Chemical mediation of interactions among marine organisms
Valerie J.
Paul
a and
Melany P.
Puglisi
b
aSmithsonian Marine Station at Fort Pierce, 701 Seaway Drive, Fort Pierce, FL 34949
bSchool of Pharmacy, Lake Erie College of Osteopathic Medicine, 1858 West Grandview Blvd., Erie, PA 16509-1025
First published on 22nd January 2004
Abstract
Covering: the literature up to 2003
This review covers the recent marine chemical ecology literature for macroalgae, sponges, octocorals and other benthic invertebrates; 332 references are cited.
 Valerie J. Paul | Valerie J. Paul is currently Head Scientist at the Smithsonian Marine Station at Fort Pierce, Florida. She received her B.A. from the University of California, San Diego in 1979 with majors in Biology and Studies in Chemical Ecology and her Ph.D. in Marine Biology in 1985 from the University of California, San Diego, Scripps Institution of Oceanography. Dr Paul joined the faculty of the University of Guam Marine Laboratory in 1985, served as Director of the Laboratory from 1991-1994, and as full Professor from 1993-2002. Valerie’s research interests include marine chemical ecology, marine plant-herbivore interactions, coral reef ecology, and marine natural products. She was elected a Fellow of the American Association for the Advancement of Science in 1996 and was elected and served as chairperson of the Marine Natural Products Gordon Research Conference in 2000 (vice-chair in 1998). She currently serves on the editorial boards of the journals Coral Reefs and Journal of Natural Products. She is the author or co-author of over 140 research papers and review articles. |
 Melany P. Puglisi | Melany P. Puglisi is currently an assistant professor of natural products chemistry and medicinal chemistry in the Pharmacy School at Lake Erie College of Osteopathic Medicine (LECOM), Erie, PA. She received her B.S. from Southampton College, Long Island University in 1991 in Chemistry, her M.S. from the University of Guam Marine Laboratory in Biology in 1995, and her Ph.D. in Pharmacognosy specializing in the field of Marine Chemical Ecology in 2001 from the University of Mississippi, School of Pharmacy. Dr Puglisi recently joined the faculty of LECOM in 2002 after completing her post-doctoral research in marine chemical ecology at the University of California at San Diego, Scripps Institution of Oceanography. Melany’s post-doctoral research focused on marine microbial chemical defenses of macroalgae. Her current research interests include marine microbial chemical ecology and marine natural products. |
1 Introduction
In this report, we review recent progress in the field of marine chemical ecology emphasizing the defensive functions of marine natural products (secondary metabolites) against predators, competitors, fouling organisms and microorganisms. Research in this field has advanced significantly since the early studies in the 1980's. Numerous reviews and two books have addressed marine chemical ecology over the past 20 years.1–9 A major focus in marine chemical ecology has been on how chemical defenses of macroalgae and invertebrates mediate predator–prey and competitive interactions. This field developed rapidly as natural product chemists and marine ecologists entered into productive collaborations to address questions of how invertebrates and algae use natural products for chemical defense against consumers. Significant contributions in this field were made by several groups of chemical and biological collaborators. Among these was a collaboration between members of John Faulkner's group and ecologist Janice Thompson. Faulkner, Thompson and coworkers conducted some of the first ecologically sound studies of sponge chemical defenses looking at the natural products chemistry and ecology of marine sponges in Southern California.10–13 Faulkner's interest in sponge chemical ecology was long-standing resulting in recent work concerning the potential origin of sponge metabolites from cyanobacteria and symbiotic microorganisms.14–16 Late in his career, John Faulkner established another collaboration with Margo Haygood to investigate symbiotic bacteria in macroorganisms as sources of bioactive metabolites.17–20Another focus of the Faulkner group was the chemical defense of marine molluscs, specifically, chemical studies of sea hares, nudibranchs, and pulmonate molluscs. Molluscs are among the best studied groups of marine invertebrates in terms of chemical defense. Excellent reviews have covered the chemical ecology of marine molluscs;21–25 therefore, we will only discuss this subject in the introduction. Faulkner22 comprehensively reviewed the chemical ecology of marine molluscs in 1992, Pawlik26 covered the topic in his 1993 review on invertebrate chemical defenses, and Avila23 reviewed natural products and chemical defenses of opisthobranch molluscs in 1995. The feeding ecology27 and chemical defenses28 of sacoglossans have been recently reviewed as have the chemical defenses of sea hares.29
Other important contributors to the early studies of chemical ecology of marine organisms were John Coll and Paul Sammarco who studied the ecological roles of soft corals (alcyonaceans) on the Great Barrier Reef, Australia,30–31 and William Fenical with phycological collaborators James Norris, Mark Littler and their graduate students including Mark Hay, who studied seaweed chemical defense with an emphasis on tropical algae.32–33 In the mid to late 1980's, Fenical explored the chemical defenses of Caribbean gorgonian corals collaborating with ecologists Drew Harvell34–35 and Joseph Pawlik.26
Chemical ecology has developed into a broad research area encompassing studies of the chemical mediation of a variety of ecological interactions among organisms.36 Marine chemical ecology includes studies of the biochemistry of marine plant–animal and animal–animal interactions, mate recognition, reproductive cues, and settlement cues.2,24,37–38 This field also includes research into the chemical recognition of prey items and chemotaxis (directed movement oriented by chemical gradients). Excellent recent reviews have covered these areas of marine chemical ecology; therefore we will only address them briefly in the introduction.
Chemotactic behaviors of organisms ranging in size from bacteria and plankton39 to large mobile predators40 are usually mediated by nutrients and other primary metabolites.39,41 Primary metabolites also mediate behavioral and physiological processes of larval settlement and metamorphosis.8,42 Biochemical investigations of cues for settlement and metamorphosis of marine invertebrate larvae have demonstrated chemical specificity for some marine larvae.38,42 Studies have also addressed the chemical nature and functions of sex pheromones43–45 and cues for synchronization of spawning and timing of release of larvae.24 While these metabolites are often released into seawater at concentrations below what is necessary for chemical characterization, experiments have demonstrated that their presence in seawater is essential for spawning and reproduction.
The distribution and abundance of mycosporine-like amino acids, considered to be produced as sunscreens in a variety of marine organisms, have received increasing attention in the literature.7,46–47 These and other UV-absorbing molecules such as scytonemin,48–49 and possibly even brown algal phlorotannins50 illustrate the variety of roles that natural products play in marine organisms.
Several areas of marine chemical ecology are poised for rapid growth as we develop better methods and tools for the study of compounds present in small quantities such as in planktonic organisms or marine invertebrate larvae.51 Some marine invertebrate larvae employ chemical defenses against predators,6,52 but the chemical identities of the deterrent compounds have rarely been elucidated. Toxic bloom-forming microalgae are known to have harmful effects on aquatic organisms53 as well as humans.54 The biosynthesis, molecular pharmacology and mechanisms of toxicity have been extensively studied.54,55 Recently, Landsberg53 reviewed the effects of harmful microalgal blooms on aquatic organisms, and Hay and Kubanek56 discussed the community and ecosystem level consequences of these toxins. Yet few researchers have directly examined the functions and fate of microalgal toxins in ecological interactions in the plankton, and the natural functions of these compounds as predator deterrents or in other roles have rarely been demonstrated experimentally.53,57–58
It is our goal in this review to summarize recent developments in the field and focus on areas that have not been covered in other recent reviews, including the chemical ecology of benthic marine invertebrates26 and the emerging field of marine microbial chemical ecology.5 In addition, we will highlight the important contributions made by Professor D. John Faulkner and coworkers to the field of marine chemical ecology.
2 Macroalgae
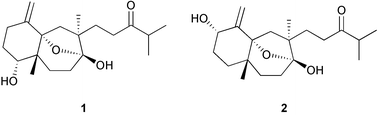
Chemical defenses of marine macroalgae (commonly called seaweeds) have been studied extensively during the past 20 years. Seaweed chemical ecology includes studies of the macroscopic, multicellular, marine, green, brown, and red algae; also included in this topic are the benthic, filamentous cyanobacteria (blue-green algae). Seaweeds ranging from the polar waters of the Antarctic25 to the tropics1,59 are known to produce natural products that function as chemical defenses. Several excellent reviews have been published over the years on this topic.1–3,60–61Recently, Paul, Cruz-Rivera, and Thacker59 discussed ecological and evolutionary perspectives of chemical defenses of macroalgae and benthic cyanobacteria toward herbivores. They reviewed the types of natural products found in the various groups of algae, the diversity, ecology and biogeography of herbivores, and the way algal natural products mediate interactions between herbivores and their algal prey. Most research on seaweed–herbivore interactions has focused on seaweed traits that affect how herbivores choose among different species of algae. The best understood ecological function of secondary metabolites in seaweeds is their ability to deter feeding by herbivores. Many examples of the feeding-deterrent effects of algal natural products have been published,59 although we still know relatively little about specific compounds involved in defense for many species of algae and cyanobacteria. Usually the presence or absence of deterrent secondary metabolites in seaweeds correlates well with the susceptibility of seaweeds toward generalist herbivores. Seaweeds that are least susceptible to grazing often employ chemical and structural defenses.62–64 The types of experiments used to measure feeding cues (either stimulants or deterrents) involve offering consumers extracts or purified metabolites at natural concentrations in otherwise palatable foods and measuring consumption relative to untreated controls.65 A recent example demonstrates the feeding deterrent effect of a mixture of isolinearol 1 and linearol 2 from Dictyota cervicornis toward the herbivorous gastropod Astraea latispina.66 Feeding assays can be used for bioassay-guided isolation of active compounds in a manner similar to studies of the pharmacological activities (cytotoxicity, antimicrobial activity, etc.) of natural products. For the results of these assays to be valid, it is important that the nutritional quality of the test food mimic that of the organisms from which the extract was obtained, because compounds that deter feeding in lower quality foods may be ineffective if placed in higher quality foods.67–70
There is considerable variation in the responses of different types of herbivores to different compounds, even closely related ones. Compounds that differ slightly in chemical structure can vary greatly in their deterrent effects.1,61 These results reinforce the importance of field and laboratory testing against natural herbivores to assess the activities of individual compounds.65 Moreover, different species of herbivores can respond differently to the same compounds.1,71,72 For example, cyanobacterial extracts and compounds that effectively deter generalist herbivores73,74 can stimulate or deter feeding by the specialist sea hare Stylocheilus longicauda depending on their concentration.75,76 Herbivore preference or tolerance for different seaweeds and their secondary metabolites can vary even among populations,77 and recent studies show that genetic variation can explain feeding preferences of different populations of a herbivorous amphipod Ampithoelongimana.77–78
Research emphasis has shifted from the isolation and testing of specific algal metabolites to demonstrate that they function as defensive agents to studies of spatial and temporal patterns in the production of algal defensive compounds. Studies of intraspecific variation, inducible defenses, and changes in metabolite production and allocation in response to abiotic factors (light, UV radiation, nutrients, desiccation) have given us insight into the natural adaptive functions of algal natural products.2,59,79 Van Alstyne, Dethier, and Duggins80 recently reviewed spatial patterns in macroalgal chemical defenses, including intraspecific patterns and biogeographical patterns among species. They also discussed the effects of environmental factors such as nutrients and light on the production and allocation of chemical defenses.
Intraspecific chemical variation occurs at a number of levels including within and among individuals and among populations within a single species. Within an individual algal thallus, defensive compounds have been shown to occur at the highest concentrations on the algal surface,81 in reproductive structures,82–84 in new growth (usually the apical tips),85–88 or, in the case of the brown alga Dictyota ciliolata, in older parts of the alga.89 Chemical variation also occurs among individuals within a population. This has been observed for Halimeda spp.85,90 and the red alga Portieria hornemannii.91,92 Some of this variation may be related to the age of individual thalli.86,90 Attempts have been made to explain the patterns of allocation of chemical defenses in relation to models developed by researchers studying the chemical ecology of terrestrial plants. Cronin79 recently discussed the marine chemical ecology literature for seaweeds and marine invertebrates with respect to resource allocation to chemical defenses and chemical defense patterns in relation to defense theory. Models proposed to describe intraspecific patterns of secondary metabolite allocation include the optimal defense theory, the growth differentiation balance hypothesis, the carbon-nutrient balance hypothesis, and the environmental stress theory.
Optimal Defense Theory (ODT) proposes that chemical defenses are costly, and plants allocate secondary metabolites to parts of the plant that are most valuable to the plant or most susceptible to herbivores.93,94 Because this theory encompasses both evolutionary and ecological time scales it can explain intraspecific, interspecific, and biogeographical variation in chemical defenses. Examples where seaweeds preferentially defend younger or more vulnerable portions of the thallus,85–87,95–96 reproductive structures,82–84 or the more exposed portions of the plant, such as the upright fronds of Caulerpa spp., which contain high concentrations of caulerpenyne,97–98 support the ODT. Pavia et al.99 used demographic elasticity analyses to determine the fitness value of different parts of the perennial brown seaweed Ascophyllum nodosum and then compared the susceptibility to herbivory and concentration of phlorotannins of the different plant parts. Their predictions of fitness values fit well with the distribution of phlorotannins and results of feeding preference experiments. Reproductive tissues of A. nodosum had the lowest fitness values and the lowest levels of phlorotannins99 in support of ODT.
The growth-differentiation balance hypothesis (GDBH) proposes that acquired resources are allocated between growth processes and differentiation (including cellular specialization and production of defensive chemicals).100 The GDBH predicts that actively growing parts of the thallus should be less defended than older, differentiated parts, thus predicting an opposite pattern from ODT. The predictions of the GDBH seem to hold for some brown algae such as Dictyota ciliolata and Zonaria angustata that allocate terpenes and phlorotannins, respectively, to older regions of their thalli.89,101 In contrast, many of the larger kelps have higher levels of phlorotannins in actively growing parts (intercalary meristems), holdfasts, and stipes than in infertile, nongrowing tissue.82,95,102 Cronin and Hay89 and Cronin79 offer an explanation for these differing patterns in seaweeds. Kelps and coenocytic green algae have more developed translocation systems than most other macroalgae,103 which might mean that the elevated concentrations of secondary metabolites found in growing tissues were produced in differentiated tissues and transported there. There is presently no direct evidence to support this hypothesis, since it is only known in a few cases where natural products are biosynthesized within algal thalli.81,101,104
The carbon-nutrient balance hypothesis (CNBH), proposed by Bryant et al.105 to explain how resource availability affects the phenotypic expression of chemical defenses, suggests that the allocation of resources to chemical defenses will change as environmental conditions such as light or nutrient availability change. For example, when nutrient levels are low and restrict growth, increases in light levels result in excess carbon that can be used for production of carbon-based secondary metabolites. The predictions of the model depend on the relative availability of carbon and nitrogen to the plants. The model fits observations on phlorotannin production in brown algae in response to changing nutrient and light levels reasonably well,106 with polyphenolics present in higher concentrations under nitrogen deficiency,80,107–111 but not in all cases.112–113 Exposure to sunlight had a positive effect on phlorotannin content in natural populations of two brown algae, Ascophyllumnodosum and Fucus vesiculosus, as predicted by the CNBH. In a manipulative experiment conducted in outdoor aquaria, only F. vesiculosus responded to changes in light intensity.113 The CNBH does not appear to have predictive value for other types of seaweed compounds such as terpenes.91,107 A recent review114 shows that the CNBH fails to predict outcomes of nutrient interactions with terrestrial plant chemical defenses so often that it can no longer be considered a useful predictive tool. The authors argue that many of the fundamental assumptions of the CNBH are not true based on what we now know about plant physiology and the production of secondary metabolites. They propose that ODT, which is based on evolution and adaptation, is a more robust model.114
Environmental Stress Theory (EST) suggests that environmental stresses, which may either reduce growth due to inadequate nutrient supply or cause damage due to adverse conditions such as desiccation or UV exposure, will affect predator–prey interactions. Environmental stress often results in increased palatability to consumers, which may be due to either increases in nutritive value or decreases in defenses in the affected plants.79,93,115 The impact of abiotic stressors on marine algae has rarely been investigated, but two studies demonstrated that algae became more susceptible to consumers after desiccation.116–117 Cronin and Hay117 traced the changes in palatability of Dictyota ciliolata to lower levels of dictyol metabolites in desiccated algae. UV-exposed algae also had lower levels of dictyols compared to individuals protected from UV, but the amphipod Ampithoe longimana did not distinguish between these treatments.117 These results contrast with those from a study exposing Ascophyllum nodosum to UV radiation in an experiment designed to determine the effects of UV-B radiation and simulated herbivory on phlorotannin production.50 Phlorotannin concentrations increased in algae exposed to UV radiation.50,112 The authors suggested that phlorotannins function as inducible screens against harmful UV radiation because phlorotannins absorb UV-B radiation. UV-exposed algae were still more susceptible to grazing by the isopod Idotea granulosa, despite their increased phlorotannin levels, than the controls.50
Variation in types and concentrations of secondary metabolites also occurs among populations of seaweeds growing in different habitats. In most cases we do not know if variation in concentrations and types of secondary metabolites results from herbivore-induced chemical defenses, localized selection resulting in high levels of defense, genetic differences or other factors not related to herbivory. Populations of Halimeda from habitats where herbivory is intense tend to contain higher levels of the more potent deterrent halimedatrial than do populations from areas of low herbivory.85 Other green algae such as Penicillus, Udotea, Rhipocephalus, and Caulerpa also often produce higher concentrations or different types of secondary metabolites in populations from herbivore-rich reef habitats than in populations from herbivore-poor areas such as reef flats or seagrass beds.118–119 Shallow and deep water populations of the brown alga Stypopodium zonale produced different secondary metabolites.120 The red alga Portieria hornemanni is well known to vary in its composition of halogenated monoterpenes among different collection sites in the tropical Pacific;91,121 this variation does not appear to be environmentally mediated.92 Different chemotypes of Laurencia nipponica were shown to be genetically distinct based on results of intra- and interpopulational crosses between female and male gametophytes in laboratory culture studies.122
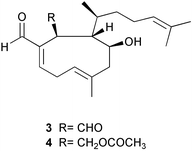
In a study of biogeographical variation in seaweed chemical defenses, Bolser and Hay123 discovered that the sea urchin Arbacia punctulata discriminated as strongly between two North Carolina populations of the brown alga Dictyota menstrualis as it did between temperate and tropical collections of different species of Dictyota. D. menstrualis plants collected from an inshore jetty were preferred over those from an offshore reef. Recently, Taylor et al.124 showed that the herbivorous amphipod Ampithoe longimana consistently preferred plants from inshore populations relative to offshore populations for three species of brown algae, Dictyota menstrualis, Spatoglossumschroederi, and Sargassum filipendula. Additional studies were conducted with D. menstrualis to determine what traits were responsible for these differences in susceptibility to amphipods. Crude nutritional properties such as organic and protein content were not good predictors of these differences in palatability. Bioassay-guided fractionation of lipid soluble crude extracts of D. menstrualis from the two sites indicated that differences in palatability were due to differences in the dictyol terpenes present in the two populations. Offshore algae contained higher concentrations of 4β-hydroxydictyodial A 3 and 18,O-dihydro-4β-hydroxydictyodial A 4 and other minor terpenes that were not identified than inshore algae. Amphipods (and grazing scars) were significantly more abundant on plants at one inshore site than at any other inshore or offshore site, suggesting that the inshore-offshore pattern was not due to herbivore-induced defenses. The investigators also showed that this variance had dramatic effects on herbivore fitness by raising juvenile amphipods on inshore versus offshore plant material. Amphipod survivorship, growth and ovulation were significantly reduced on the offshore compared to the inshore algal material.
