray (EDAX) spectroscopy of the same region was carried out.‡ After treatment of the electrocyte with 1, the relative semi-quantitative contents [Kα (wt%)] of oxygen, sodium, and chloride ions were 39, 17, and 15, respectively. Compared to the negative control, the peak for Na+ is five times larger, while for oxygen, the variation is not significant. Additionally, the Ca2+ peak is twice as large for the treated electrocyte compared to the negative control, but that for K+ is six times smaller. The simultaneous increase in the Na+ and Ca2+ concentrations and the decrease in the K+ concentration is good evidence for cationic channel activation by the porphyrin derivative. However, the most important change is the appearance of a large new peak corresponding to the Cl− anion.
 | ||
| Fig. 4 Scanning electron micrographs of the non-innervated face of an electrocyte before (a) and after (b) treatment with 1 in CHCl3. | ||
Treatment of electrocytes with systems I and II [(I) 7.8 × 10−5 M of 1 in CHCl3–EtOH–imidazole buffer; (II) 3.9 × 10−4 M of 1 in xylene–EtOH–imidazole buffer] produces similar behavior to that observed after treatment with the CHCl3 solution of 1. In both cases, the EDAX patterns shows a new peak corresponding to the chloride anion (Fig. 5). The Kα values for this element are 47 (system I) and 33 wt% (system II), in spite of the higher concentration of 1 in system II (5 times higher) compared to system I. These results constitute suggestive evidence for chloride channel activation as a consequence of the penetration of 1 into the electrocytes. The massive intracellular accumulation of Cl− and, particularly, the influx of Na+ lead to cell swelling, and eventually a necrotic response from the cells.17,18 It should be pointed out that chloride channel activation is not usual for weakly electric fish. However, the presence of a voltage-gated chloride channel was demonstrated in the non-innervated plasma membrane of electrocytes of Torpedo, a strongly electric fish.19
 | ||
| Fig. 5 Energy-dispersive X-ray spectra and electron micrographs (×80) of electric organ segments treated with 1 in chloroform–EtOH–imidazole buffer (a) and xylene–EtOH–imidazole buffer (b). | ||
The activation of the chloride channel by 1 may constitute an alternative for the treatment of cystic fibrosis, which is related to a dysfunction of chloride ion transport.20 In contrast, the increase in the Ca2+ concentration in the electrocytes suggests the participation of an apoptotic mechanism.1 Moreover, the interaction of 1 with DNA, and even the low amount of mitochondrial-bound porphyrin, may contribute to cell death by an apoptotic mechanism after excitation with light.
The photosensitizer used in this work has liquid crystalline properties.21,22 It would be very interesting to compare these results with those obtained using another photosensitizer with a similar chemical structure but no mesomorphic properties.
Notes and references
- N. L. Oleinick, R. L. Morris and I. Belichenko, The role of apoptosis in response to photodynamic therapy: what, where, why, and how, Photochem. Photobiol. Sci., 2002, 1, 1–21 RSC.
- S. Rywkin, E. Ben-Hur, Z. Malik, A. M. Prince, Y. S. Li, M. E. Kenney, N. L. Oleinick and B. Horowitz, New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates, Photochem. Photobiol., 1994, 60, 165–170 CAS.
- M. Merchat, P. Giacomoni, A. Villanueva, G. Bertoloni and G. Jori, Photosensitization of bacteria to visible light by meso-substituted porphyrins, J. Braz. Chem. Soc., 1995, 6, 123–125 Search PubMed.
- K. Kassab, D. Dei, G. Roncucci, G. Jori and O. Copellotti, Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis, Photochem. Photobiol. Sci., 2003, 2, 668–672 RSC.
- Z. Malik, T. Babushkin, S. Sher, J. Hanania, H. Ladan, Y. Nitzan and S. Salzberg, Collapse of K+ and ionic balance during photodynamic inactivation of leukemic cells, erythrocytes and Staphylococcus aureus, Int. J. Biochem., 1993, 25, 1399–1406 Search PubMed.
- E. Reddi, M. Ceccon, G. Valduga, G. Jori, J. Bommer, F. Elisei, L. Latterini and U. Mazzucato, Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins, Photochem. Photobiol., 2002, 75, 462–470 CrossRef CAS.
- J. Moan and T. Christensen, Porphyrins as tumor localizing agents and their possible use in photochemotherapy of cancer, Tumor Res., 1980, 15, 1–10 Search PubMed.
- E. Weizman, C. Rothmann, L. Greenbaum, A. Shainberg, M. Adamek, B. Ehrenberg and Z. Malik, Mitochondrial localization and photodamage during photodynamic therapy with tetraphenylporphines, J. Photochem. Photobiol., B, 2000, 59, 92–102 CrossRef CAS.
- A. Graham, G. Li, Y. Chen, J. Morgan, A. Oseroff, T. Dougherty and R. Pandey, Structure-activity relationship of new octaethylporphyrin-based benzochlorins as photosensitizers for photodynamic therapy, Photochem. Photobiol., 2003, 77, 561–566 CAS.
- K. Berg and J. Moan, Lysosomes and microtubules as targets for photochemotherapy of cancer, Photochem. Photobiol., 1997, 65, 403–409 CAS.