Variation in production of polyphenolics by brown algae has received the most attention of any group of algal secondary metabolites.106,125,126 This is largely because polyphenolics, as a class of compounds, can be assayed by colorimetric techniques such as the Folin–Denis or Folin–Ciocalteu assays126–128 which quickly yield information about the intraspecific and interspecific patterns of variation of this group of compounds (including monomeric and polymeric phenols). Unfortunately, colorimetric assays do not provide information regarding the concentration of individual compounds or, at the least, specific data regarding changes in relative concentrations of compounds in the different size classes of polymeric phenols. Polyphenolics have been found to vary within individual thalli depending on tissue type,82,95,101,102,129–131 among size classes of algae,132–134 among individual thalli within and between populations,134–136 and seasonally.137 Several reviews have addressed chemical defenses of brown algal phlorotannins.4,80,106,126
Recent studies continue to increase our understanding of intraspecific variation in polyphenolics in brown algae. In a study of brown algae from the Pacific Northwest, four common invertebrate herbivores were offered juvenile and adult algae in feeding experiments; the susceptibility of these algae to herbivores depended on both the algal stage and species of herbivore.138 In a follow-up study, Van Alstyne et al.133 investigated whether differences in susceptibility of juveniles and adults to herbivores could result from chemical, morphological, or nutritional differences. They examined preferences of four common herbivores for juvenile and adult tissues of eight common brown algae. Paired juvenile and adult tissues were offered in laboratory assays. Juvenile algae were not highly preferred by herbivores, and phlorotannin concentrations were higher in juveniles of four algal species and lower in juveniles of only one species. Nitrogen levels were similar in juveniles and adults of three species and higher in juveniles of two species. Preferences between juvenile and adult algae appear to be determined by a combination of chemical and morphological traits and the different responses of various consumers to these algal traits.
Toth and Pavia139 tested whether habitat choice and food choice on the kelp Laminaria hyperborea were the same for two gastropods, the patellid limpet Ansates pellucida and the littorinid snail Lacuna vincta. L. vincta preferred new fronds of L. hyperborea as both habitat and food. A. pellucida preferred to reside on old fronds but did not differentiate between new and old fronds as food. There was no overall difference in tissue nitrogen or phlorotannin content between new and old fronds of the kelp, so these chemical factors did not explain food and habitat choices for these herbivores.
Other recent studies have examined nutrient enrichment and its effects on growth and phlorotannin production by brown algae. Pfister and Van Alstyne140 fertilized the intertidal kelp Hedophyllum sessile with both nitrogen and phosphorus in field experiments. They used Osmocote timed-release fertilizer to enrich the water column at the bases of newly recruited individuals for approximately 2 months during the summer growing season. Nutrient enrichment did not increase frond growth, tissue carbon and nitrogen were unaffected, and the concentrations of polyphenolics were unchanged by the nutrient enrichment. The results suggested that this intertidal kelp is not limited by nitrogen or phosphorus in its wave-washed environment. Van Alstyne and Pelletreau141 grew Fucus gardneri embryos in cultures enriched in either nitrate, phosphorus or iron. Nitrogen enrichment significantly enhanced growth rates and reduced phlorotannin concentrations. Iron enrichment alone had no effect on phlorotannin concentrations, but affected the shape rather than the overall size of embryos, resulting in embryos that were shorter and wider than embryos grown without iron. Phosphorus enrichment had no effect on growth but significantly lowered phlorotannin concentrations. Enrichment with phosphorus and iron had a strong negative effect on phlorotannin concentrations, and Van Alstyne and Pelletreau141 suggested that the interaction of these two nutrients may have caused physiological stress in the embryos resulting in lower production of secondary metabolites.
Herbivore-induced defenses142 are another important factor influencing spatial variation in algal chemical defenses. Van Alstyne et al.80 reviewed what was known about inducible defenses in brown algae as well as examples where herbivore-induced defenses did not occur after natural or artificial grazing. Simulated herbivory (mechanical wounding) has been shown to induce phlorotannin production in some studies109,111,130,143–145 but not in others.50,146 Phlorotannin induction can occur rapidly, within 1–3 days of wounding.111,145 Pavia and Toth147 found that a few weeks of grazing by the periwinkle snail Littorina obtusata could induce the production of phlorotannins in Ascophyllum nodosum, but grazing by the isopod Idotea granulosa and simulated herbivory caused no significant changes in phlorotannin levels. They proposed that patterns of grazing by L. obtusata, which lives and feeds on a few species of fucoid algae, could be an important factor in explaining natural variation in the levels of phlorotannins in A. nodosum.147 Induction by some herbivores and not others suggests that herbivore specific cues are involved. Toth and Pavia148 tested whether waterborne chemical cues might be important in induction of phlorotannins in A nodosum. They found that waterborne cues from actively feeding periwinkles, L. obtusata, could induce phlorotannin production in unharmed individuals of the seaweed, which reduced the palatability of the seaweed to subsequent grazing by the herbivore. Waterborne cues from amphipod grazing did not induce resistance to herbivores, but direct grazing did, in a study with the brown alga Sargassum filipendula.149 In another study by Toth and Pavia,131 phlorotannin production in the kelp Laminaria hyperborea was not induced as a result of artificial damage or grazing by the patellid limpet Ansatespellucida or the littorinid snail Lacuna vincta. Instead, these herbivores decreased the phlorotannin content of grazed kelps relative to ungrazed controls. Arnold et al.150 showed that a single brief exposure to airborne methyl jasmonate resulted in an increase in polyphenolic content of apical tissues of Fucusvesiculosus after 12 days. The induced response was similar to induced responses to real or simulated herbivory in this alga.
Arnold and Targett151 recently suggested that ecological theories that assume a trade-off between growth and defense may not apply to phlorotannins because these compounds can have both primary and secondary functions in brown algae. Phlorotannins are known to have primary functions including serving as components of brown algal cell walls, precursors to holdfast adhesive, and possibly in brown algal reproduction in addition to secondary functions such as herbivore deterrents, antimicrobial agents, and UV screens. Arnold and Targett151 propose that the extractable phlorotannins sequestered in physodes serve as secondary metabolites and later as primary metabolites when they fuse with cell membranes and become relatively unreactive cell wall components. They have also shown that metabolic turnover of polyphenolics in tropical brown algae is relatively rapid under both laboratory and field conditions152 suggesting that synthesis and metabolism of these compounds may occur at rates higher than predicted. Their model suggests that phlorotannin production and accumulation may not be driven by secondary functions but rather by the need for the compounds in cell wall construction. This proposal may explain apparent trade-offs between phlorotannin accumulation and growth and the induction of phlorotannin production after simulated or natural grazing without the need to use growth/defense trade-offs as predictors of metabolite cost.
The majority of studies of brown algal defenses and phlorotannin concentrations have been correlative.4,126 The effectiveness of phlorotannins from the brown alga Fucus vesiculosus as defenses against grazing by sea urchins has recently been questioned.153 In a series of feeding assays, researchers found that galactolipids rather than phlorotannins deterred feeding by the sea urchin Arabaciapunctulata. Uncharacterized non-phenolic compounds in the aqueous extract also had deterrent effects.153 The role of phlorotannins in herbivore defense has been previously discussed and both the characteristics of the phlorotannins (including molecular weight of polymers) and characteristics of the herbivore (gut pH, digestive mechanisms) can be important in explaining herbivores' responses to these compounds.126
There has been surprisingly little evidence showing that inducible defenses occur in seaweeds other than brown algae. In addition to the examples of phlorotannin induction, two other examples of inducible chemical defenses have been reported in brown algae. Dictyota menstrualis increased levels of dictyol terpenes in response to grazing by the amphipod Ampithoe longimana, which made the seaweed less susceptible to further grazing by the amphipod.154 Among-site differences in amphipod densities, grazing scars, seaweed defensive chemistry and palatability were clearly documented in one year, but patterns were less clear in two subsequent years, illustrating the variability and complexity among patterns of grazing and algal response.154 Constitutive and inducible defenses were studied in the brown seaweed Sargassum filipendula as defenses toward amphipod grazing.149,155 Different parts of S. filipendula thalli varied widely in palatability to grazing by Ampithoe longimana, with top blades of the seaweed more palatable than older portions at the base of the seaweed. The bottom stipes, which anchor the seaweed to the sea floor, were defended constitutively by their toughness and not secondary metabolites. The top stipes became more resistant after amphipod grazing,149 and this induced defense was not due to toughness or other structural properties, but seemed to be due to increased chemical defenses based on tests with crude lipophilic extracts of different parts of the thallus.155 Compounds responsible for this induced resistance were not identified. The investigators suggested that the compounds responsible for this induced resistance were probably not phlorotannins because phlorotannin concentrations are very low in S. filipendula and levels of phlorotannins do not relate to feeding by this herbivore.106,155
Induced defenses in macroalgae usually occur over a period of days145 to weeks144,155 following damage. The production of activated defenses (wound-induced biotransformation)156 is an alternative way that herbivores can influence the potency of chemical defenses in algae. Activation occurs within seconds of injury and appears to be an enzymatically mediated transformation whereby precursor compounds are converted to more deterrent ones. Only the portion of the alga in the immediate vicinity of the injury is affected. These conversions occur after any mechanical injury and could occur when a fish bites or chews on algae156 or even during post-ingestive processes.157 Activation is a common type of chemical defense in some terrestrial plants. Examples include plants that produce HCN from organic precursors,158,159 plants that convert glucosinolates to thiocyanates and isothiocyanates160,161 after tissue damage and the activation of oleuropein, 5 a phenolic secoiridoid glycoside with strong protein denaturing activity.162 In these cases, precursor compounds are compartmentalized and physically separated from the enzymes that activate them; however, we know little about the mechanisms involved in activation in marine plants.
Because the process of activation is so rapid, it may be a common defense mechanism in habitats where herbivory is intense and herbivores are large and mobile. In some coral reef habitats, such as shallow reef slopes, herbivory is intense but highly variable over short periods of time (i.e. when grazing fishes bite seaweeds and swim away) and herbivores such as fishes and urchins are large and can rapidly and completely consume macroalgae. Thus, we might expect activated defenses to be more common in tropical algae.
Cetrulo and Hay163 conducted a survey of activated defenses in seaweeds and also looked for geographic differences in the frequency of activated defenses between tropical and temperate algae. Thin-layer chromatography of organic extracts indicated that damaging the algae before extraction caused detectable chemical changes in 70% of the algae they studied. They tested the extracts of 42 species of activated and non-activated algae in fish and sea urchin feeding assays and found that extracts of activated algae of 17% of tropical and temperate species were more deterrent than extracts of non-activated algae. Extracts of four species were more palatable after they were damaged. There was no greater incidence of activated defenses in tropical than in temperate algae.163 Unfortunately, many activated defenses are unstable compounds that are not amenable to testing in feeding assays, so this type of study may underestimate the frequency of activated defenses.
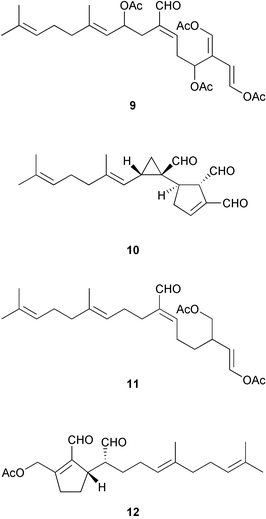
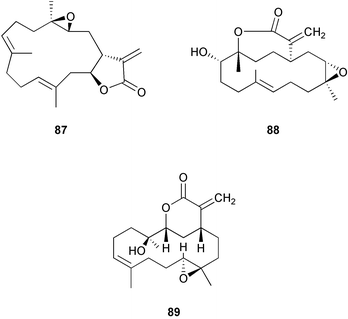
Wound-activated transformation of caulerpenyne 6 to oxytoxins 1 7 and 2 8 and related acetoxy aldehydes, which results from deacetylation of caulerpenyne, has been described for Mediterranean Caulerpa taxifolia (Scheme 1).164 Activation of caulerpenyne to reactive aldehydes such as the oxytoxins, which are presumably more potent defensive compounds, had been looked for but not observed in Caulerpa prolifera in the Mediterranean.165 Further investigation of this process suggests that, in wounded algae, esterases act on caulerpenyne 6 by removing the three acetate residues to rapidly yield the reactive aldehydes.166 More than 50% of stored caulerpenyne was converted to aldehydes within 1 minute for three species of Caulerpa that occur in the Mediterranean including invasive C. taxifolia and C. racemosa and indigenous C. prolifera.166 A similar wound-activated transformation has been previously reported for green algae of the genus Halimeda.156 In many species of Halimeda, the diterpene bis-enol acetate halimedatetraacetate 9 converts to the aldehyde halimedatrial 10 when algae are damaged. Halimedatrial is a more potent toxin and feeding deterrent than its precursor halimedatetraacetate.156 Similarly, Udotea flabellum shows a wound-activated conversion from udoteal 11 to petiodial 12.167 Activated chemical defenses appear to be common among green seaweeds of the families Caulerpaceae and Udoteaceae.
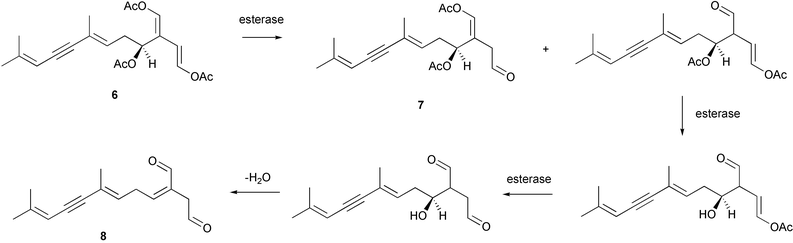 | ||
| Scheme 1 | ||
Another example of an activated defense, which has been recently described for temperate macroalgae, is the biotransformation of dimethylsulfoniopropionate (DMSP) to acrylic acid and dimethylsulfide (DMS) by the enzyme DMSP lyase.157,168 This transformation occurs after damage to the algae and is especially prevalent in many species of green (especially the Ulvophyceae) and red macroalgae. Species of algae containing DMSP tend to be consumed at lower rates by sea urchins than species without it.157 This DMSP-cleavage reaction has also been reported in unicellular phytoplankton and hypothesized to be an activated defense system.169,170 Van Alstyne and coworkers demonstrated that both products of the cleavage reaction, acrylic acid and DMS, functioned as feeding deterrents toward sea urchins, while the precursor DMSP was a feeding attractant.157,168
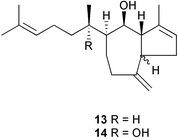
Several obvious gaps in our knowledge of seaweed–herbivore interactions exist. One is lack of direct evidence for the physiological effects of algal chemical defenses toward herbivores (post-ingestive effects). Natural products may act by directly affecting consumer physiological processes or by indirectly reducing overall food consumption, which would reduce nutrient acquisition. Targett and Arnold171 review the effects of secondary metabolites on digestion in marine herbivores. A recent study by Cruz-Rivera and Hay70 examined the separate and interactive effects of prey nutritional quality and chemical defenses on feeding behavior and overall performance of six different crustacean consumers (5 amphipods, 1 isopod). The chemical defenses tested were the dictyol diterpenes, pachydictyol A 13 and dictyol E 14, of Dictyotamenstrualis. Dictyols deterred feeding by all species of amphipods in low or high quality foods, but did not affect feeding by the isopod. Dictyols decreased fitness (survivorship, growth, or reproduction) in only 3 of the 5 amphipod species; the effects were often less pronounced in foods with higher nutritional quality. Low nutritional quality alone negatively influenced fecundity and growth of most species of consumers. Cruz-Rivera and Hay70 stress the importance of studying the interactive effects of chemical defenses and prey nutritional characteristics for understanding food selection by consumers.
Algal chemical defenses may have indirect positive and negative effects on associated species.59 Associational refuges and defenses have been used to describe interactions in which one species gains protection from natural enemies by living close to a deterrent species.172 Most discussions of this topic have focused on sessile organisms living in close proximity to each other; however, motile organisms can also benefit from algal chemical defenses. Small grazers, such as amphipods, polychaetes, crabs, and molluscs, often live and feed on chemically defended plants.24,173 Chemically-rich plants are frequented less often by omnivorous consumers and thus provide a refuge from predation for the small consumers inhabiting them.24,172–173
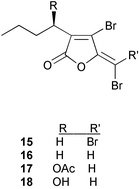
The literature describing interactions between seaweeds and microorganisms, specifically, studies addressing antifouling and chemical defense against potential pathogens, is sparse compared to that of seaweed–herbivore interactions. The late emergence of this field is proposed to be at least partially due to the difficulties associated with experimental design including methods for culturing marine microorganisms and the identification of microorganisms that have a significant impact on the ecology of marine macroorganisms.5 In studies of the red alga Delisea pulchra, researchers developed methods that unequivocally demonstrated that halogenated furanones 15–18 produced by the alga mediate the formation of the bacterial film on the surface.174 This represents one of the best understood examples of secondary metabolite mediation of surface colonization. The mechanism of furanone interference with bacterial colonization has been recently reviewed.8,9 Halogenated furanones, located in cells at the surface of D. pulchra, are similar in structure to the acylated homoserine lactones (AHLs), bacterial signaling molecules, and act at the LuxR homologous receptor protein in Gram-negative bacteria interfering with the binding of AHLs.175 AHLs regulate the swarming and biofilm formation in Gram-negative bacteria, therefore, interference by halogenated furanones prevents colonies forming on the surface of the alga. Recently, this mechanism of bacterial colonization interference of quorum sensing by halogenated furanones has been explored as a potential mechanism to control bacteria that cause human disease.176 A synthetic analog of the D. pulchra furanone metabolites disrupted the quorum sensing system of Pseudoalteromonas aeruginosa PAO1 and inhibited the expression of virulence factors.
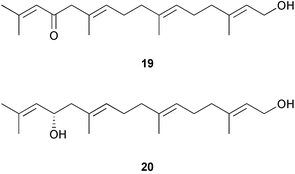
A survey of extracts from twenty brown algae abundant in South Africa, the Atlantic shores of Europe and the Mediterranean Sea against a panel of typical fouling organisms demonstrated that 10 of the extracts, mostly extracts of Bifurcaria bifurcata collected in different regions, exhibited activity against marine bacteria, fungi, or diatoms.177 Eleganolone 19, eleganediol 20, and other related diterpenes subsequently isolated from B. bifurcata exhibited variable activities in the panels suggesting that small modifications to a parent structure can provide a marine organism with defenses against multiple pathogens. In another survey, four red algae were investigated for antifouling activity against the cyprid larvae of Balanus improvisus.178 The extracts from all four species surveyed inhibited the attachment of larvae, however, when the ciprid larvae were offered whole algae to settle upon, the larvae only avoided settling on Chondrus crispus. Walters et al.179 surveyed common Hawaiian macroalgae for their effects on settlement by larvae of two species of marine invertebrates, the polychaete tube worm Hydroides elegans and the bryozoan Bugula neritina. They found that larvae responded both positively and negatively to waterborne cues from 12 species of macroalgae. Waterborne cues from Dictyota sandvicensis were toxic to both species of larvae; cues from Halimeda discoidea, Sphacelaria tribuloides, and Ulvareticulata inhibited settlement.
With the increase of disease outbreak in benthic marine organisms on coral reefs in the past decade,180 the focus of seaweed chemical defense in algae has turned to defense against potential pathogenic marine microorganisms. While this topic was recently reviewed by Engel et al.,5 the significance of the recent advances in this area merit some discussion in this report.
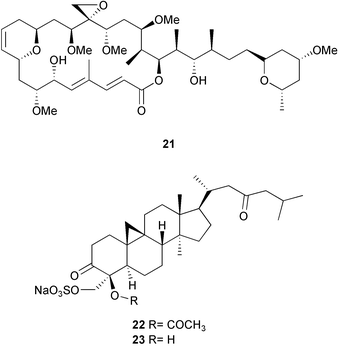
Outbreaks of disease on coral reefs have had devastating effects resulting in the mass mortality of individual populations over short periods of time; however, they appear to be rare occurrences suggesting that marine organisms maintain effective defenses against disease causing microorganisms.5,181,182 Recently studies of seaweed chemical defense using bioassays developed to test naturally occurring concentrations of algal extracts and purified compounds against potentially harmful marine microorganisms have led to the isolation of new, potent antifungal metabolites from the brown alga Lobophora variegata181 and Penicillus capitatus.182 Lobophorolide 21, a macrolide from L. variegata (or associated microorganisms) displayed potent antifungal activities against the marine fungi Dendrophiella salina and Lindra thallasiae. Capisterones A 22 and B 23, triterpene sulfate esters from P. capitatus, displayed selective, potent activity against L. thallasiae. D. salina is a fungal saprophyte that is involved in the decomposition of plant material. L. thallasiae is an opportunistic pathogen attacking weakened or damaged tissues in many species of marine plants. Although field studies have not been conducted to confirm the laboratory results, the authors of these studies propose that lobophorolide 21 and the capisterones may play a role in protecting the algae from fungal infection. Because marine plants are continually exposed to potential pathogens and other harmful microorganisms present in seawater they propose that there may be high selective pressure for plants to produce chemical defenses against infection. Further studies of this nature will undoubtedly yield novel metabolites from marine algae.
It is noteworthy that some natural products that are known to function as feeding deterrents also show antimicrobial and antifouling activities.183–186 Some compounds may function simultaneously as defenses against pathogens, fouling organisms and herbivores, thereby increasing the adaptive value of the compounds.118,184,185,187
3 Sponges
Our knowledge of chemical defenses of marine sponges (Phylum Porifera) is the most extensive of all the marine invertebrates.188 Sessile marine invertebrate chemical defenses were last comprehensively reviewed by Paul1 and Pawlik,26 and a considerable amount of progress has been made in understanding the ecological roles of invertebrate chemical defenses since that time. Of all marine organisms, sponges have yielded the greatest number and diversity of natural products,188–190 and we now know that many of these compounds function in defenses against predators, competitors, and microorganisms.2,5,26First, we would be remiss if we did not mention the important early work by Faulkner and coworkers on this topic, which were some of the first ecologically sound studies of sponge chemical defenses.10–13 Forty different sponges from San Diego, CA, were collected, and extracts were tested for suppression of bacterial and fungal growth. Many of the extracts and some pure compounds were tested for inhibition of settlement and metamorphosis of marine invertebrate larvae, behavioral modifications of adult invertebrates (a hydroid, a bryozoan, a sea star, and keyhole limpet), and feeding deterrence toward several fishes.11 Sponge extracts and metabolites showed a broad range of activities; most had activity in at least one but usually more than one assay. This study illustrated the multiple functions that sponge natural products could play in the environment.
In a recent survey conducted with temperate sponge assemblages, Wright et al.191 found that distribution and abundance of sponges differed in two habitats that had different levels of predation by sea urchins. Extracts of four sponges from each of the two habitats were tested for their effects on feeding by the sea urchin Centrostephanus rodgersii. Three of four sponges from the urchin barrens where urchin predation was greatest had deterrent extracts, while none of the sponges from the kelp bed site with lower urchin densities had deterrent extracts.191 The study demonstrated that sponges with chemical defenses against sea urchins predominated in the high-density urchin habitat, illustrating the importance of predation in influencing the distribution of sponges between habitats.
On tropical reefs, fish predation on sponges by angelfishes, trunk fishes, file fishes, and even parrotfishes can be quite intense with some sponges being rapidly consumed in field experiments in the Florida Keys and Caribbean.192–195 Predation likely plays an important role in structuring sponge communities,191,194–195 which makes defense by chemical or other means very important in reef habitats characterized by high numbers of predators.
Pawlik and coworkers have conducted surveys of the chemical defenses of large numbers of Caribbean sponges from reef, mangrove, and seagrass habitats toward different consumers in laboratory assays. Organic extracts from 71 Caribbean sponges were tested toward the common bluehead wrasse Thalassomabifasciatum.196 The majority of sponge extracts (69%) were deterrent toward this generalist predator, but extracts of some sponges, including some common reef sponges, were highly palatable. There was no relationship between sponge color and deterrency, suggesting that sponges are not aposematic to visual predators such as reef fishes. Some variability in the palatability of extracts was observed for several sponges that were collected at different sites.196 In addition, sponges with palatable extracts did not differ from those with deterrent extracts in nutritional value or structural materials.197 Another survey of extracts from 30 Caribbean sponge species assayed for their effects on feeding by the omnivorous hermit crab Paguristes punticeps, also demonstrated that twenty-six sponges (87%) were chemically defended against the hermit crab.198 A few results differed between the two surveys. Two sponges that were consistently palatable to T. bifasciatum deterred P. punticeps, and several species that were consistently deterrent to T. bifasciatum varied in their ability to deter P. punticeps depending on site of collection.
Feeding deterrent properties of extracts of 16 species of sponges from a variety of reef, mangrove, soft bottom and seagrass habitats in Bermuda toward two omnivorous fishes were examined.199 Six of 16 sponges had at least one extract that deterred feeding by the sergeant major Abudefduf saxatilus while only one of 16 had an extract that deterred feeding by the Bermuda bream Diplodus bermudensis. Deterrent activity was relatively low compared to other surveys;196,198 however, the inclusion of aqueous extracts and use of different species of fish preclude direct comparisons. Nonetheless, some of the same sponges, including Aplysina fistularis, Dysidea etheria, and Ircinia felix, deterred fish feeding in both studies.196,199
In another study, two species of sea stars, Echinaster echinophorus and E. sentus, were offered pieces of 6 mangrove and 5 reef sponges in pairwise assays in laboratory aquaria.200 Both species exhibited similar preferences. Pairwise assays of organic extracts of the same sponges were tested to determine whether chemical defenses of the sponges were responsible for the preferences observed. Of the mangrove sponges, only extracts of Dysidea etheria deterred feeding by E. echinophorus. Three extracts of reef sponges deterred feeding by E. echinophorus confirming the effectiveness of chemical defenses of some Caribbean reef sponges toward invertebrate predators.
In similar surveys, spicules from sponges and spiculated spongin did not deter feeding by consumers such as Thalassomabifasciatum,197,201Paguristes punticeps,198 the sea star Echinaster echinophorus,200 or in field assays with natural assemblages of reef fishes,201 suggesting that sponge spicules play little role in defense against predators. Some recent evidence suggests that these results cannot be extended to all predators. A comparison of structural defenses was conducted by testing spicules from 6 Caribbean and 6 Red Sea sponges toward the Caribbean bluehead wrasse Thalassomabifasciatum and the Red Sea wrasse T. klunzingeri.202T. klunzingeri was deterred by spicules of 4 of 6 Red Sea sponges and 2 of 6 Caribbean sponges, whereas T. bifasciatum was deterred by spicules of only one Red Sea sponge. These results show that different fish species can be affected differently by sponge spicules in their diets. Hill and Hill203 suggested that structural defenses occur in the tropical sponge Anthosigmella varians, but this was not demonstrated experimentally in feeding experiments. Their study showed that spicule concentration was a plastic morphological trait that could be induced by damage (clipping with scissors).
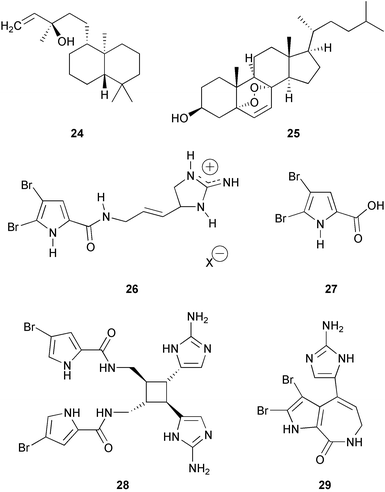
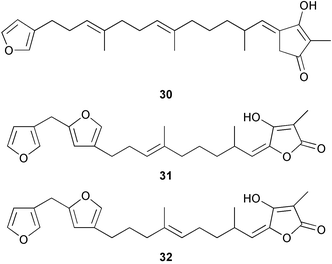
The compounds responsible for chemical defenses in several Caribbean sponges have been isolated. The crude non-polar extract of Aplysilla glacialis, a major diterpene manoöl 24, and cholesterol endoperoxide 25 deterred feeding by natural assemblages of fishes in the Bahamas.204 Sterol endoperoxides were also isolated from the mucus of the sponge, which is secreted in large amounts when the sponge is disturbed.204 Oroidin 26 and 4,5-dibromopyrrol-2-carboxylic acid 27 were identified as the deterrent metabolites in the active fractions of Agelas clathrodes.205 These two major compounds were also present in A. wiedenmayeri but at different relative concentrations.206 Dimeric bromopyrrole alkaloids dominated by sceptrin 28 serve as chemical defenses against reef fish for the related sponge A. conifera.206 Stevensine 29, another compound in the oroidin class of brominated pyrroles, was primarily responsible for chemical defense of the sponge Axinella corrugata.207 Seven other related pyrrole- and imidazole-containing alkaloids as well as synthetic analogs were tested for fish feeding inhibition activity to explore structure–activity relationships of the pyrrole group and the importance of an imidazole group for defense.208 The pyrrole moiety was necessary for feeding deterrent activity, and addition of bromine to the pyrrole group enhanced activity.206 Various functionalized imidazoles were not deterrent by themselves, but compounds containing both functional groups were highly deterrent.208 The brominated pyrrole alkaloids appear to be an important and broadly distributed group of compounds functioning as chemical defenses in sponges in the families Axinellidae and Agelasidae. Many of these brominated pyrroles also inhibited attachment of the marine bacterium Vibrio harveyi in assays designed to examine bacterial colonization.209
Furanosesterterpene tetronic acids are common in sponges of the genus Ircinia. Mixtures of these compounds as well as purified variabilin 30 from Ircinia felix deterred feeding by the wrasse Thalassoma bifasciatum in aquarium assays.210 Natural mixtures of volatiles of I. felix obtained from ground sponge and pure dimethylsulfide were tested in the same type of aquarium assays and had no effect on feeding by T. bifasciatum.210 In similar experiments with pure compounds from three related species of Ircinia, ircinin I 31 and II 32 from I. oros and variabilin 30 from I. spinulosa deterred feeding by T. bifasciatum.211
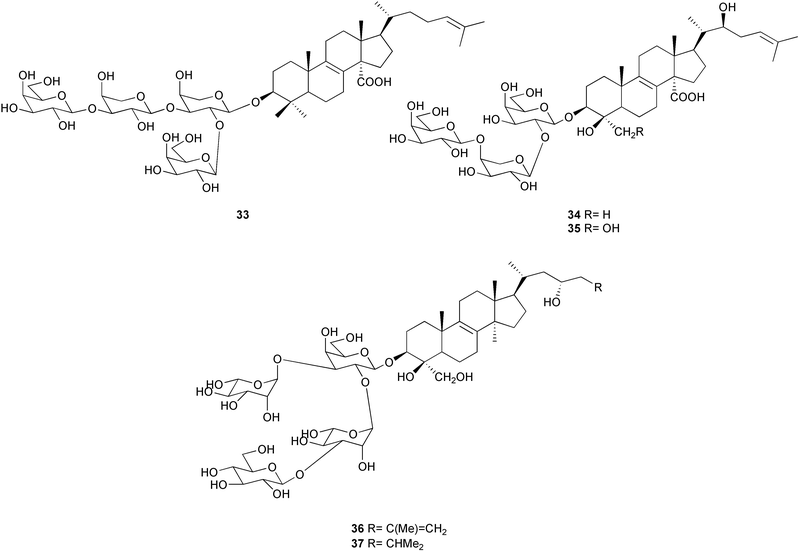
Triterpene glycosides have also been demonstrated to function as chemical defenses in two species of sponge belonging to different orders, Erylus formosus and Ectyoplasia ferox.212–213 Formoside 33 was the major deterrent compound in E. formosus, with other minor triterpene glycosides present. Ectyoplasia ferox contained ectyoplasides A 34 and B 35 and feroxosides A 36 and B 37. Formoside 33, as well as mixtures of triterpene glycosides, deterred feeding by reef fish in aquarium and field assays. The concentration of formoside 33 was lower in the top layer of Erylus formosus than in the inner layer, while concentrations of triterpene glycosides were higher in the top layer of Ectyoplasia ferox than in the inner third layer. The triterpene glycosides inhibited marine bacterial attachment, settlement by fouling organisms, and sponge overgrowth.213 This study adds to the growing list of examples of multiple functions for natural products from marine invertebrates.11,209,214–216
Intraspecific variation in the production of chemical defenses has not been studied as thoroughly for sponges as for seaweeds. Environmentally induced variation has been reported for furanoditerpene composition of the marine sponge Rhopaloeides odorabile on the Great Barrier Reef, Australia.217 Diterpenes were most concentrated on the surface of the sponge. Sponges transplanted to unshaded areas at shallow depths (5 m) contained the highest levels of diterpenes,217 suggesting that illumination influenced diterpene production in this sponge. In contrast, the natural product profile of Aplysina cavernicola did not change following transplantation to shallower, more light-exposed sites relative to sponges from the original location.218
Individual variation in defensive chemistry has been reported for several sponges. Feeding deterrence of extracts of Xestospongia muta varied among collection sites219 but could not be related to the size or sterol composition220 of sponges. The deterrent compounds in X. muta are not known but are moderately polar components of the extracts.219 Sceptrin 28 concentrations were higher in Agelas conifera individuals collected from the Southern Bahamas Islands relative to individuals from the middle Bahamas.206 Oroidin 26 concentrations varied by an order of magnitude among individuals of Agelas wiedenmayeri collected in the Florida Keys and Bahamas.206 Stevensine 29 also varied considerably among individuals of Axinella corrugata collected at different sites in the Florida Keys and Bahamas.207 The reasons for this variation among individuals collected at different sites have not been explored.