- M. Schaffer, P. M. Schaffer, L. Corti, M. Gardiman, G. Sotti, A. Hofstetter, G. Jori and E. Duhmke, Photofrin as a specific radiosensitizing agent for tumors: studies in comparison to other porphyrins, in an experimental in vivo model, J. Photochem. Photobiol., B, 2002, 66, 157–164 CrossRef CAS.
- C. Ometto, C. Fabris, C. Milanesi, G. Jori, M. J. Cook and D. A. Russell, Tumour-localizing and -photosensitizing properties of a novel zinc(II)-octadecyl-phthalocyanine, Br. J. Cancer, 1996, 74, 1891–1899 CAS.
- C. Fabris, C. Ometto, C. Milanesi, G. Jori, M. J. Cook and D. A. Russell, Tumour-localizing and tumour-photosensitizing properties of Zinc(II)-octapentyl-phthalocyanine, J. Photochem. Photobiol., B, 1997, 39, 279–284 CrossRef CAS.
- M. Prado Figueroa, A. Vidal and F. J. Barrantes, Ultrastructure of Psammobatis extenta (Rajidae) electrocytes and cytochemical localization of acetylcholinesterase, acetylcholine receptor and F-actin, Biocell, 1995, 19, 113–123 Search PubMed.
- A. Vidal, M. Prado Figueroa, M. E. Eberwein, E. Kreda and F. J. Barrantes, Co-distribution of tropomyosin and α-actinin with actin in Psammobatis extenta electrocytes brings out their similarity with muscle fiber cytoplasm, Comp. Biochem. Physiol., A, 1997, 116, 113–118 CrossRef CAS.
- D. J. Ball, S. Mayhew, S. R. Wood, J. Griffiths, D. I. Vernon and S. B. Brown, A comparative study of the cellular uptake and photodynamic efficacy of three novel zinc phthalocyanines of differing charge, Photochem. Photobiol., 1999, 69, 390–396 CrossRef CAS.
- Y. Okada and E. Maeno, Apoptosis, cell volume regulation and volume-regulatory chloride channels, Comp. Biochem. Physiol., A, 2001, 130, 377–383 Search PubMed.
- L. F. Barros, T. Hermosilla and J. Castro, Necrotic volume increase and the early physiology of necrosis, Comp. Biochem. Physiol., A, 2001, 130, 401–409 Search PubMed.
- M. White and C. Miller, Probes of the conduction process of a voltage-gated Cl− channel from Torpedo electroplax, J. Gen. Physiol., 1981, 78, 1–18 Search PubMed.
- E. M. Schwiebert, L. Cid-Soto, D. Stafford, M. Carter, C. Blaisdell, P. Zeitlin, W. Guggino and G. Cutting, Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 3879–3884 CrossRef CAS.
- Y. Shimizu, M. Miya, A. Nagata, K. Ohta, A. Matsumura, I. Yamamoto and S. Kusabayashi, Mesomorphic phase transitions of 5,10,15,20-tetrakis(4-n-dodecylphenyl)porphyrin, Chem. Lett., 1991, 25–28 CAS.
- Y. Shimizu, M. Miya, A. Nagata, K. Ohta, I. Yamamoto and S. Kusabayashi, Mesomorphic phase transitions of tetraphenylporphyrins with four long aliphatic chains, Liq. Cryst., 1993, 14, 795–805 CAS.
Footnotes |
| † The tetraphenylporphyrin derivative 1 was synthesized following the literature procedure.21,22 The purity was monitored by 1H-NMR spectroscopy and elemental analysis. For 1, λmax(CHCl3) = 418 nm. This compound has already been studied in regard to its thermal properties. It was found to exhibit two discotic lamellar phases. The phases change and the transition temperatures are: C 31 °C DL 52 °C DL′ 155 °C isotropic.21,22 |
| ‡ Cryostat sections of about 10 µm were incubated for 3 min with a 1.2 × 10−4 M solution of 1 in chloroform. Sections were fixed for 5 min at 4 °C in a mixture of 3.7% formaldehyde and 0.5% glutaraldehyde in 0.05 M phosphate buffer, pH 7.4. In another experiment, sections were treated for 1 min with 1 diluted in CHCl3 (1 µg in 10 µL) and then in 90 µL of a mixture of 70% ethanol and 3 mM imidazole buffer, pH 7.2, yielding a final concentration of 1 of 1.2 × 10−5 M. Treatment of sections in xylene was exactly the same, but the concentration of 1 was 5 times higher. After fixation, sections were washed in PBS or imidazole buffer for 5 min, mounted with Citifluor and observed with a Nikon Optiphot epifluorescence microscope equipped with filter G and a 580W supplementary filter. Photomicrographs were taken using a Nikon camera and Ilford HP 35 400 ASA film. As a negative control, similar sections were treated as described above, but omitting 1. They did not exhibit any fluorescence. For EDAX-SEM analysis, electric organ segments of 1 × 2 mm were treated with 1 under similar conditions as described for the fluorescence microscopy samples. After evaporation of solvents, samples were metallized with gold (200 Å) in a Ted Pella Pelco 3 sputter coater and oriented for observation with JEOL 35 or Philips 500 scanning electron microscopes equipped with an EDAX Si(Li) energy-dispersive X-ray detector. Microanalysis was carried out at the non-innervated face of the electrocytes. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2004 |