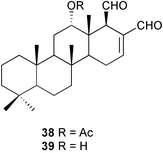
While knowledge of intraspecimen variation of secondary metabolites may increase our understanding of predator–prey relationships, such variation has been studied for only a few sponges. The tropical Pacific sponge Cacospongia sp. showed variation in levels of secondary metabolites and structural materials (fiber and ash content) in different parts of the sponge.221 Structural material was highest at the base of the sponge. Filtered extract and desacetylscalaradial 38 were highest in the tips of the branches. Scalaradial 39 also tended to be higher in tips of the sponge, but concentrations were highly variable. Cacospongia sp. extracts deterred feeding by reef fishes at the lowest concentration found in the sponge. The specialist nudibranch Glossodoris pallida preferred bases of the sponge over tips, and avoidance of high levels of compounds could be a reason for this selection.221
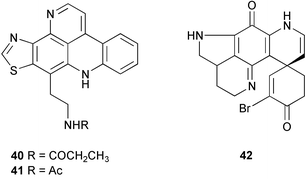
Other studies have also found higher concentrations of secondary metabolites in the most exposed surface layers of the sponge (ectosome) or in tips of branches. The tropical Pacific sponge Oceanapia sp. has an unusual growth form with a turnip-shaped base that is buried in substrate and upright fistules that protrude from the sand with a small fragile capitum on top of each fistule. Concentrations of the major pyridoacridine alkaloids kuanoniamine C (dercitamide) 40 and kuanoniamine D 41 showed a sharp increase from the basal root to the capitum of the sponge.222 The pure pyridoacridines deterred feeding by natural assemblages of reef fishes at fistule concentrations demonstrating the defensive function of the compounds. The Antarctic sponge Latrunculia apicalis has a spherical shape, and concentrations of the major alkaloid discorhabdin G 42 are greatest in the outermost surface layer (periphery) of the sponge.223 The compound appears to serve as a chemical defense against predation by sea stars, which are major predators in Antarctic benthic environments. Both of these studies support Optimal Defense Theory, with highest concentrations in those parts of the sponge that are most vulnerable to predators.222–223 In contrast, extracts from ectosome (periphery) and endosome (interior) of 6 Red Sea sponges did not differ in their ability to deter feeding by the wrasse Thalassoma klunzingeri or the sea urchin Diadema setosum.224
The defense of sponge larvae has been examined in only a few studies. Lindquist and Hay225 examined the palatability of a variety of invertebrate larvae toward different fish consumers. Brooded larvae of Caribbean sponges, gorgonians, temperate hydroids and a bryozoan were unpalatable to fishes. Extracts from larvae of three sponges, a bryozoan and a hydroid were all deterrent to fishes, illustrating that there was a chemical basis for this unpalatability, whereas the adults of only three of these species (two sponges and the hydroid) deterred fish feeding. Similarly, a variety of marine invertebrate larvae were also unpalatable to corals and sea anemones.226 The deterrent properties of two Mediterranean sponges, Crambe crambe and Dysidea avara, were studied at three stages of their life cycles, larvae, recruits and adults.227Crambe crambe was effectively defended as an adult from grazing by the sea urchin Paracentrotus lividus, but larvae and young recruits were readily eaten by the fish Parablennius incognitus. In contrast, Dysidea avara larvae and recruits were avoided by the fish but adult tissues and extracts were readily eaten by the sea urchin. These studies illustrated that larvae and adults can differ in the effectiveness of their chemical defenses toward different predators.
Seasonal variation in chemical defense has been examined for a few sponges. Turon et al.228 measured toxicity in the sponge Crambe crambe on a monthly basis by Microtox® analysis as an approximation of secondary metabolite variation.229 Highest levels of toxicity were found in summer and autumn months, and overall toxicity was higher in the periphery of the sponge relative to the interior.228 Antibiotic activity of fragments of the sponge Aplysina fistularis was studied over an annual cycle.230 Peak antibiotic activity against some microorganisms but not others occurred in April and May.
Geographical variation in sponge chemical defenses has been studied in only a few cases. Burns et al.224 compared chemical defenses of 17 common Caribbean sponges toward the Caribbean wrasse Thalassoma bifasciatum and the Red Sea wrasse T. klunzingeri. Extracts ranged from highly palatable to highly deterrent, but both fish species responded to the extracts in similar ways. The authors suggested that there were general responses by fish predators to sponge chemical defenses, regardless of geographic origin.224 Becerro et al.231 tested the hypothesis that tropical species have evolved more effective chemical defenses to deter predators because predation is higher in tropical than in temperate habitats. There is little direct evidence that tropical species are better defended against predators than temperate species.123,163 Extracts from twenty common sponge species from tropical Guam and temperate western Mediterranean (NE Spain) were tested in field-based feeding experiments with large and small fish predators in both geographic areas. All of the sponges investigated showed deterrent properties against some predators in the field-based assays. Tropical and temperate sponges had comparable deterrence suggesting that chemical defenses from both tropical and temperate sponges were equally effective against predators.231
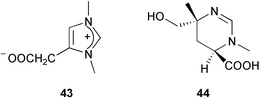
In addition to predator defense, sponge extracts have been shown to display antifouling, antimicrobial, and allelopathic activities. For example, zooanemonin 43 and 3,4,5,6-tetrahydromethyl-3,6-dimethyl-4-pyrimidinecarboxylic acid 44 isolated from Protophlitaspongia aga collected in Palau have been reported to inhibit the settlement of cyprid larvae from Balanus amphirite.232
Recently, with the development of new marine microbiological techniques, the focus of sponge chemical ecology is moving toward antimicrobial activities of sponge extracts and metabolites. Extracts of 33 Caribbean sponges were tested against 8 marine bacteria, including Bacillus spp., Vibrio parahaemolyticus, V. alginolyticus, Deleya marina, and Listonella anguillarum; 6 of the bacterial strains were isolated from sponges or seawater in the Bahamas. Approximately half of the extracts exhibited antibiotic activity by the standard agar disc-diffusion method against at least one bacterial strain.216 Interestingly, all of the species yielding antibacterial extracts also deterred feeding by reef fishes.196 A recent study examined bacterial attachment of the motile marine bacterium Vibrio harveyi on agar blocks containing extracts of 26 different Caribbean sponges and found that 20 of these extracts significantly decreased bacterial settlement relative to controls. Nine extracts almost completely inhibited bacterial attachment.209 Four species, which did not show antibiotic activity in a previous study,216 inhibited bacterial attachment.
Several other studies have examined antimicrobial activities of sponge extracts and the relationship to bacterial films on the sponge surfaces. Extracts of the Mediterranean encrusting sponge Crambe crambe had strong antimicrobial activity against marine bacteria, and no bacteria were present on the sponge surface. Two other sponges, Ircinia fasciculata and Spongia officinalis, had lower levels of antibiotic activity than C. crambe and appeared to gain no protection against surface bacteria.233 Extracts of the sponge Ircinia ramosa were tested for antibacterial activity toward isolates of marine bacteria (including some isolated from the sponge surface) during two collection periods (January and May). Extracts were broadly deterrent against isolates of marine bacteria; however, differences were noted in the chemical nature and antibiotic activity of extracts collected during January and May suggesting an environmental influence on the production of antibacterial compounds by the sponge.234 In a similar study, 2-octaprenyl-1,4-hydroquinone 45 from Ircinia spinulosa was reported to inhibit the growth of marine fungi and bacteria.211 Ircinin I 31 and II 32 from I. oros and 2-octaprenyl-1,4-hydroquinone 45 from I. spinulosa also inhibited the attachment of microalgae to experimental petri dishes.211
Studies of antimicrobial activity of 11 dominant Red Sea sponges against a panel of marine bacteria showed that sponge extracts exhibited a great deal of variability in antimicrobial activity.235 Eight of 11 sponge extracts inhibited at least one bacterial isolate. Amphimedon viridis had the highest antimicrobial activity, and no bacteria were observed on surfaces of this sponge. Bioassay-guided fractionation yielded the pyridinium alkaloids halitoxin 46 and amphitoxin 47 as potent antimicrobial compounds. Amphitoxin 47 has also been previously shown to deter feeding by the bluehead wrasse Thalassoma bifasciatum.236 Interestingly, the compounds showed specific rather than broad spectrum activity against marine bacteria. Strains of bacteria from seawater surrounding the sponges were inhibited while strains associated with the sponge were resistant to the compounds. The researchers suggested that this selectivity might allow certain bacteria to live in association with the sponge while preventing microbial pathogenesis.235 This rapidly growing field of sponge-antimicrobial interactions has been recently reviewed by Engel et al.5
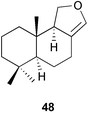
Competition for space among organisms on tropical reefs has often been hypothesized to be mediated by allelopathic interactions, but the secondary metabolites involved in these interactions have rarely been identified. Thacker et al.215 studied competition and the compounds involved in allelopathic interactions between a Dysidea sp. and Cacospongia sp. The sponge Dysidea sp. overgrows Cacospongia sp and causes necrosis. In field assays, crude extracts of Dysidea sp. and the major metabolite 7-deacetoxyolepupuane 48 both caused necrosis in Cacospongia when they were incorporated into agar strips and placed in contact with Cacospongia for 7 days. In addition to its role in competition, 7-deacetoxyolepupuane 48 deterred feeding by natural assemblages of reef fishes as well as a spongivorous angelfish, illustrating the multiple ecological roles that a single secondary metabolite may play.215 Engel and Pawlik237 tested extracts of 20 Caribbean sponges in Florida for their ability to deter overgrowth by 3 sponges in field assays. Of the sponge extracts tested, 30% inhibited sponge growth. Approximately half of the extracts had no effect on overgrowth, and 3 extracts actually promoted overgrowth.
Activated defenses (wound-induced transformations) have been reported for sponges of the genus Aplysina.238–240 Isofistularin-3 49 and aerophobin 2 50 are brominated isoxazoline alkaloids that are presumably rapidly converted to aeroplysinin-1 51 and a dienone 52 when sponges are wounded (Scheme 2). The products of the conversion have been shown to be more active against microorganisms238–239 and fish predators240 than the isoxazoline precursors. Recently Puyana et al.241 investigated the occurrence of activated defenses in species of Aplysina from the Caribbean. They did not find evidence for activated defenses based on LC-MS analyses of sponge extracts after sponges were stabbed with a scalpel; however, differences in the methods used between their study and those conducted previously preclude direct comparison of results. For activated defenses to occur, tissues of organisms must be wounded by crushing or grinding to break down the compartmentalization that separates enzyme and substrates. Such methods have been used to study activated defenses in marine algae156,164 but not in marine sponges based on reported methods.238–242
 | ||
| Scheme 2 | ||
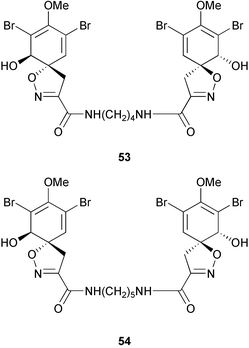
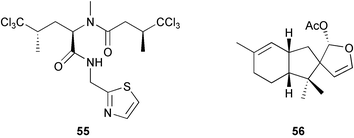
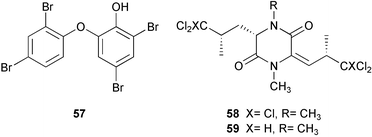
The abundance and diversity of marine natural products and the relationship of some of these to known cyanobacterial and bacterial metabolites has led to the suggestion that symbiotic microorganisms may be responsible for the production of secondary metabolites rather than the sponges themselves.17,243–244 This question of secondary metabolite localization in sponge cells was first addressed for Aplysina fistularis.10 Using dispersive X-ray microanalysis, Thompson and colleagues10 demonstrated that two brominated compounds, aerothionin 53 and homoaerothionin 54, are localized in the spherulous cells of the sponge. These results suggest that the sponge rather than symbionts produces these compounds. More recent contributions of John Faulkner's group have demonstrated that some sponge metabolites are localized in symbiotic cyanobacterial or bacterial cells,14–15,243–247 which are prevalent in some sponges of the genus Dysidea248 (Lamellodysidea249). A study of the sponge Dysidea herbacea collected from Heron Island, Australia using flow-cytometry to separate symbiotic cyanobacterial cells, Oscillatoria spongeliae, from the sponge cells demonstrated that the polychlorinated metabolites, including the major metabolite 13-demethylisodysidenin 55, were localized in the cyanobacterial cells while the sesquiterpenes, including spirodysin 56, were localized in the sponge cells.244 In a second study of D. herbacea collected in Palau also employing flow-cytometry to separate, in this case, the O. spongeliae, sponge cells and heterotrophic bacterial cells, 2-(2′,4′-dibromophenyl)-4,6-dibromophenol 57 was localized in the O. spongeliae, although large crystals of this compound were found throughout the sponge.245 A similar study of D. herbacea collected at One Tree Island, Australia, which used density gradient centrifugation rather than flow-cytometry to separate cell types, confirmed spirodysin 56 to be localized in the archaeocytes and choanocytes of the sponge, and demonstrated that the diketopiperazines, dihydrodysamide C 58 and didechlorodihydrodysamide C 59, are localized in O. spongeliae.250
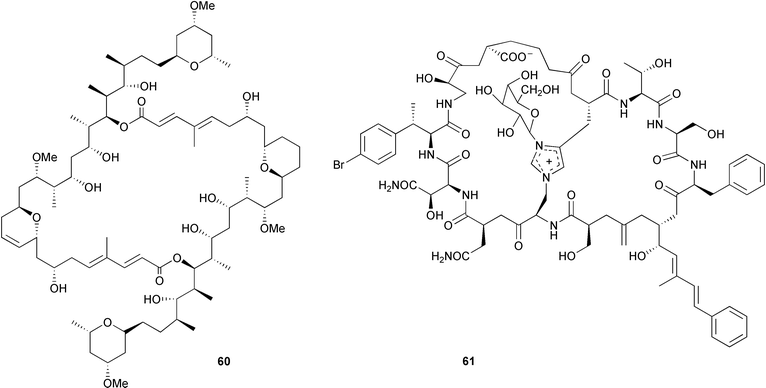
More recently the focus of Faulkner's group turned to the lithistid sponges, known to be chemically rich with many of the metabolites resembling complex bacterial metabolites.14–15,246–247 Cell separation by density gradient centrifugation resulted in the localization of swinholide A 60 in a mixed population of unicellular heterotrophic bacteria cells found in Theonella swinhoei14 and the bicyclic glycopeptide theopalauamide 61 in a filamentous bacterial symbiont.14,247
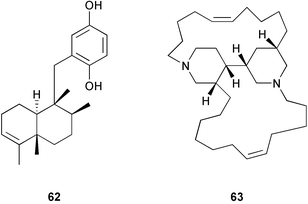
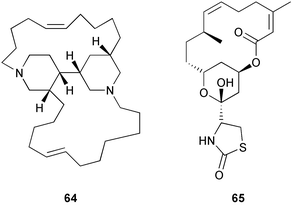
Other recent studies of metabolite localization in sponges demonstrate that major secondary metabolites are often not found in the symbionts.16–17,251–254 Cell fractionation and Ehrlich staining showed that the defensive furanosesquiterpenes found in Dysidea fragilis were located in large spherular sponge cells.253 Cell fractionation on Ficoll gradients showed that avarol 62 from Dysidea avara was located within choanocytes.254 Density gradient cell fractionation studies of Haliclona sp. showed that the alkaloids, haliclonacyclamines A 63 and B 64, are localized within the sponge cells rather than an associated dinoflagellate.251 Studies of Amphimedon terpenensis showed that brominated long-chain fatty acids were associated with sponge cells, not cyanobacterial or bacterial cells.255 Studies of Negombata magnifica252 and Oceanapia sagittaria16 have also demonstrated that latrunculin B 65 and dercitamide 40 are localized in the bacteria-free sponge cells.
The results from sponge localization experiments raise many questions about whether some metabolites may serve as sponge chemical defenses.256 Few ecological studies convincingly demonstrate that compounds produced by a symbiont chemically defend the host against infection or predation. Future ecological studies to explore the role of metabolites in sponge chemical defense will require researchers to address the questions of localization of metabolites and the exposure of metabolites to a potential predator.
4 Octocorals
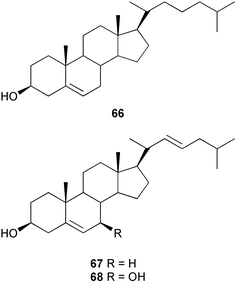
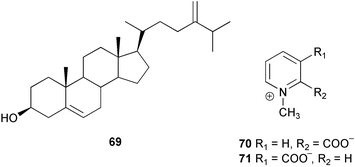
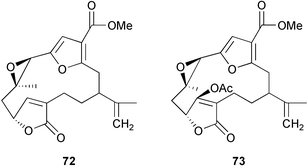
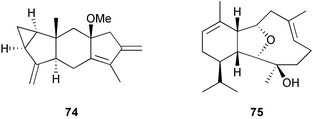
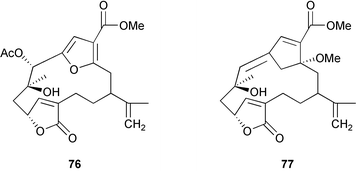
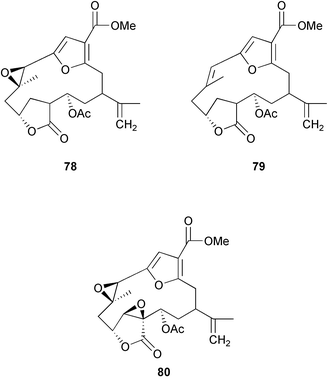
Chemical ecology studies of Alcyonarians (Octocorallia), especially the Alcyonacea (soft corals) and Gorgonacea (gorgonian corals including sea whips and fans), have largely focused on predator defense. Numerous feeding studies with crude extracts and secondary metabolites from alcyonaceans and gorgonian corals have shown that with the exception of a few specialist predators, fishes and invertebrates do not readily consume soft corals, sea fans, and sea whips.257–271 For example, crude extracts from three species of Antarctic soft corals caused tube-foot retraction by the sea stars Perknaster fuscus and Odontaster validus.264 In a subsequent study, cholesterol 66, 22-dehydrocholesterol 67, 22-dehydro-7β-hydroxycholesterol 68, and 24-methylenecholesterol 69 were isolated as the active metabolites from Alcyonium paessleri while homarine 70 and trigonelline 71 were isolated as the active metabolites from Gersemia antarctica.272 Ecologically relevant concentrations of these metabolites were found in the seawater surrounding the soft corals posing a potential barrier to predators. Extracts of the surrounding seawater also inhibited the growth of three sympatric microbes.Chemical defenses isolated from gorgonians and alcyonaceans are usually terpenoids.26,30–31,34–35,188,261,273–278 For example, briarane and asbestinane diterpenes from different populations of the gorgonian Briareum asbestinum colonies were reported to deter fish feeding.273 Moreover, the larvae of this species synthesize the asbestinane diterpenes.279 Similarly, pukalide 72 and 11β-acetoxypukalide 73 were found in the eggs of the Pacific soft coral Sinularia polydactyla.280 The concentration of pukalide 72 was similar to that of the adults while the concentration of 11β-acetoxypukalide 73 was much lower. Recently, the sesquiterpene heterogorgiolide 74 and a known eunicellane diterpenoid 75 from Heterogorgia uatumani were reported to be unpalatable to natural assemblages of fishes in Brazil.265 A similar study of the endemic Brazilian gorgonian Lophogorgia violacea using field feeding assays for bioassay-guided fractionation yielded a mixture of 5 furanocembranoid diterpenes that appear to deter fish predation in an additive manner.267 This mixture included two new furanocembranoids, 7-acetoxy-hydroxylophotoxin 76 and 3-methoxy-8-hydroxylophotoxin 77 and the known compounds, lophotoxin 78, deoxylophotoxin 79 and 13-acetoxy-11β, 12β-epoxypukalide 80.
Crude extracts from common species of gorgonians collected in the Caribbean,281–282 Singapore,283 and the Pacific,270,284 have been surveyed for predator deterrent activity in laboratory assays against a generalist predator or in field assays against natural assemblages of reef fishes. The earliest survey demonstrated that approximately 60% of the extracts tested deterred feeding by Thalassoma bifasciatum at natural concentrations determined as the percentage of dry mass.281 Recently, O'Neal and Pawlik282 repeated this survey of the crude extracts and sclerites from Caribbean gorgonians reporting that 100% of the extracts assayed deterred T. bifaciatum. O'Neal and Pawlik282 attributed the disparity to two changes in the experimental methods: (1) extracting samples immediately and (2) assaying samples at natural volumetric concentration, an assay technique developed by Harvell et al.34 to account for the variation in volume of water in the gorgonian tissue. In the previous study,281 gorgonian samples were dried in the sun for later extraction and assays were conducted with krill by incorporating extracts at natural gravimetric concentrations. Koh et al.283 and Eve284 employed similar methods to those of O'Neal and Pawlik282 when surveying 8 species of gorgonians from Singapore and 65 species from the Pacific, respectively, and reported 100% success in feeding deterrence by the crude extracts that were assayed. Another recent study also reported an 86% success rate in fish feeding deterrence against reef fishes in field assays of the crude extracts from common shallow water gorgonians of Guam, however, these authors employed a modified gravimetric method to determine the natural concentration of crude extract in the polyps.270 Briefly, this method involved removing the coenenchyme of the gorgonian from the gorgonin skeleton, oven drying, determining the dry mass of the tissue, and soaking the gorgonian pieces in bleach to obtain the sclerites. Natural concentrations were calculated as the percentage of dry mass of the organic tissue of the gorgonian (total dry mass − sclerite mass).270 Sclerites from the Caribbean282 and Singapore283 rarely deterred fish feeding while sclerites from 5 of the 7 species surveyed in Guam deterred fishes in field assays.270
Several alcyonaceans and gorgonian corals are reported to produce structural (i.e. sclerites) defenses in addition to chemical (i.e. secondary metabolites) defenses against predation.34,261,285–286 Only one gorgonian, the Pacific sea whip Viminella sp., in the family Ellisellidae, has been shown to rely solely upon sclerites to deter feeding by generalist predators.270 The importance of the size, shape and the density of sclerites to unpalatability has been addressed for Briareum asbestinum.286–287 Short, densely packed sclerites in colonies at shallow depths deterred fishes while longer, more loosely associated sclerites in colonies at deeper depths did not.286–287 One of the most interesting aspects of the structural defenses of B. asbestinum is that simulated predator damage to colonies resulted in the induction of the smaller predator-deterrent sclerites.285 In a more recent study, Puglisi et al.270 conducted feeding experiments with sclerites powdered in a mortar and pestle versus whole sclerites from five Pacific gorgonian corals. The results from these assays showed that fishes preferred the controls over both treatments; size and shape account for some of the observed feeding deterrence activity.270
Induction of chemical defense in marine macroorganisms has rarely been demonstrated.2 Recently, Thorton and Kerr288 reported that high levels of predation by the mollusc Cyphoma gibbosum induced pseudopterosin C 81 production by Pseudopterogorgia elisabethae. While some experimental evidence suggests that C. gibbosum may use biotransformation enzymes to detoxify gorgonian metabolites,262 this is the first instance where a specialist has been shown to induce the production of a gorgonian metabolite. Interestingly, induction did not occur after high levels of predation by the butterfly fish Chaetodon capistratus or artificial wounding of the sea fan. However, similar results to C. gibbosum predation were obtained in response to decreased levels of UV/VIS radiation.288
In addition to predator defense, gorgonian and soft coral extracts and metabolites have also been implicated as anti-fouling agents.275,289–291 Both chloroform and aqueous methanol extracts from the Antarctic soft corals Alcyonium paessleri and Gersemia antarctica were reported to inhibit bacterial attachment.291 In addition, the aqueous methanol extracts inhibited the growth of three sympatric bacteria. The muricins 82–85, pregnene glycosides isolated from the gorgonian Muricea fruticosa inhibited the growth of the marine diatom Phaeodactylum tricornutum, a potential fouling organism, in laboratory assays at natural concentrations.289 Diterpenes from the soft coral Sinularia flexibilis inhibited the development of eggs and larvae of the corals Acropora tenuis and Montipora digitatain vitro.292
Several surveys of antimicrobial activities of crude extracts from Caribbean gorgonian corals have been conducted.269,292–294 Results of a survey of the non-polar and polar extracts from seven Caribbean gorgonians in the family Plexauridae and one gorgonian in the family Gorgonidae showed that non-polar fractions were the most effective in the growth inhibition of 3 species of marine bacteria and 2 species of non-marine bacteria.293 In a broad survey of 39 Caribbean sea fans and sea whips, crude extracts were screened for antibacterial activity by the standard agar disc-diffusion method against a host of marine bacteria isolated from the surfaces of living colonies of Briareum asbestinum and decaying gorgonians and three bacterial species known to be pathogenic to marine invertebrates.294 Extracts from Briareum asbestinum exhibited little or no inhibition to bacteria isolated from conspecific colonies in the field. Overall, only 15% of the extracts showed antimicrobial activity; however, extracts from Pseudopterogorgia spp. were quite active. Extracts from 3 species of Pseudopterogorgia inhibited the growth (zone of inhibition > 5 mm) of most of the bacterial strains tested.294 Extracts of various reproductive and developmental stages (including 1–2-day and 3–6-day old embryos) of the Red Sea soft coral Parerythropodium fulvum fulvum exhibited antimicrobial activity against several co-occurring and potentially pathogenic marine bacteria.295 Bioassay-guided fractionation of extracts indicated that antimicrobial activity was found in fractions of different polarities and was due to trace amounts of compounds that were highly potent.295
Recently, the role of chemical defense in gorgonians against marine microorganisms has become of great interest due to outbreaks of disease in Caribbean populations of Gorgonia spp. caused by the fungal pathogen Aspergillus sydowii.296 Kim et al.296 have shown that crude extracts from the tips of healthy Gorgonia spp. colonies are more resistant to the fungal pathogen A. sydowii than extracts from other parts of the colony and that crude extracts inhibit spore formation at natural concentrations. Slattery266 found that fungal infection increased the susceptibility of Gorgonia ventalina to grazing by Cyphoma gibbosum. The furano-germacrene julieannafuran 86263 decreased in concentration in infected colonies. The compound was deterrent to fish but only at levels found in pre-infected colonies.266 In a more recent survey, crude extracts from Echinogorgia sp. C, C. cf. umbraculum and S. suberosa were active against potentially invasive fungi at or near natural concentrations.269
Intra-specific, spatial, and temporal chemical variation has been studied for soft corals and gorgonians. Concentrations and types of secondary metabolites have been reported to vary based on colony size,297 depth,273,298 and proximity to competitors.299 Site specific differences suggest that terpene levels may be influenced by environmental conditions.297,300
Crude extract concentrations vary among different parts of colonies, and some parts of soft coral or gorgonian colonies may rely more upon chemical defense while others rely more on structural defense.35,261,301–302 Natural assemblages of coral reef fishes were deterred by extracts from three species of the Pacific alcyonacean Sinularia at the higher concentrations found in the polyp-bearing tissues at the top of colonies, while feeding was not affected by the lower concentration in the bottom portion of the colony.301 The inverse was observed for sclerites, suggesting that the lower portion of the colony relies on structural defenses.301 The Caribbean gorgonians Pseudopterogorgia spp. also had higher concentrations of metabolites at the tips of colonies.34–35 In Gorgonia ventalina, the crude extracts are reported to be uniform throughout the colony.261,266 However, as previously discussed, crude extracts from the tips of healthy Gorgonia spp. colonies exhibited more antifungal activity against Aspergillus sydowii than extracts from other parts of the colony.296 A recent study of Pacific gorgonians270 showed that crude extract concentrations varied significantly among base, mid-axis and tips for four species, Annella mollis, A. reticulata, Subergorgia suberosa, and Viminella sp., yet crude extracts from the mid-axis and tip portions of the colonies of Annella mollis, A. reticulata, and Subergorgia suberosa deterred fish feeding while the crude extract from Viminella sp. was ineffective as a feeding deterrent.268,270 In addition, only the crude extracts from the tips of Rumphella sp. and Astrogorgia sp. deterred predation, however, these two species did not exhibit concentration gradients.270
A variety of different metabolites have been reported from similar species of gorgonians and soft corals collected from geographically discrete regions.190 A biogeographic comparison of Sinularia maxima and S. polydactyla throughout the tropical Pacific demonstrated that concentrations of the major metabolites pukalide 72 and 11β-acetoxypukalide 73 exhibited a two- to five-fold difference among collection sites within the region.300 Temporal differences in concentrations of these compounds were observed for S. maxima and S. polydactyla over a two-year period, however the authors were unable to find any seasonal patterns. In the same study, concentrations of pukalide 72 were reported to change as much as 27% in transplant experiments. These changes correlated well with the levels of predation at the transplant sites suggesting that the differences observed in soft coral metabolites may be due to local predation pressure.300
A geographic comparison of colonies of the gorgonian Briareum asbestinum from two regions within the Caribbean, the Bahamas and St. Croix, showed that the two chemically defended populations produced different classes of diterpenes.273 More recently, a study of the defenses of Annella mollis, A. reticulata and Rumphella sp. addressed geographic differences in the palatability of crude extracts of colonies collected from two distant tropical islands in the Pacific Ocean, Guam and Lizard Island, Australia.268 Reciprocal feeding assays conducted in the laboratory and the field at both islands with crude extracts from different parts of colonies demonstrated that the production of chemical defenses of all three species are conserved among populations at these two islands.268 In another recent study, Dube et al.303 addressed the geographic variation of chemical resistance of Gorgonia ventalina colonies from the Florida Keys and San Salvador, Bahamas to the fungal pathogen Aspergillus sydowii. The results of this study demonstrated that antifungal activity varied significantly among sites in the Florida Keys but did not vary among sites at San Salvador. The authors suggest that differences may not have been observed due to the close proximity of the sites at San Salvador. In addition, smaller colonies were determined to exhibit more resistance to A. sydowii than more mature colonies.303
The question concerning the origin of metabolites has also been recently addressed for the soft coral Lobophytum compactum.304 In this study, the authors inoculated freshly metamorphosed polyps of identical genetic background with different strains of zooxanthellae. Analysis by electro-spray and Fourier-transform mass spectrometry indicated that the host soft coral was responsible for the production of the major metabolite, isolobophytolide 87. However, the authors also suggested that zooxanthellae may have an indirect effect on the production of isolobophytolide 87 because zooxanthellae contribute to the primary metabolism of the host and, in the instance of soft coral bleaching, L. compactum may not have the resources to invest in the production of this metabolite.304 In simulated bleaching experiments with L. compactum and Sinularia flexibilis, the concentrations of isolobophytolide 87 and sinulariolide 88 decreased within the tissues of the bleached colonies, respectively, while the concentration of flexibilide 89, known to exhibit antimicrobial activity, increased in the tissues of S. flexibilis.305 Algal overgrowth commonly associated with coral bleaching did not occur and the authors suggested that the soft corals may alter secondary metabolite production to prevent fouling.
5 Ascidians (Tunicates)
The ascidians (members of the class Ascidiacea within the chordate subphylum Tunicata) are rich in nitrogenous secondary metabolites188,306 that can deter feeding by predators.26,52,188 Both secondary metabolites and inorganic acids have been proposed to protect adult ascidians from predation.26,307,308 Pisut and Pawlik309 tested the effectiveness of organic extracts from 17 species of tropical and warm temperate ascidians to deter feeding by Thalassoma bifasciatum. They also assessed the effects that lowered pH (pH < 3.0), similar to levels found in the tunics of some ascidians, had on fish feeding. They found that 16 of 17 species had deterrent extracts from some part of the body. Nine species sequestered acid in their tunics at levels that deterred T. bifasciatum. Many species had both deterrent extracts and acidic tunics that functioned as predator defenses.309 Ecological roles of ascidian metabolites have been investigated in relatively few studies compared with other benthic invertebrates. Ascidian compounds can effectively defend against predation by generalist fishes and invertebrates26,52,310–313 and against fouling organisms.314–317 Ascidian larvae have also been shown to be effectively chemically defended against predators.6,52,312,318 Similarity has been found in the natural products chemistry of ascidian adults and larvae.52Little is known about chemical variation in ascidians. Lindquist et al.52 quantified six didemnin cyclic peptides in different colonies collected on one patch reef in the Bahamas and found large differences in peptide concentrations among colonies. Pisut and Pawlik309 tested extracts from tunics, viscera, and gonads in laboratory feeding assays. They found that the location of deterrent compounds was greatest in the gonads for three solitary ascidians, suggesting that defenses are passed on to eggs or larvae.
The origin of metabolites in Lissoclinum spp. has been recently revisited in a study of Lissoclinum patella.319 Chemical analysis of carefully separated Prochloron cells and the tunic of L. patella showed that the cyclic peptides patellamides A–C 90–92 could not always be extracted from the Prochloron cells, suggesting that the compounds are either produced in the tunic or transferred from the Prochloron cells to the tunic. Previous studies of related species have provided inconclusive results as to the origin of metabolites;320–324 the authors suggest that variable results have been observed because the methods lack an essential step of careful dissection of the tunic material.319
6 Other invertebrates
Unlike other well-studied invertebrates such as sponges and soft corals, predator defenses of marine worms have not been the subject of many studies. In a survey of feeding deterrent properties of 11 worm species collected from the southern Florida coast using the wrasse Thalassoma bifasciatum, the extracts of Cirriformia tentaculata, Ptychodera bahamensis, and Eupolymnia crassicornis were unpalatable and chemically defended.325 In the case of E. crassicornis only the extract from the exposed portion (i.e. tentacles) of the animal was unpalatable to fish and crabs.325,326 Bioassay-guided isolation of C. tentaculata extracts led to the isolation of predator deterrent 2-alkylpyrrole sulfamates 93–95.325,327 In a second survey of 16 species of worms collected from a Georgia mud-flat, only one species, Saccoglossus kowalevskii, was unpalatable to two sympatric fish, Fundulus heteroclitus and Leisostomus xanthurus.328 Bioassay-guided fractionation yielded 2,3,4-tribromopyrrole 96 as the active constituent in S. kowalevskii. In further assays with worms from South Atlantic Bight known to produce brominated metabolites and pure compounds produced by marine worms against sympatric fishes, the brominated compounds failed to consistently deter fish feeding. The authors suggest that predator deterrent activity of 2,3,4-tribromopyrrole is unusual because other brominated metabolites seldom functioned as chemical defenses against predators.328Other groups of benthic invertebrates including bryozoans and echinoderms have also been demonstrated to be chemically defended.188 While bryozoans have been studied for their natural products chemistry,188,329 less is known about the natural functions of these compounds.330 Echinoderms, known for their production of saponins,188 have also been shown to be chemically defended against consumers.331–332
7 Conclusions
The early contributions of John Faulkner, William Fenical, John Coll and their students and colleagues were invaluable to the development of the field of marine chemical ecology. John Faulkner's research greatly advanced our understanding of the chemical ecology of marine molluscs and sponges. We now have a clear understanding that most benthic marine organisms produce an array of secondary metabolites as chemical defenses against large and small predators and competitors for space and resources, and that some of these metabolites may be involved with reproduction, settlement, and metamorphosis. Chemical ecologists addressing questions in the new field of marine microbial chemical ecology are beginning to explore the role of chemical mediation of marine microorganisms and the role of secondary metabolites in preventing infection and large disease outbreaks.There is ample research that demonstrates qualitative and quantitative variation in secondary metabolite production within and among populations, both temporally and spatially. However, while these studies can show patterns of variation, few studies sufficiently address the causes and consequences of this variability. Future studies of chemical variation are necessary to address the question of whether we can attribute these differences to local pressures by predators and competitors, environmental factors, or genetic variation. In addition, as chemical ecologists continue to isolate active metabolites from crude extracts, we will gain a better understanding of structure–activity relationships and the complexity of chemical defenses in marine organisms, which is crucial to understanding the physiological effects of chemical defenses in predator–prey and pathogenic interactions. Advances in analytical and natural products chemistry and new molecular techniques will provide new tools for chemical ecologists to address these more complex questions. Marine chemical ecology will continue to benefit greatly from collaborations between chemists and biologists, including ecologists, molecular biologists, and microbiologists. Interdisciplinary approaches are the key to addressing complex questions in this field of research.
8 References
- Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992 Search PubMed.
- M. E. Hay, J. Exp. Mar. Biol. Ecol., 1996, 200, 103 CrossRef CAS.
- Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001 Search PubMed.
- T. M. Arnold and N. M. Targett, J. Chem. Ecol., 2002, 28, 1919 Search PubMed.
- S. Engel, P. R. Jensen and W. Fenical, J. Chem. Ecol., 2002, 28, 1971 Search PubMed.
- N. Lindquist, J. Chem. Ecol., 2002, 28, 1987 Search PubMed.
- J. M. Shick and W. C. Dunlap, Annu. Rev. Physiol., 2002, 64, 223 CrossRef CAS.
- P. D. Steinberg, R. De Nys and S. Kjelleberg, J. Chem. Ecol., 2002, 28, 1935 Search PubMed.
- P. D. Steinberg and R. de Nys, J. Phycol., 2002, 38, 621 CrossRef CAS.
- J. E. Thompson, K. D. Barrow and D. J. Faulkner, Acta Zool., 1983, 64, 199.
- J. E. Thompson, R. P. Walker and D. J. Faulkner, Mar. Biol., 1985, 88, 11 CAS.
- J. E. Thompson, Mar. Biol., 1985, 88, 23 CAS.
- R. P. Walker, J. E. Thompson and D. J. Faulkner, Mar. Biol., 1985, 88, 27 CAS.
- C. A. Bewley, N. D. Holland and D. J. Faulkner, Experientia, 1996, 52, 716 Search PubMed.
- C. A. Bewley and D. J. Faulkner, Angew. Chem., Int. Ed., 1998, 37, 2162 CrossRef.
- C. E. Salomon, T. Deerinck, M. H. Ellisman and D. J. Faulkner, Mar. Biol., 2001, 139, 313 Search PubMed.
- D. J. Faulkner, M. K. Harper, M. G. Haygood, C. E. Salomon and E. W. Schmidt, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel, 2000, p. 107 Search PubMed.
- E. W. Schmidt, A. Y. Obratsova, S. K. Davidson, D. J. Faulkner and M. G. Haygood, Mar. Biol., 2000, 136, 969 CrossRef CAS.
- M. G. Haygood, E. W. Pavia, S. K. Davidson and D. J. Faulkner, J. Mol. Microbiol. Biotechnol., 1999, 1, 33 Search PubMed.
- M. G. Haygood, E. W. Schmidt, S. K. Davidson and D. J. Faulkner, in Molecular Marine Biology, ed. D. Bartlett, Horizon Science Press, New York, 2000p. 61 Search PubMed.
- P. Karuso, in Bioorganic Marine Chemistry, Volume 1, ed. P. J. Scheuer, Springer-Verlag, Berlin, 1987, p. 31 Search PubMed.
- D. J. Faulkner, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992, p. 119 Search PubMed.
- C. Avila, Oceanogr. Mar. Biol. Ann. Rev., 1995, 33, 487 Search PubMed.
- J. J. Stachowicz, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 157 Search PubMed.
- C. D. Amsler, K. B. Iken, J. B. McClintock and B. J. Baker, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 267 Search PubMed.
- J. R. Pawlik, Chem. Rev., 1993, 93, 1911 CrossRef CAS.
- S. I. Williams and D. I. Walker, Oceanogr. Mar. Biol. Ann. Rev., 1999, 37, 87 Search PubMed.
- G. Cimino and M. T. Ghiselin, Chemoecology, 1998, 8, 51 CAS.
- P. M. Johnson and A. O. D. Willows, Mar. Fresh. Behav. Physiol., 1999, 32, 147 Search PubMed.
- J. C. Coll, Chem. Rev., 1992, 92, 613 CrossRef CAS.
- P. W. Sammarco and J. C. Coll, Mar. Ecol. Prog. Ser., 1992, 88, 93 Search PubMed.
- J. N. Norris and W. Fenical, in Atlantic Barrier Reef Ecosystem Carrie Bow Cay, Belize, Volume 1, Structure and communities, ed. K. Ruetzler and I. G. Macintyre, Smithson. Contrib. Mar. Sci. 12, 1982, p. 417 Search PubMed.
- M. E. Hay, Oecologia, 1984, 64, 396.
- C. D. Harvell, W. Fenical and C. H. Greene, Mar. Ecol. Prog. Ser., 1988, 49, 287 Search PubMed.
- C. D. Harvell and W. Fenical, Limnol. Oceanogr., 1989, 34, 382 Search PubMed.
- J. B. Harborne, Nat. Prod. Rep., 2001, 18, 361 RSC.
- R. K. Zimmer and C. A. Butman, Biol. Bull., 2000, 198, 168 Search PubMed.
- M. G. Hadfield and V. J. Paul, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 431 Search PubMed.
- C. D. Amsler and K. B. Iken, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 413 Search PubMed.
- M. J. Weissburg, M. C. Fenner, D. P. Pisut and D. L. Smee, J. Chem. Ecol., 2002, 28, 1953 Search PubMed.
- H. G. Trapido-Rosenthal, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 463 Search PubMed.
- P. D. Steinberg, R. de Nys and S. Kjelleberg, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 355 Search PubMed.
- W. Boland, in Chemical Ecology: The Chemistry of Biotic Interaction, ed. T. Eisner and J. Meinwald, National Academy Press, Washington D. C., 1995, p. 87 Search PubMed.
- N. Asai, N. Fusetani, S. Matsunaga and J. Sasaki, Tetrahedron, 2000, 56, 9895 CrossRef CAS.
- K. C. Buresch, J. G. Boal, J. Knowles, J. Debrose, A. Nichols, A. Erwin, S. D. Painter, G. T. Nagle and R. T. Hanlon, J. Chem. Ecol., 2003, 29, 547 Search PubMed.
- D. Karentz, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 481 Search PubMed.
- W. C. Dunlap and J. M. Shick, J. Phycol., 1998, 34, 418 CrossRef.
- P. J. Proteau, W. H. Gerwick, F. Garcia-Pichel and R. W. Castenholz, Experientia, 1993, 49, 825 Search PubMed.
- S. Brenowitz and R. W. Castenholz, FEMS Microbiol. Ecol., 1997, 24, 343 CrossRef CAS.
- H. Pavia, G. Cervin, A. Lindgren and P. Aberg, Mar. Ecol. Prog. Ser., 1997, 157, 139 Search PubMed.
- M. E. Hay, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992, p. 93 Search PubMed.
- N. Lindquist, M. E. Hay and W. Fenical, Ecol. Monogr., 1992, 62, 547 Search PubMed.
- J. H. Landsberg, Rev. Fish. Sci., 2002, 10, 113 Search PubMed.
- Marine Toxins, ed. S. Hall and G. Strichartz, ACS Symposium Series 418, 1990 Search PubMed.
- Y. Shimizu, Chem. Rev., 1993, 93, 1685 CrossRef CAS.
- M. E. Hay and J. Kubanek, J. Chem. Ecol., 2002, 28, 2001 Search PubMed.
- J. T. Turner and P. A. Tester, Limnol. Oceanogr., 1997, 42, 1203 Search PubMed.
- S. G. Bullard and M. E. Hay, Limnol. Oceanogr., 2002, 47, 1456 Search PubMed.
- V. J. Paul, E. Cruz-Rivera and R. W. Thacker, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 227 Search PubMed.
- M. E. Hay and W. Fenical, Annu. Rev. Ecol. Syst., 1988, 19, 111 Search PubMed.
- M. E. Hay and P. D. Steinberg, in Herbivores: Their Interactions with Secondary Plant Metabolites. Volume II, Ecological and Evolutionary Processes, ed. G. A. Rosenthal and M. R. Berenbaum, Academic Press, San Diego, 1992, p. 371 Search PubMed.
- V. J. Paul and M. E. Hay, Mar. Ecol. Prog. Ser., 1986, 33, 255 Search PubMed.
- M. E. Hay, Proc. 8th Int. Coral Reef Symp., 1997, 1, 713 Search PubMed.
- V. J. Paul, Proc. 8th Int. Coral Reef Symp., 1997, 1, 707 Search PubMed.
- M. E. Hay, J. J. Stachowicz, E. Cruz-Rivera, S. Bullard, M. S. Deal, N. Lindquist, in Methods in Chemical Ecology, Volume 2, Bioassay Methods, ed. K. F. Haynes and J. G. Millar, Chapman & Hall, Norwell, MA, 1998, p. 39 Search PubMed.
- R. C. Pereira, M. D. Pinheiro, V. L. Teixeira and B. A. P. da Gama, Braz. J. Biol., 2002, 62, 33 Search PubMed.
- J. E. Duffy and V. J. Paul, Oecologia, 1992, 90, 333.
- M. E. Hay, Q. E. Kappel and W. Fenical, Ecology, 1994, 75, 1714 Search PubMed.
- S. C. Pennings, S. R. Pablo, V. J. Paul and J. E. Duffy, J. Exp. Mar. Biol. Ecol., 1994, 180, 137 CrossRef.
- E. Cruz-Rivera and M. E. Hay, Ecol. Monogr., 2003, 73, 483 Search PubMed.
- M. E. Hay, J. E. Duffy, C. A. Pfister and W. Fenical, Ecology, 1987, 68, 1567 Search PubMed.
- S. C. Pennings, S. R. Pablo and V. J. Paul, Limnol. Oceanogr., 1997, 42, 911 Search PubMed.
- D. G. Nagle and V. J. Paul, J. Exp. Mar. Biol. Ecol., 1998, 29, 225.
- D. G. Nagle and V. J. Paul, J. Phycol., 1999, 35, 1412 CrossRef CAS.
- D. G. Nagle, F. T. Camacho and V. J. Paul, Mar. Biol., 1998, 132, 267 CrossRef.
- E. Cruz-Rivera and V. J. Paul, Proc. 9th Int. Coral Reef Symp., 2000 Search PubMed.
- E. E. Sotka and M. E. Hay, Ecology, 2002, 83, 2721 Search PubMed.
- E. E. Sotka, Mar. Ecol. Prog. Ser., 2003, 256, 305 Search PubMed.
- G. Cronin, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 325 Search PubMed.
- K. L. Van Alstyne, M. N. Dethier and D. O. Duggins, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 301 Search PubMed.
- S. A. Dworjanyn, R. de Nys and P. D. Steinberg, Mar. Biol., 1999, 133, 727 CrossRef CAS.
- P. D. Steinberg, Science, 1984, 223, 405.
- D. W. Phillips and G. H. N. Towers, J. Exp. Mar. Biol. Ecol., 1982, 58, 285 CrossRef CAS.
- D. J. Carlson, J. Lubchenco, M. A. Sparrow and C. D. Trowbridge, J. Chem. Ecol., 1989, 15, 1321 Search PubMed.
- V. J. Paul and K. L. Van Alstyne, Coral Reefs, 1988, 6, 263 CAS.
- M. E. Hay, V. J. Paul, S. M. Lewis, K. Gustafson, J. Tucker and R. N. Trindell, Oecologia, 1988, 75, 233.
- K. D. Meyer and V. J. Paul, Mar. Biol., 1995, 122, 537 CAS.
- S. C. Pennings, M. P. Puglisi, T. J. Pitlik, A. C. Himaya and V. J. Paul, Mar. Ecol. Prog. Ser., 1996, 134, 49 Search PubMed.
- G. Cronin and M. E. Hay, Oecologia, 1996, 105, 361.
- V. J. Paul and K. L. Van Alstyne, Proc. 6th Int. Coral Reef Symp., 1988, 3, 133 Search PubMed.
- M. P. Puglisi and V. J. Paul, Mar. Biol., 1997, 128, 161 CrossRef CAS.
- D. B. Matlock, D. W. Ginsburg and V. J. Paul, Hydrobiologia, 1999, 398/399, 267 Search PubMed.
- D. F. Rhoades, Am. Nat., 1985, 125, 205 CrossRef.
- J. K. Nitao and A. R. Zangerl, Ecology, 1987, 68, 521 Search PubMed.
- S. Tugwell and G. M. Branch, J. Exp. Mar. Biol. Ecol., 1989, 129, 219 CrossRef CAS.
- R. De Nys, P. D. Steinberg, C. N. Rogers, T. S. Charlton and M. W. Duncan, Mar. Ecol. Prog. Ser., 1996, 130, 135 Search PubMed.
- K. D. Meyer and V. J. Paul, Mar. Ecol. Prog. Ser., 1992, 82, 249 Search PubMed.
- P. Amade and R. Lemee, Aquat. Toxicol., 1998, 43, 287 CrossRef CAS.
- H. Pavia, G. B. Toth and P. Aberg, Ecology, 2002, 83, 891 Search PubMed.
- D. A. Herms and W. J. Mattson, Q. Rev. Biol., 1992, 67, 283 Search PubMed.
- A. G. B. Poore, Mar. Ecol. Prog. Ser., 1994, 107, 113 Search PubMed.
- K. L. Van Alstyne, J. J. McCarthy III, C. L. Hustead and L. J. Kearns, J. Phycol., 1999, 35, 483 CrossRef CAS.
- C. S. Lobban and P. J. Harrison, Seaweed Ecology and Physiology, Cambridge University Press, Cambridge, 1994 Search PubMed.
- D. N. Young, B. M. Howard and W. Fenical, J. Phycol., 1980, 16, 182 CAS.
- J. P. Bryant, F. S. Chapin III and D. R. Klein, Oikos, 1983, 40, 357 CAS.
- P. D. Steinberg, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992, p. 51 Search PubMed.
- G. Cronin and M. E. Hay, Oikos, 1996, 77, 93 CAS.
- H. Ilvessalo and J. Tuomi, Mar. Biol., 1989, 101, 115 CAS.
- J. L. Yates and P. Peckol, Ecology, 1993, 74, 1757 Search PubMed.
- T. M. Arnold, C. E. Tanner and W. I. Hatch, Mar. Ecol. Prog. Ser., 1995, 123, 177 Search PubMed.
- P. Peckol, J. M. Krane and J. L. Yates, Mar. Ecol. Prog. Ser., 1996, 138, 209 Search PubMed.
- H. Pavia and E. Brock, Mar. Ecol. Prog. Ser., 2000, 193, 285 Search PubMed.
- H. Pavia and G. B. Toth, Hydrobiologia, 2000, 440, 299 Search PubMed.
- J. G. Hamilton, A. R. Zangerl, E. H. DeLucia and M. R. Berenbaum, Ecology Lett., 2001, 4, 86 Search PubMed.
- T. C. R. White, Oecologia, 1984, 63, 90.
- P. E. Renaud, M. E. Hay and T. M. Schmitt, Oecologia, 1990, 82, 217.
- G. Cronin and M. E. Hay, Ecology, 1996, 77, 1531 Search PubMed.
- V. J. Paul and W. Fenical, Mar. Ecol. Prog. Ser., 1986, 34, 157 Search PubMed.
- V. J. Paul, M. M. Littler, D. S. Littler and W. Fenical, J. Chem. Ecol., 1987, 13, 1171 Search PubMed.
- W. H. Gerwick, W. Fenical and J. N. Norris, Phytochemistry, 1985, 24, 1279 CrossRef CAS.
- A. A. L. Gunatilaka, V. J. Paul, P. U. Park, M. P. Puglisi, A. D. Gitler, D. S. Eggleston, R. C. Haltiwanger and D. G. I. Kingston, J. Nat. Prod., 1999, 62, 1376 CrossRef CAS.
- M. Masuda, T. Abe, S. Sato, T. Suzuki and M. Suzuki, J. Phycol., 1997, 33, 196 CAS.
- R. C. Bolser and M. E. Hay, Ecology, 1996, 77, 2269 Search PubMed.
- R. B. Taylor, N. Lindquist, J. Kubanek and M. E. Hay, Oecologia, 2003, 136, 412 Search PubMed.
- P. D. Steinberg, Oecologia, 1989, 78, 374.
- N. M. Targett and T. M. Arnold, J. Phycol., 1998, 34, 195 CrossRef CAS.
- K. L. Van Alstyne, J. Chem. Ecol., 1995, 21, 45 Search PubMed.
- J. L. Stern, A. E. Hagerman, P. Steinberg, F. C. Winter and J. A. Estes, J. Chem. Ecol., 1996, 22, 1273 Search PubMed.
- J. Tuomi, H. Ilvessalo, P. Niemela, S. Siren and V. Jormalainen, Bot. Mar., 1989, 32, 505 Search PubMed.
- K. L. Van Alstyne, Mar. Ecol. Prog. Ser., 1989, 56, 169 Search PubMed.
- G. B. Toth and H. Pavia, Mar. Biol., 2002, 140, 403 Search PubMed.
- A. Denton, A. R. O. Chapman and J. Markham, Mar. Ecol. Prog. Ser., 1990, 65, 103 Search PubMed.
- K. L. Van Alstyne, S. L. Whitman and J. M. Ehlig, Mar. Biol., 2001, 139, 201 Search PubMed.
- H. Pavia, G. B. Toth, A. Lindgren and P. Aberg, Phycologia, 2003, in press Search PubMed.
- H. Pavia and P. Aberg, Hydrobiologia, 1996, 326/327, 199 Search PubMed.
- K. L. Van Alstyne, J. J. McCarthy III, C. L. Hustead and D. O. Duggins, Mar. Biol., 1999, 133, 371 CrossRef CAS.
- P. D. Steinberg, Oecologia, 1995, 102, 169.
- K. L. Van Alstyne, J. M. Ehlig and S. L. Whitman, Mar. Ecol. Prog. Ser., 1999, 180, 179 Search PubMed.
- G. B. Toth and H. Pavia, J. Mar. Biol. Ass. U. K., 2002, 82, 3859/1–5 Search PubMed.
- C. A. Pfister and K. L. Van Alstyne, J. Phycol, 2003, 39, 285.
- K. L. Van Alstyne and K. N. Pelletreau, Mar. Ecol. Prog. Ser., 2000, 206, 33 Search PubMed.
- R. Karban and I. T. Baldwin, Induced Responses to Herbivory, The University of Chicago Press, Chicago, 1997 Search PubMed.
- K. L. Van Alstyne, Ecology, 1988, 69, 655 Search PubMed.
- P. Peckol and J. L. Yates, Proc. 8th Int. Coral Reef Symp., 1997, 2, 1259 Search PubMed.
- K. Hammerstrom, M. N. Dethier and D. O. Duggins, Mar. Ecol. Prog. Ser., 1998, 165, 293 Search PubMed.
- P. D. Steinberg, Mar. Ecol. Prog. Ser., 1994, 112, 129 Search PubMed.
- H. Pavia and G. Toth, Ecology, 2000, 81, 3212 Search PubMed.
- G. B. Toth and H. Pavia, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14418 CrossRef CAS.
- E. E. Sotka, R. B. Taylor and M. E. Hay, J. Exp. Mar. Biol. Ecol., 2002, 277, 1 CrossRef.
- T. M. Arnold, N. M. Targett, C. E. Tanner, W. I. Hatch and K. E. Ferrari, J. Phycol., 2001, 37, 1026 CrossRef CAS.
- T. M. Arnold and N. M. Targett, Oikos, 2003, 100, 406.
- T. M. Arnold and N. M. Targett, J. Chem. Ecol., 2000, 26, 1393 Search PubMed.
- M. S. Deal, M. E. Hay, D. Wilson and W. Fenical, Oecologia, 2003, 136, 107 Search PubMed.
- G. Cronin and M. E. Hay, Ecology, 1996, 77, 2287 Search PubMed.
- R. B. Taylor, E. Sotka and M. E. Hay, Oecologia, 2002, 132, 68 Search PubMed.
- V. J. Paul and K. L. Van Alstyne, J. Exp. Mar. Biol. Ecol., 1992, 160, 191 CrossRef CAS.
- K. L. Van Alstyne and L. T. Houser, Mar. Ecol. Prog. Ser., 2003, 250, 175 Search PubMed.
- E. E. Conn, in Herbivores: their interaction with secondary plant metabolites, ed. G. A. Rosenthal and D. H. Janzen, Academic Press, New York, 1979, p. 387 Search PubMed.
- D. S. Seigler, in Herbivores: their interaction with secondary plant metabolites, Vol. I. The chemical participants, 2nd edition, ed. G. A. Rosenthal and M. R. Berenbaum, Academic Press, San Diego, 1991, p. 35 Search PubMed.
- F. S. Chew, in Biologically active natural products: potential use in agriculture, ed. E. D. Cutler, American Chemical Society Symposium Series No. 380, 1988, p. 155 Search PubMed.
- S. Louda and S. Mole, in Herbivores: their interaction with secondary plant metabolites, Vol. I. The chemical participants, 2nd edition, ed. G. A. Rosenthal and M. R. Berenbaum, Academic Press, San Diego, 1991, p. 123 Search PubMed.
- K. Konno, C. Hirayama, H. Yasui and M. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9159 CrossRef CAS.
- G. L. Cetrulo and M. E. Hay, Mar. Ecol. Prog. Ser., 2000, 207, 243 Search PubMed.
- V. Jung and G. Pohnert, Tetrahedron, 2001, 57, 7169 CrossRef CAS.
- M. Gavagnin, A. Marin, F. Castelluccio, G. Villani and G. Cimino, J. Exp. Mar. Biol. Ecol., 1994, 175, 197 CrossRef.
- V. Jung, T. Thibaut, A. Meinesz and G. Pohnert, J. Chem. Ecol., 2002, 28, 2091 Search PubMed.
- V. J. Paul, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992, p. 24 Search PubMed.
- K. L. Van Alstyne, G. V. Wolfe, T. L. Freidenburg, A. Neill and C. Hicken, Mar. Ecol. Prog. Ser., 2001, 213, 53 Search PubMed.
- G. V. Wolfe and M. Steinke, Limnol. Oceanogr., 1996, 41, 1151 Search PubMed.
- G. V. Wolfe, M. Steinke and G. O. Kirst, Nature, 1997, 387, 894 CrossRef CAS.
- N. M. Target and T. M. Arnold, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 391 Search PubMed.
- M. E. Hay, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Comstock Publishing Associates, Ithaca, 1992, p. 93 Search PubMed.
- M. E. Hay and W. Fenical, Oceanography, 1996, 9, 10 Search PubMed.
- R. Maximilien, R. De Nys, C. Holmstrom, L. Gram, S. Kjelleberg and P. D. Steinberg, Aquat. Microb. Ecol., 1998, 15, 233 Search PubMed.
- M. Manefield, T. B. Rasmussen, M. Hentzer, J. B. Andersen, P. Steinberg, S. Kjelleberg and M. Givskov, Microbiology, 2002, 148, 1119 Search PubMed.
- M. Hentzer, H. Wu, J. B. Anderson, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffen, M. Manefield, J. W. Costeron, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby and M. Givskov, EMBO J., 2003, 22, 3803 CrossRef CAS.
- C. Hellio, H. Thomas-Guyon, G. Culioli, L. Piovetti, N. Bourgougnon and Y. Le Gal, Biofouling, 2001, 17, 189 Search PubMed.
- G. M. Nylund and H. Pavia, Mar. Biol., 2003, 143, 875 Search PubMed.
- L. J. Walters, M. G. Hadfield and C. M. Smith, Mar. Biol., 1996, 126, 383.
- C. D. Harvell, K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhaus, R. M. Overstreet, J. W. Porter and G. R. Vasta, Science, 1999, 285, 1505 CrossRef CAS.
- J. Kubanek, P. R. Jensen, P. A. Keifer, M. Cameron Sullards, D. O. Collins and W. Fenical, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6916 CrossRef CAS.
- M. P. Puglisi, L. T. Tan, P. R. Jensen and W. Fenical, Tetrahedron, in press Search PubMed.
- R. De Nys, P. D. Steinberg, P. Willemsen, S. A. Dworjanyn, C. L. Gabelish and R. J. King, Biofouling, 1995, 8, 259 Search PubMed.
- T. M. Schmitt, M. E. Hay and N. Lindquist, Ecology, 1995, 76, 107 Search PubMed.
- T. M. Schmitt, N. Lindquist and M. E. Hay, Chemoecology, 1998, 8, 125 CrossRef CAS.
- R. De Nys, S. A. Dworjanyn and P. D. Steinberg, Mar. Ecol. Prog. Ser., 1998, 162, 79 Search PubMed.
- M. E. Hay, J. Piel, W. Boland and I. Schnitzler, Chemoecology, 1998, 8, 91 CAS.
- M. K. Harper, T. S. Bugni, B. R. Copp, R. D. James, B. S. Lindsay, A. D. Richardson, P. C. Schnabel, D. Tasdemir, R. M. VanWagoner, S. M. Verbitseki and C. M. Ireland, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 3 Search PubMed.
- C. M. Ireland, B. R. Copp, M. P. Foster, L. A. McDonald, D. C. Radisky and J. C. Swersey, in Marine Biotechnology, Volume 1, Pharmaeutical and Bioactive Natural Products, ed. D. H. Attaway and O. R. Zaborsky, Plenum Press, New York, 1993, p. 1 Search PubMed.
- MarinLit, Chem. Dept., University of Canterbury, NZ, 2003 Search PubMed.
- J. T. Wright, K. Benkendorff and A. R. Davis, J. Exp. Mar. Biol. Ecol., 1997, 213, 199 CrossRef.
- J. L. Wulff, in Sponges in time and space, ed. R. W. M. van Soest, T. M. G. van Kempen and J. C. Braekman, A. A. Balkema, Rotterdam, 1994, p. 265 Search PubMed.
- J. L. Wulff, Mar. Biol., 1997, 129, 41 CrossRef.
- M. Dunlap and J. R. Pawlik, Mar. Biol., 1996, 126, 117.
- J. R. Pawlik, Limnol. Oceanogr., 1998, 43, 1396 Search PubMed.
- J. R. Pawlik, B. Chanas, R. J. Toonen and W. Fenical, Mar. Ecol. Prog. Ser., 1995, 127, 183 Search PubMed.
- B. Chanas and J. R. Pawlik, Mar. Ecol. Prog. Ser., 1995, 127, 195 Search PubMed.
- B. Waddell and J. R. Pawlik, Mar. Ecol. Prog. Ser., 2000, 195, 125 Search PubMed.
- J. B. McClintock, D. Swenson, H. Trapido-Rosenthal and L. Banghart, J. Chem. Ecol., 1997, 23, 1607 Search PubMed.
- B. Waddell and J. R. Pawlik, Mar. Ecol. Prog. Ser., 1996, 195, 133 Search PubMed.
- B. Chanas and J. R. Pawlik, Oecologia, 1996, 107, 225.
- E. Burns and M. Ilan, Mar. Ecol. Prog. Ser., 2003, 252, 115 Search PubMed.
- M. S. Hill and A. L. Hill, Biol. Bull., 2000, 202, 86 Search PubMed.
- S. C. Bobzin and D. J. Faulkner, J. Chem. Ecol., 1992, 18, 309 Search PubMed.
- B. Chanas, J. R. Pawlik, T. Lindel and W. Fenical, J. Exp. Mar. Biol. Ecol., 1996, 208, 185 CrossRef.
- M. Assmann, E. Lichte, J. R. Pawlik and M. Koeck, Mar. Ecol. Prog. Ser., 2000, 207, 255 Search PubMed.
- D. M. Wilson, M. Puyana, W. Fenical and J. R. Pawlik, J. Chem. Ecol., 1999, 25, 2811 Search PubMed.
- T. Lindel, H. Hoffmann, M. Hochgurtel and J. R. Pawlik, J. Chem. Ecol., 2000, 26, 1477 Search PubMed.
- S. R. Kelly, P. R. Jensen, T. P. Henkel, W. Fenical and J. R. Pawlik, Aquat. Microb. Ecol., 2003, 31, 175 Search PubMed.
- J. R. Pawlik, G. McFall and S. Zea, J. Chem. Ecol., 2002, 28, 1103 Search PubMed.
- M. Tsoukatou, C. Hellio, C. Vagias, C. Harvala and V. Roussis, Z. Naturforsch., C: Biosci., 2002, 57, 161 Search PubMed.
- J. Kubanek, J. R. Pawlik, T. M. Eve and W. Fenical, Mar. Ecol. Prog. Ser., 2000, 207, 69 Search PubMed.
- J. Kubanek, K. E. Whalen, S. Engel, S. R. Kelly, T. P. Henkel, W. Fenical and J. R. Pawlik, Oecologia, 2002, 131, 125 Search PubMed.
- M. A. Becerro, X. Turon and M. J. Uriz, J. Chem. Ecol., 1997, 23, 1527 Search PubMed.
- R. W. Thacker, M. A. Becerro, W. A. Lumbang and V. J. Paul, Ecology, 1998, 79, 1740 Search PubMed.
- R. W. Newbold, P. R. Jensen, W. Fenical and J. R. Pawlik, Aquat. Microb. Ecol., 1999, 19, 279 Search PubMed.
- J. E. Thompson, P. T. Murphy, P. R. Bergquist and E. A. Evans, Biochem. Syst. Ecol., 1987, 15, 595 CrossRef CAS.
- C. Thoms, M. Horn, M. Wagner, U. Hentschel and P. Proksch, Mar. Biol., 2003, 142, 685 Search PubMed.
- B. Chanas and J. R. Pawlik, Proc. 8th Int. Coral Reef Symp., 1997, 2, 1363 Search PubMed.
- R. G. Kerr and M. Kelly-Borges, in Sponges in time and space, ed. R. W. M. van Soest, T. M. G. van Kempen and J. C. Braekman, A. A. Balkema, Rotterdam, 1994, p. 65 Search PubMed.
- M. A. Becerro, V. J. Paul and J. Starmer, Mar. Ecol. Prog. Ser., 1998, 168, 187 Search PubMed.
- P. Schupp, C. Eder, V. J. Paul and P. Proksch, Mar. Biol., 1999, 135, 573 CrossRef CAS.
- F. B. Furrow, C. D. Amsler, J. B. McClintock and B. J. Baker, Mar. Biol., 2003, 143, 443 Search PubMed.
- E. Burns, I. Ifrach, S. Carmeli, J. R. Pawlik and M. Ilan, Mar. Ecol. Prog. Ser., 2003, 252, 105 Search PubMed.
- N. Lindquist and M. E. Hay, Ecol. Monogr., 1996, 66, 431 Search PubMed.
- N. Lindquist, Mar. Biol., 1996, 126, 745.
- M. J. Uriz, X. Turon, M. A. Becerro and J. Galera, J. Exp. Mar. Biol. Ecol., 1996, 205, 187 CrossRef.
- X. Turon, M. A. Becerro and M. J. Uriz, Oikos, 1996, 75, 33.
- R. Marti, A. Fontana, M. J. Uriz and G. Cimino, J. Chem. Ecol., 2003, 29, 1307 Search PubMed.
- M. Betancourt-Luzano, F. Gonzalez-Farias, B. Gonzalez-Acosta, A. Garcia-Gasca and J. R. Bastida-Zavala, J. Exp. Mar. Biol. Ecol., 1998, 223, 1 CrossRef.
- M. A. Becerro, R. W. Thacker, X. Turon, M. J. Uriz and V. J. Paul, Oecologia, 2003, 135, 91 Search PubMed.
- T. Hattori, S. Matsuo, K. Adachi and Y. Shizuri, Fish. Sci., 2001, 67, 690 Search PubMed.
- M. A. Becerro, N. I. Lopez, X. Turon and M. J. Uriz, J. Exp. Mar. Biol. Ecol., 1994, 179, 195 CrossRef.
- N. L. Thakur and A. C. Anil, J. Chem. Ecol., 2000, 26, 57 Search PubMed.
- D. Kelman, Y. Kashman, E. Rosenberg, M. Ilan, I. Ifrach and Y. Loya, Aquat. Microb. Ecol., 2001, 24, 9 Search PubMed.
- S. Albrizio, P. Ciminiello, E. Fattorusso, S. Magno and J. R. Pawlik, J. Nat. Prod., 1995, 58, 647 CrossRef CAS.
- S. Engel and J. R. Pawlik, Mar. Ecol. Prog. Ser., 2000, 207, 273 Search PubMed.
- R. Teeyapant and P. Proksch, Naturwissenschaften, 1993, 80, 360.
- B. Weiss, R. Ebel, M. Elbrachter, M. Kirchner and P. Proksch, Biochem. Syst. Ecol., 1996, 24, 1 CrossRef CAS.
- R. Ebel, M. Brenzinger, A. Kunze, H. J. Gross and P. Proksch, J. Chem. Ecol., 1997, 23, 1451 Search PubMed.
- M. Puyana, W. Fenical and J. R. Pawlik, Mar. Ecol. Prog. Ser., 2003, 246, 127 Search PubMed.
- R. Teeyapant, P. Kreis, V. Wray, L. Witte and P. Proksch, Z. Naturforsch., C: Biosci., 1993, 84, 644 Search PubMed.
- J. D. Faulkner, M. D. Unson and C. A. Bewley, Pure Appl. Chem., 1994, 66, 1983 CAS.
- M. D. Unson and D. J. Faulkner, Experientia, 1993, 49, 349 Search PubMed.
- M. D. Unson, N. Holland and D. J. Faulkner, Mar. Biol., 1994, 119, 1 CAS.
- C. A. Bewley and D. J. Faulkner, J. Org. Chem., 1994, 59, 4849 CrossRef CAS.
- E. W. Schmidt, C. A. Bewley and D. J. Faulkner, J. Org. Chem., 1998, 63, 1254 CrossRef CAS.
- R. W. Thacker and S. Starnes, Mar. Biol., 2003, 142, 643 Search PubMed.
- S. de C. Cook and P. R. Bergquist, in Systema Porifera: A Guide to the Classification of Sponges, ed. J. N. A. Hooper and R. W. M. Van Soest, Kluwer Academic/Plenum Publishers, New York, 2002, p. 1061 Search PubMed.
- A. E. Flowers, M. J. Garson, R. E. Webb, E. J. Dumdei and R. D. Charan, Cell Tissue Res., 1998, 292, 597 CrossRef CAS.
- M. J. Garson, A. E. Flowers, R. L. Webb, R. D. Charan and E. J. McCaffrey, Cell Tissue Res., 1998, 293, 365 CrossRef CAS.
- O. Gillor, S. Carmeli, Y. Rahamin, Z. Fishelson and M. Ilan, Mar. Biotech., 2000, 2, 213 Search PubMed.
- A. Marin, M. D. Lopez, M. A. Esteban, J. Meseguer, J. Munoz and A. Fontana, Mar. Biol., 1998, 131, 639 CrossRef CAS.
- M. J. Uriz, X. Turon, J. Galera and J. M. Tur, Cell Tissue Res., 1996, 285, 519 CrossRef.
- M. J. Garson, M. P. Zimmermann, C. N. Battershill, J. L. Holden and P. T. Murphy, Lipids, 1994, 29, 509 Search PubMed.
- M. A. Becerro, M. J. Uriz and X. Turon, Recent Res. Dev. Ecol., 2001, 1, 81 Search PubMed.
- H. R. Lasker, Mar. Ecol. Prog. Ser., 1985, 21, 213 Search PubMed.
- C. D. Harvell and T. H. Suchanek, Mar. Ecol. Prog. Ser., 1987, 38, 37 Search PubMed.
- H. R. Lasker and M. A. Coffroth, Mar. Ecol. Prog. Ser., 1988, 43, 285 Search PubMed.
- J. L. Ruesink and C. D. Harvell, Mar. Ecol. Prog. Ser., 1990, 65, 265 Search PubMed.
- K. L. Van Alstyne and V. J. Paul, Coral Reefs, 1992, 11, 155.
- N. H. Vrolijk and N. M. Targett, Mar. Ecol. Prog. Ser., 1992, 88, 237 Search PubMed.
- G. Cronin, M. E. Hay, W. Fenical and N. Lindquist, Mar. Ecol. Prog. Ser., 1995, 119, 177 Search PubMed.
- M. Slattery and J. B. McClintock, Mar. Biol., 1995, 122, 461.
- L. F. Maia, R. d. A. Epifanio, T. Eve and W. Fenical, J. Nat. Prod., 1999, 62, 1322 CrossRef CAS.
- M. Slattery, Chemoecology, 1999, 9, 97 CrossRef.
- R. d. A. Epifanio, L. F. Maia and W. Fenical, J. Braz. Chem. Soc., 2000, 11, 584 Search PubMed.
- M. P. Puglisi, V. J. Paul and M. Slattery, Mar. Ecol. Prog. Ser., 2000, 207, 263 Search PubMed.
- L. L. Koh, T. K. Tan, L. M. Chou and N. K. C. Goh, J Exp. Mar. Biol. Ecol., 2002, 273, 121 CrossRef.
- M. P. Puglisi, V. J. Paul, J. Biggs and M. Slattery, Mar. Ecol. Prog. Ser., 2002, 239, 105 Search PubMed.
- D. Kelman, Y. Benayahu and Y. Kashman, J. Exp. Mar. Biol. Ecol., 1999, 238, 127 CrossRef CAS.
- M. Slattery, M. T. Hamann, J. B. McClintock, T. L. Perry, M. P. Puglisi and W. Y. Yoshida, Mar. Ecol. Prog. Ser., 1997, 161, 133 Search PubMed.
- C. D. Harvell, W. Fenical, V. Roussis, J. L. Ruesink, C. C. Griggs and C. H. Greene, Mar. Ecol. Prog. Ser., 1993, 93, 165 Search PubMed.
- J. C. Coll, B. F. Bowden, A. Heaton, P. J. Scheur, M. K. W. Li, J. Clardy, G. K. Schulte and J. Finer-Moore, J. Chem. Ecol., 1989, 15, 1177 Search PubMed.
- J. C. Coll, B. F. Bowden, D. M. Tapiolas, R. H. Willis, P. Djura, M. Streamer and L. Trott, Tetrahedron, 1985, 41, 1085 CrossRef CAS.
- P. W. Sammarco and J. C. Coll, in Bioorganic Marine Chemistry, Volume 2, ed. P. J. Scheuer, Springer-Verlag, Berlin, 1988, p. 87 Search PubMed.
- W. Fenical and J. R. Pawlik, Mar. Ecol. Prog. Ser., 1991, 75, 1 Search PubMed.
- J. R. Pawlik and W. Fenical, Mar. Ecol. Prog. Ser., 1992, 87, 183 Search PubMed.
- C. D. Harvell, J. M. West and C. Griggs, Invertebr. Reprod. Dev., 1996, 30, 239 Search PubMed.
- M. Slattery, G. A. Hines, J. Starmer and V. J. Paul, Coral Reefs, 1999, 18, 75 CrossRef.
- J. R. Pawlik, M. T. Burch and W. Fenical, J. Exp. Mar. Biol. Ecol., 1987, 108, 55 CrossRef.
- W. O'Neal and J. R. Pawlik, Mar. Ecol. Prog. Ser., 2002, 240, 117 Search PubMed.
- L. L. Koh, N. K. C. Goh, L. M. Chou and Y. W. Tan, J Exp. Mar. Biol. Ecol., 2000, 251, 103 CrossRef.
- T. Eve, Ph.D. Dissertation, University of California, San Diego, 2001 Search PubMed.
- J. M. West, Biol. Bull., 1997, 192, 279 Search PubMed.
- J. M. West, Evol. Ecol., 1998, 12, 803 Search PubMed.
- J. M. West, C. D. Harvell and A.-M. Walls, Mar. Ecol. Prog. Ser., 1993, 94, 61 Search PubMed.
- R. S. Thorton and R. Kerr, J. Chem. Ecol., 2002, 28, 2083 Search PubMed.
- M. M. Bandurraga and W. Fenical, Tetrahedron, 1985, 41, 1057 CrossRef CAS.
- D. J. Gerhart, D. Rittsschoff and S. W. Mayo, J. Chem. Ecol., 1988, 14, 1905 Search PubMed.
- M. Slattery, J. B. McClintock and J. N. Heine, J. Exp. Mar. Biol. Ecol., 1995, 190, 61 CrossRef CAS.
- T. L. Aceret, P. W. Sammarco and J. C. Coll, Mar. Biol., 1995, 122, 317 CAS.
- K. Kim, Coral Reefs, 1998, 13, 75.
- P. R. Jensen, C. D. Harvell, K. Wirtz and W. Fenical, Mar. Biol., 1996, 125, 411.
- D. Kelman, A. Kushmaro, Y. Loya, Y. Kashman and Y. Benayahu, Mar. Ecol. Prog. Ser., 1998, 169, 87 Search PubMed.
- K. Kim, C. D. Harvell, P. D. Kim, G. W. Smith and S. M. Merkel, Mar. Biol., 2000, 136, 259 CrossRef.
- M. Maida, A. R. Carroll and J. C. Coll, J. Chem. Ecol., 1993, 19, 2285 Search PubMed.
- D. Kelman, Y. Benayahu and Y. Kashman, J. Chem. Ecol., 2000, 26, 1123 Search PubMed.
- P. A. Leone, B. F. Bowden, A. R. Carroll and J. C. Coll, Mar. Biol., 1995, 122, 675 CAS.
- M. Slattery, J. Starmer and V. J. Paul, Mar. Biol., 2001, 138, 1183 Search PubMed.
- K. L. Van Alstyne, C. R. Wylie and V. J. Paul, J. Exp. Mar. Biol. Ecol., 1994, 178, 17 CrossRef CAS.
- C. R. Wylie and V. J. Paul, J. Exp. Mar. Biol. Ecol., 1989, 129, 141 CrossRef.
- D. Dube, K. Kim, A. P. Alker and C. D. Harvell, Mar. Ecol. Prog. Ser., 2002, 231, 139 Search PubMed.
- K. Michalek-Wagner, D. J. Bourne and B. F. Bowden, Mar. Biol., 2001, 138, 753 Search PubMed.
- K. Michalek-Wagner and B. F. Bowden, J. Chem. Ecol., 2000, 26, 1543 Search PubMed.
- B. S. Davidson, Chem. Rev., 1993, 93, 1771 CrossRef CAS.
- D. Stoecker, Mar. Ecol. Prog. Ser., 1980, 3, 257 Search PubMed.
- S. L.-M. Teo and J. S. Ryland, Mar. Biol., 1994, 120, 297.
- D. P. Pisut and J. R. Pawlik, J. Exp. Mar. Biol. Ecol., 2002, 270, 203 CrossRef CAS.
- V. J. Paul, N. Lindquist and W. Fenical, Mar. Ecol. Prog. Ser., 1990, 59, 109 Search PubMed.
- J. B. McClintock, J. Heine, M. Slattery and J. Weston, J. Exp. Mar. Biol. Ecol., 1991, 147, 163 CrossRef CAS.
- N. Lindquist, M. E. Hay and W. Fenical, Ecology, 1995, 76, 1347 Search PubMed.
- H. Vervoort, P. R. Jensen and W. Fenical, Mar. Ecol. Prog. Ser., 1998, 164, 221 Search PubMed.
- A. R. Davis, Mar. Biol., 1991, 111, 375.
- A. R. Davis and J. B. Bremner, in Recent Advances in Marine Biotechnology, Volume III, ed. M. Fingerman, R. Nagabhushanam and M. F. Thompson, Science Publishers, Inc., New Hampshire, 1999, p. 259 Search PubMed.
- M. Wahl, P. R. Jensen and W. Fenical, Mar. Ecol. Prog. Ser., 1994, 110, 45 Search PubMed.
- P. J. Bryan, J. B. McClintock, M. Slattery and D. P. Rittschof, Biofouling, 2003, 19, 235 Search PubMed.
- C. M. Young and B. L. Bingham, Mar. Biol., 1987, 96, 539 CAS.
- C. E. Salomon and D. J. Faulkner, J. Nat. Prod., 2002, 65, 689 CrossRef CAS.
- W. E. G. Muller, A. Maidhof, R. K. Zahn, J. Conrad, T. Rose, P. Stephanovich, I. Muller, U. Friese and G. Uhlenbruck, Biol. Cell, 1984, 51, 381.
- B. M. Degan, C. J. Hawkins, M. F. Lavin, E. J. Mc Caffrey, D. L. Parry, A. L. van den Breck and D. J. Watters, J. Med. Chem., 1989, 32, 1349 CrossRef CAS.
- B. M. Degan, C. J. Hawkins, M. F. Lavin, E. J. Mc Caffrey, D. L. Parry, A. L. van den Breck and D. J. Watters, J. Med. Chem., 1989, 32, 1354 CrossRef CAS.
- J. F. Baird, C. Grivois, J. F. Verbist, C. Debitus and J. B. Carre, J. Mar. Biol. Assoc., U. K., 1990, 70, 741 Search PubMed.
- H. L. Sings and K. L. Rinehart, J. Ind. Microbiol. Biotech., 1996, 17, 385 CrossRef CAS.
- C. E. Kicklighter, J. Kubanek, T. Barsby and M. E. Hay, Mar. Ecol. Prog. Ser., 2003, 263, 299 Search PubMed.
- G. R. Gaston and M. Slattery, Bull. Mar. Sci., 2002, 70, 891 Search PubMed.
- T. Barsby, C. E. Kicklighter, M. E. Hay, M. C. Sullards and J. Kubanek, J. Nat. Prod., 2003, 66, 1110 CrossRef CAS.
- C. E. Kicklighter and M. E. Hay, Limnol. Oceaonogr., in press Search PubMed.
- R. G. Kerr, A. C. Kohl and J. M. Boehnlein, in Recent Advances in Marine Biotechnology, Volume 6, Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science publishers, Inc., Enfield, 2001, p. 149 Search PubMed.
- A. J. Blackman and J. T. Walls, Stud. Nat. Prod. Chem., 1995, 17, 73 Search PubMed.
- P. J. Bryan, J. B. McClintock and T. S. Hopkins, J. Exp. Mar. Biol. Ecol., 1997, 210, 173 CrossRef CAS.
- J. B. McClintock, A. R. Mahon, K. J. Peters, C. D. Amsler and B. J. Baker, Antarct. Sci., 2003, 15, 339 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |