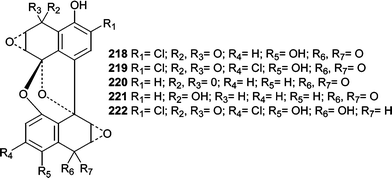Marine-derived fungi: a chemically and biologically diverse group of microorganisms
Tim S.
Bugni
and
Chris M.
Ireland
*
Department of Medicinal Chemistry, University of Utah, Salt Lake City, UT 84112, USA. E-mail: cireland@pharm.utah.edu; Fax: 801-585-6208; Tel: 801-581-8305
First published on 9th January 2004
Abstract
Covering: through 2002
A diverse array of secondary metabolites have been isolated and characterized from marine-derived fungi. The structures and biological activities of these metabolites are presented. Additionally, some basic principles of mycology are covered. Overall, 273 structures are presented and the review contains 162 references.
 Tim S. Bugni | Dr Bugni received a B.S. degree in Chemistry from Montana Tech of the University of Montana in 1995. He worked as a research specialist for three years at Montana Tech under the supervision of Dr Andrea A. Stierle and Professor Donald B. Stierle. He received a Ph.D. degree in Medicinal Chemistry from the University of Utah in 2003 under the direction of Professor Chris M. Ireland. Currently, he is a Postdoctoral Research Chemist at Scripps Institution of Oceanography under the guidance of Professor William Fenical. |
 Chris M. Ireland | Dr Ireland received a B.A. degree in Chemistry from UCSD in 1973 and a Ph.D. degree in Oceanography from Scripps Institution of Oceanography in 1977 under the direction of Professor D. John Faulkner. He worked for two years at the University of Hawaii as an NIH postdoctoral fellow under the guidance of Professors Paul Scheuer and Mike Hadfield, followed by a three-month visit with Professor Ken Rinehart at the University of Illinois, Urbana-Champaign. In March 1980, he joined the faculty of the University of Connecticut, School of Pharmacy as an Assistant Professor. In 1983 he moved to the University of Utah, College of Pharmacy, where his current appointment is Professor and Chair, Department of Medicinal Chemistry. |
1 Introduction
Marine microorganisms have become an important source of pharmacologically active metabolites. Published reviews show the importance of these organisms as potential sources of pharmaceutical leads.1–8 More specifically, fungi from the marine environment have shown great potential as suggested by the diversity of secondary metabolites summarized in this review.The intent of this review is to summarize the new marine-derived fungal metabolites that have been reported in the literature. Every attempt was made to thoroughly search the available literature. Compounds that have novel carbon skeletons and/or biomedical potential will be discussed in greater detail. The sections of this review that cover ecological roles of fungi in the marine environment and taxonomic considerations of marine-derived fungi are intended to be introductory. In addition, most fungi will be referred to as “marine-derived” since the marine ecosystem is comprised of fungi that are obligate marine fungi and facultative species that are found in terrestrial ecosystems as well. A more detailed discussion about marine fungi and obligate marine species has been presented by Kohlmeyer and Kohlmeyer, 1979.9 Briefly, obligate marine fungi grow and sporulate in a marine environment while facultative marine fungi are from terrestrial or freshwater habitats, but may also grow in the marine environment.
2 Historical perspective
Historically, fungi have influenced many aspects of human culture and development. One of the most notable effects of fungi on human culture is St. Anthony's fire which was caused by ergot alkaloids found in Claviceps purpurea-infected rye. During the middle ages, ergotism outbreaks were severe and resulted in mania, hallucinations, swollen flesh, and loss of limbs. Although ergot alkaloids cause detrimental effects at high doses, they have medicinally useful properties, such as inducing labor, at low doses. Further studies on the use of ergotamine, a major active constituent of C. purpurea, demonstrated ergotamine to be an effective migraine treatment and increased our understanding of migraine headaches while aiding the design of effective treatments.10Although the ergot alkaloids have interesting medicinal properties, antibiotic production by fungi provided the stimulus for an entire era of drug discovery research. The earliest report indicating the antibacterial potential of fungi occurred in 1876 when Tyndall described the antagonistic effect of a Penicillium sp. on bacteria.11 In 1896, Gosio reported that he had isolated small amounts of a crystalline substance that had antibacterial properties. The compound was later confirmed to be mycophenolic acid.11 Although there were numerous reports describing antibiotic properties of fungi in the late 1800s and early 1900s, the landmark discovery came in 1929 when Sir Alexander Fleming described the effects of Penicillium notatum and penicillin on bacteria. Unfortunately, the importance of his work was not fully realized until the early 1940s when a group at Oxford began investigating penicillin for use as an antibiotic in humans. After the potential of penicillin was elaborated, Giuseppe Brotzu, in 1945, began investigating seawater samples near a sewage outlet in Sardinia for antibiotic producing microbes.12 Brotzu hypothesized that self-purification of water might be due in part to bacterial antagonism. He obtained a fungus from a seawater sample that exhibited antibacterial activity and noted that it was similar to Cephalosporium acremonium (now named Acremonium chrysogenum). This observation led to the discovery of cephalosporin C. In the intervening years, the actinomycetes became the primary focus of drug discovery work, but interest in fungi resurged when cyclosporin A was isolated from Tolypocladium inflatum in 1976 and was approved for clinical use as an immunosuppressant in 1983. However, the rediscovery of high numbers of previously described metabolites has to some extent precluded the study of traditional terrestrial sources of fungi and led researchers to explore unique habitats, such as the marine environment, for fungi with potentially new biosynthetic diversity.
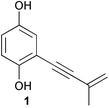
Although cephalosporin C was isolated from a marine-derived fungus, studies reporting chemistry from marine-derived fungi were rare until the 1990s. A few studies were performed between 1970 and 1990 followed by a nearly exponential growth through the 1990s. Although siccayne (1) was most likely the first antibiotic isolated from an obligate marine fungus, the compound had been previously isolated from a terrestrial species.13 Overall, research on marine-derived fungi has led to the discovery of 272 new natural products including many that have novel carbon skeletons, thus, providing evidence that marine-derived fungi have the potential to be a rich source of pharmaceutical leads.
3 Taxonomy
The proper identification and classification of fungi is critical to the study of natural products. Without proper identification and preservation of fungal isolates, chemical investigations of fungi become difficult if not impossible to reproduce. Additionally, proper characterization of fungi combined with reported chemistry can provide additional tools for mycologists to classify fungi.The field of mycology has traditionally studied organisms from three separate Kingdoms, Protozoa, Straminipila, and Fungi (true fungi). Cellular slime molds and plasmodial slime molds (myxomycetes) belong to the Kingdom Protozoa. The straminipiles, closely related to true fungi, include the oomycetes (water molds). The oomycetes were originally classified under the Kingdom Fungi, but were reclassified under the Kingdom Chromista in 1995,14 and more recently reclassified in the new Kingdom Straminipila.15 The common name “zoosporic fungi” is still used to describe the straminipiles. Major distinctions that separate straminipiles from true fungi are the presence of biflagellate zoospores, cell walls that contain cellulose (true fungi do not contain cellulose), and oomycetous cell wall proteins that contain hydroxyproline as opposed to proline for the true fungi. The Kingdom Fungi16 (true fungi) includes the following four major phyla: Chytridiomycota, Zygomycota, Ascomycota and the Basidiomycota. The deutoromycetes or “fungi imperfecti” are an additional group within the Kingdom Fungi, but they are not recognized as a formal phylum.
Although fungi have historically been identified and classified primarily by morphological characteristics, mycologists now employ a number of techniques to help identify fungi and to organize fungal systematics. Morphology still plays a major role in taxonomy, but the additional use of molecular data is becoming more common. Molecular data such as DNA GC content, RAPD (random amplified polymorphic DNA) fingerprints, RFLP (restriction fragment length polymorphism) analyses, and the use of different DNA sequences are now common and combined with WWW searchable databases have made identification of known species more consistent, faster, and easier for the non-specialist.17–19 Additionally, DNA macroarray techniques have also been utilized for field identification of marine yeasts.20
The use of ribosomal DNA (rDNA) sequences has become one of the most useful techniques to aid in fungal identification and to study phylogenetics. The ribosomal gene cluster (see Fig. 1) is comprised of three regions coding for the 5.8S, 18S, and 28S ribosomal RNA genes. Although these genes are present as tandem repeats, they evolve as a single unit even though the rates of evolution vary within individual regions of the ribosomal RNA gene cluster and therefore can be used to compare organisms at several levels.21 Since the ITS regions are fairly divergent and vary between species within a genus, they can be very useful in taxonomy. Using ITS1 and ITS4 primers, the ITS1-5.8S-ITS2 region (∼550 base pairs) can be amplified by PCR and subsequently sequenced. The sequence can be compared to GenBank and taxonomic information obtained. Ribosomal gene sequence data has been used to study Fusarium sp.,22Aspergillus sp.,23 and Penicillium sp.24 as well as other groups. Additionally, details that describe the methods for PCR amplification of the nuclear and mitochondrial fungal ribosomal RNA genes have been summarized by White et al. including a list of common primers.25 Additional lists of primers have been published by S. W. Peterson.24 Although the ITS1-5.8S-ITS2 sequence is commonly used as a taxonomic tool, the IGS region is the most divergent and may be useful for closely related species that have identical or nearly identical ITS sequences.26
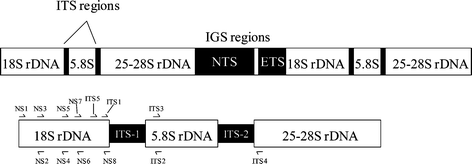 | ||
| Fig. 1 Fungal rDNA gene cluster with an expansion showing common primer sites. | ||
Chemotaxonomy could also be quite useful for researchers in the field of natural products. HPLC profiles have been generated for a number of Penicillium sp.27 and can provide information about the chemistry present. Additionally, an outline of common mycotoxins and their connection to correctly identified Penicillia and Aspergilli has been presented.28 A more detailed account of chemical approaches to taxonomy of fungi have been covered elsewhere.29 Incorporating chemotaxonomy into fungal natural products research should be very useful for comparing strains and dereplication of known metabolites.
4 Ecological roles and effects of fungi in the marine environment
Fungi are heterotrophic eukaryotes that play a major role in the decomposition of dead plant tissues (cellulose and lignan) and to a lesser extent animal tissues such as keratin and chitin. The decomposition liberates nutrients back into the ecosystem. Fungi have evolved biologically and biochemically in a diverse manner that has allowed them to utilize various solid substrates. Although many ecological roles for fungi in the terrestrial ecosystem have been described and thoroughly studied, the ecology of fungi in the marine environment has been more difficult to study. The following section will highlight several examples that demonstrate the importance of fungi in marine ecosystems.In mangrove habitats, the straminipiles play an important ecological role in recycling nutrients. Of the straminipiles, oomycetes are among the first microorganisms to colonize fallen leaves from mangrove trees.30 The oomycetes' role requires them to be highly versatile due to the ever changing environment of many mangrove habitats. A detailed review that outlines the roles of straminipiles in mangrove habitats has recently been published.31
Many studies involving the ecology of marine fungi have been initiated due to the pathogenicity of certain fungal species. For example, Caribbean sea-fan mortalities have been attributed to infection by pathogenic strains of the genus Aspergillus.32 Although Aspergillus spp. are normally considered terrestrial species, the genus is tolerant to high salt concentrations and low water activity.33 Other fungi thought to be terrestrial species can have effects on the marine ecosystem. For example, Fusarium solani is known for its ability to infect various marine crustaceans.34 The oomycete Lagenidium callinectes is known for its pathogenicity toward blue crab eggs and many cultured crab and lobster eggs.35
Although many diseases and infections in the marine environment can be attributed to fungi, other cases indicate that mutualistic relationships between a host and a fungus exist. For example, the ascomycete Turgidosculum ulvae has only been found growing in the thallus of the green alga Blidingia minima.9 The presence of T. ulvae causes infected regions to become black and leathery; invertebrates that normally graze on the alga will not eat the fungal-infected portions of the alga. Therefore, the relationship could be mutually beneficial to both organisms. In the case of the alga Ascophyllum nodosum and the endosymbiotic fungus Mycosphaerella ascophylli, both the host and the fungus appear to depend on each other for survival as neither appear to occur separately in nature.36 Endolithic fungi and algae (Ostreobium queketti) appear to coexist with live polyp tissue of the coral Porites lobata.37 Interestingly, the fungus is taxonomically similar to terrestrial Aspergilli. Another interesting observation is that species belonging to the genus Koralionastes are always found in close association with encrusting sponges.38 Future research focused on the relationships between fungi and their hosts in the marine environment will help our understanding of the marine ecosystem and could lead to improved collection methods and the isolation of chemically unexplored species.
5 Fermentation studies
Halotolerant marine fungal species have evolved unique metabolic mechanisms that are responsive to salt concentrations. For fungi to grow in the marine environment, they must have osmoregulatory mechanisms that signal the production of polyols and amino compounds in conjunction with increasing the concentration of cytoplasmic ions. Since the biosynthesis of these solutes for osmoregulation is energetically costly, fungi may exhibit decreased secondary metabolite production or slower rates of metabolite production in the presence of high salt concentrations.Interestingly, a survey of the literature shows only two reports that investigate antimicrobial metabolite production of marine-derived fungi in response to varying salt concentrations.39,40 These initial findings show that some marine-derived species exhibit increased growth with increasing seawater concentration in the medium while antimicrobial activity appeared to be maximal in media containing 25–50% seawater. Even species that would be classified as terrestrial species show these trends. However, it has been demonstrated that preculturing of some terrestrial fungi on agar containing 1.0 M sodium chloride can lead to increased growth in a salt containing production medium.41 Overall these findings suggest that marine-derived fungal metabolite production could be sensitive to seawater concentration and could have implications for drug discovery from marine-derived fungi.
5.1 Personal studies
In a study performed by our group, nine Fijian fungal isolates were selected for the fermentation study (see Table 1). Each of the nine isolates was identified at the genus level. Each isolate was grown in 15 mL of ½ concentration potato dextrose broth (PDB) that was made with varying percentages of artificial seawater (ASW) (0%, 20%, 40%, 60%, 80% and 100%) in 50 mL fermentation flasks. For conditions that were less than 100% ASW, the other percentage was distilled water. ASW was used instead of natural seawater because the exact ion concentrations are more consistent with ASW, as is media reproducibility. Identical cultures for each of the four time points at each ASW concentration were inoculated with 750 µL of 4-day seed cultures. Cultures were shaken at 200 rpm at 22 °C and harvested after 7, 10, 12, and 14 days of growth. Extracts were screened against the following infectious microorganisms: methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive S. aureus (MSSA), vancomycin-resistant Enterococcus facium (VREF), E. coli, and Candida albicans.| Isolate | Source | Genus |
|---|---|---|
| F97S11 | Aplidium sp. tunicate | Aspergillus |
| F97S28 | Didemnum sp. tunicate | Beauveria |
| F97S13 | White finger sponge | Penicillium |
| F97S44 | Acabaria sp. soft coral | Penicillium |
| F97I33 | Dendronephthya sp. soft coral | Penicillium |
| F97S75 | Zyzzya sp. sponge | Penicillium |
| F97I21 | Dendronephthya soft coral | Penicillium |
| F97S76 | Zyzzya sp. sponge | Penicillium |
| F97I14 | Palythoa sp. coelenterate | Cladosporium |
Six of the nine fungi exhibited ASW dependent metabolite production as determined by antimicrobial activity and HPLC profiles. Most fungi showed decreased metabolite production at 80 and 100% ASW. Penicillium brocae (isolate F97S76) showed interesting responses to ASW concentrations (see Table 2). When cultures were harvested at 10 days the activity profile showed that cultures in 20–60% ASW exhibited activity against MRSA while cultures containing 80 and 100% ASW exhibited activity against MSSA suggesting a change in the type of metabolites produced.
Fig. 2 shows the profound effect of ASW on metabolite production of a Penicillium sp. (isolate F97S75). The peak eluting at ∼8 min substantially increases upon addition of ASW into the growth medium followed by a marked decrease in production at 80%. Although initial MS and NMR analyses of the compound indicate that it is a secondary metabolite, the compound was not stable and decomposed prior to complete structural elucidation.
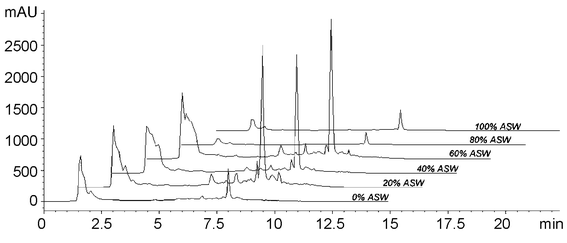 | ||
| Fig. 2 HPLC traces of isolate F97S75 extracts that were harvested on day 10. | ||
Isolate F97I21 was identified as a Penicillium sp. and shows a strong correlation between the addition of ASW and antimicrobial activity (see Fig. 3). The activity profile clearly demonstrates that active metabolites are produced in higher amounts when the medium contains 20–40% ASW. HPLC profiles (data not shown) are also consistent with the activity profile, but it is difficult to unambiguously determine which metabolite is responsible for the activity.
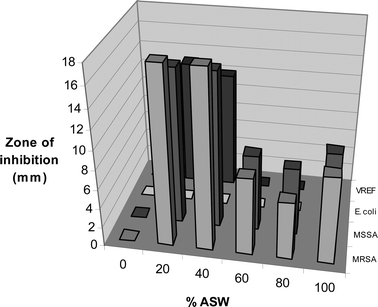 | ||
| Fig. 3 Effect of ASW on antimicrobial activity of F97I21 at day 10. | ||
Isolate F97S11, an Aspergillus niger, showed a decrease in antimicrobial activity with increasing ASW. For example, day 10 extracts showed 12 mm zones of inhibition against S. aureus at 0% ASW while yielding only 7 mm zones at 60, 80 and 100% ASW. More dramatic results were observed for the 14-day cultures, but were nonetheless consistent with the 7-day cultures (see Table 3).
When harvested after 12 and 14 days, F97S13 showed similar antimicrobial activities at 0 and 20% and 80 and 100% while exhibiting minimal antimicrobial activity at 40 and 60% (see Table 4 and Table 5). It would be interesting to see if the antimicrobial metabolites produced at higher concentrations of ASW are utilized for osmoregulation.
Similar to the results reported by Masuma et al.,39 these studies indicate that generally, fermentation of marine-derived fungi in a medium containing 20–60% ASW leads to increased production of antimicrobial metabolites. Interestingly, all isolates in this study and the identified isolates in the study by Masuma et al. are generally considered terrestrial species. These initial results indicate that fungal metabolite production for many species may be enhanced by the addition of higher concentrations of a more diverse array of ions, such as those found in ASW.
5.2 Distribution of marine-derived fungi
The distribution of fungi, as described in this section, is based solely on reports that describe chemical investigations of marine-derived fungi. Although this will provide a general bias in that many marine mycological reports will not be included, this sort of analysis will hopefully reveal areas that have not been adequately investigated and that represent valuable resources for underexplored fungal habitats and discovery of new natural products. The distribution of fungi will be categorized based on the source of the fungus and, it is hoped, will reveal trends linking fungal sources to biosynthetic diversity.The distribution of fungi has been graphically summarized based on the number of distinct genera described from a marine source in the natural product literature (See Fig. 4). From the graph, it is apparent that sponges have yielded the greatest taxonomic diversity. One key question that remains unanswered is whether or not fungi are living in sponge or other marine invertebrate tissues. If so, has the marine environment effected secondary metabolite production in these fungi? If fungi are not living in sponge tissues, then the sponges may only be harboring spores and/or fungal hyphae that remain dormant until proper nutrient conditions are encountered for growth.
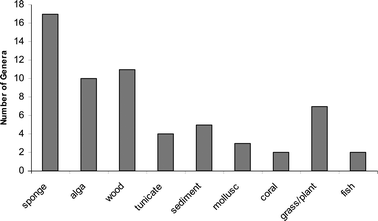 | ||
| Fig. 4 The number of distinct fungal genera based on the marine source. | ||
Fungi obtained from either sponges, algae, or wood substrates account for the majority of chemistry described (70%, see Fig. 5). Interestingly, sponge-derived fungi account for the largest number (33%) of total compounds in the literature and have the overall highest number of novel metabolites (see Fig. 6). Algicolous fungi are second accounting for 24% of the total number of compounds, but represent a slightly higher percentage (27%) of new metabolites. Additionally, the ratio of new metabolites to known metabolites is much higher for algicolous fungi (3.1 : 1) as compared to sponge-derived fungi (1.4 : 1). Unfortunately, known compounds are not always reported. Tunicate-derived fungi only account for 5% of the total number of reported compounds representing 7% of the novel compounds.
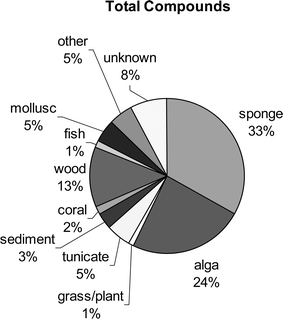 | ||
| Fig. 5 The distribution of all compounds reported from marine-derived fungi is shown as a function of the fungal source. Other represents sources that were too small to represent a significant number of metabolites. | ||
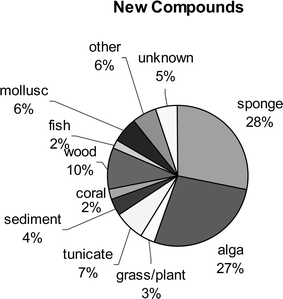 | ||
| Fig. 6 The distribution of new compounds reported from marine-derived fungi is shown as a function of the fungal source. | ||
6 Chemistry and biological activities
Overall, marine-derived fungi are a source of significant chemical diversity and this fact is supported by the 272 new compounds that have been isolated and described, thus far. As illustrated in Fig. 7, research on marine-derived fungi blossomed in the 1990s, and notably since 1998. Although the annual number of reported new compounds was highest in 1998 and 2000, the general trend indicates that the number of new metabolites is still on the rise.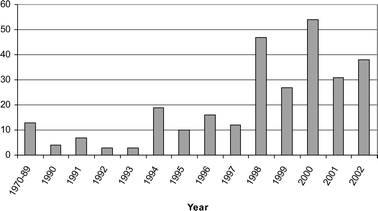 | ||
| Fig. 7 Number of new compounds reported annually. | ||
Biological activities are mainly focused in the areas of antibiotic and anticancer properties, but other selective activities include cell cycle inhibition, antagonism of platelet activating factor, antiviral activity, neuritogenic activity, phosphatase inhibition and kinase inhibition, and radical scavenging activities. The organization of the following sections is based on the source of the fungus (sponge, alga, etc.). Within each group the chemistry will be organized according to biological activity. Only compounds that were novel at the time of publication are presented unless otherwise noted.
6.1 Antimicrobial metabolites from sponge-derived fungi
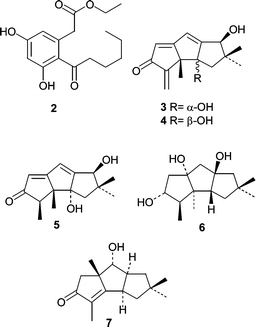
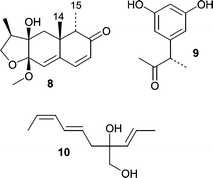
The polyketide 14,15-secocurvularin (2)42 was described as being mildly antibiotic against Bacillus subtilis when compared to tetracycline. 14,15-Secocurvularin (2) was isolated from the saltwater culture of an unidentified fungus obtained from an Indonesian encrusting sponge, Spirastrella vagabunda. The salt water culture of another unidentified fungus obtained from a Haliclona sp. sponge was shown to produce several new hirsutane sesquiterpenes hirsutanols A–C (3–5) and ent-gloeosteretriol (6).43 Hirsutanols are biosynthetically related to several compounds reported from the terrestrial fungus Coriolus consors, and this prompted the authors to investigate several C. consors isolates obtained from the ATCC. A seawater-based culture of C. consors ATCC 66132 produced hirsutanol D (7) demonstrating that the use of seawater with terrestrial fungi can yield new metabolites. Hirsutanol A (3) and ent-gloeosteretriol (6) exhibited mild antibiotic activity against B. subtilis.A Microsphaeropsis sp. was obtained from the sponge Myxilla incrustans and produced the novel metabolite microsphaeropsin (8), which is an eremophilane derivative that showed antifungal activity at the 50 µg level.44 Interestingly, microsphaeropsin (8) contains a CH3-14 and CH3-15 anti-relationship that is rare among the eremophilane terpenes. In the same report,44 two additional novel metabolites, (3S)-(3′,5′-dihydroxyphenyl)butan-2-one (9) and 2-(1′(E)-propenyl)-octa-4(E),6(Z)-diene-1,2-diol (10) were described from a Coniothyrium sp. fungus from the Carribbean sponge, Ectyoplasia ferox (the name of this sponge was misspelled in the original manuscript as Ectyplasia perox).
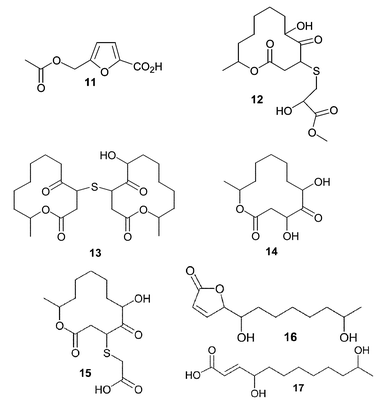
Acetyl Sumiki's acid (11) was isolated from a seawater-based fermentation of a Cladosporium herbarum isolate obtained from the marine sponge Callyspongia aerizusa.45 Both Sumiki's acid and its acetyl derivative showed activity against B. subtilis and S. aureus at 5 µg/disc. In addition, the fungus produced two novel twelve-membered macrolides, pandangolides 3 (12) and 4 (13), along with other known macrolides. Interestingly, an unidentified fungus obtained from an orange encrusting sponge from Indonesia was the original source of pandangolides 1 (14) and 2 (15) as well as the hexaketides iso-cladospolide B (16) and seco-patulolide C (17).46 In both cases the macrolides were inactive when tested against both gram-positive and gram-negative bacteria.
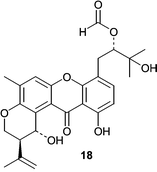
Varixanthone (18) was isolated from a sponge-derived Emericella variecolor (anamorph Aspergillus variecolor) and displayed MIC values of 12.5 µg mL−1 against E. coli, Proteus sp., B. subtilis, and S. aureus while exhibiting lower potency against Enterococcus faecalis (MIC = 50 µg mL−1).47
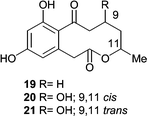
HPLC-NMR-CD was used for preliminary characterization of three active metabolites, xestodecalactones A–C (19–21) from the fungus Penicillium cf. montanense that was derived from the sponge Xestospongia exigua.48 The authors were able to collect an “online” COSY and ROESY using the WET sequence for solvent suppression, but the xestodecalactones had to be purified for a complete unambiguous structure determination. Although none of the xestodecalactones show antibacterial activity, xestodecalactone B (19) produced 25, 12, and 7 mm zones of inhibition against Candida albicans at 100, 50, and 20 µmol, respectively.
6.2 Cytotoxic metabolites from sponge-derived fungi
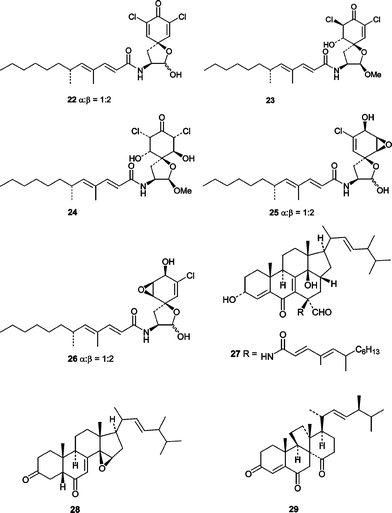
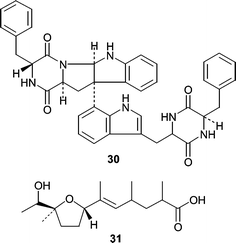
Atsushi Numata's group at Osaka University of Pharmaceutical Sciences was the first to report novel cytotoxic metabolites from a sponge-derived fungus.49 The initial report described the elucidation of the structurally unusual cytotoxic gymnastatins A (22), B (23), and C (24) that were obtained from a strain of the ascomycete Gymnascella dankaliensis derived from the sponge Halichondria japonica. The absolute stereostructures of gymnastatins A–C (22–24) along with gymnastatins D (25) and E (26) were described in a subsequent report.50 Gymnastatins A (22), B (23), and C (24) exhibited potent cytotoxicity in a P388 lymphocytic leukemia test system with ED50 values of 18, 108, and 106 ng mL−1, respectively. Gymnastatins D (25) and E (26), which lack the ketone are only weakly cytotoxic with ED50 values of 10.5 and 10.8 µg mL−1, respectively. Gymnastatins A (22) and B (23) could act as potent cytotoxins due to the presence of a Michael acceptor, but at first glance that would not explain the potent cytotoxicity observed for gymnastatin C (24). The authors have suggested that gymnastatin C (24) could form an enone system (Michael acceptor) in situ through dehydration to yield gymnastatin B (23). This hypothesis is consistent with similar ED50 values for B (23) and C (24). In addition to the gymnastatins, the unusual ergostanoids gymnasterones A (27) and B (28)51 as well as dankasterone (29)52 were isolated from G. dankaliensis and shown to be weakly cytotoxic.Asperazine (30), isolated from a Hyrtiosproteus sponge-derived Aspergillus niger showed selective cytotoxicity against leukemia cells while exhibiting no antifungal (C. albicans) or antibacterial (B. subtilis) activity, suggesting asperazine (30) has a specific mammalian target. Structurally, asperazine is a dimerized diketopiperazine biosynthetically derived from two phenylalanines (R and S) and two tryptophans (both S). A subsequent report showed that the same A. niger produced asperic acid (31).53
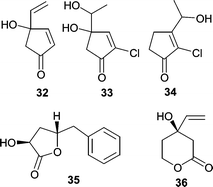
A variety of microorganisms were isolated from a Japanese specimen of Halichondria okadai, including a strain of Trichoderma harzianum (OUPS-N115) that produced trichodenones A–C (32–34) and harzialactones A (35) and B (36).54 Trichodenones A (32), B (33), and C (34) showed modest cytotoxicity against a leukemia P388 cell line with ED50 values of 0.21, 1.21, and 1.45 µg mL−1, respectively. Harzialactone B (36) showed weak activity (ED50 = 60 µg mL−1) while harzialactone A (35) was inactive.
6.3 Kinase inhibitors from sponge-derived fungi
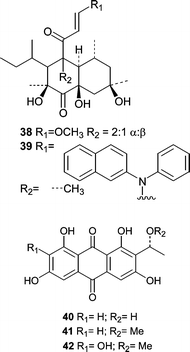
Ulocladol (37), a p56lck tyrosine kinase inhibitor, was isolated from a culture of the Ulocladium botrytis that was obtained from the sponge Myxilla incrustans.55 Ulocladol (37) reduced the activity of the enzyme to 7% at 0.02 µg mL−1 in an ELISA-based assay. Since p56lck is necessary for T-cell activation, inhibitors of the kinase could be useful for treating autoimmune diseases.56 Additionally, Holler et al.55 reported that chemical investigation of the obligate marine fungus Asteromyces cruciatus, obtained from the marine sponge Myxilla incrustans, resulted in the isolation of (+)-2,4-dimethyl-4,5-dihydrofuran-3-carbaldehyde. The only other metabolite reported for the Asteromyces genus is the known diketopiperazine gliovictin.57The Mediterranean sponge Aplysina aerophoba yielded a previously undescribed Microsphaeropsis sp. that produced two classes of compounds;58 two betaenone derivatives (38 and 39) were isolated along with three anthraquinone analogs (40–42). All five compounds were screened for kinase inhibition against PKC-ε, CDK4, and EGFR. Interestingly, 39 showed no activity while 38 inhibited all three in the low µM range. Compound 40 showed slight selectivity toward PKC-ε with an IC50 of 18.5 µM versus 43.5 and 37.5 µM against CDK4 and EGFR, respectively. The absolute stereochemistries of 40–42 were determined by comparing the experimental CD to that calculated by quantum mechanical methods.
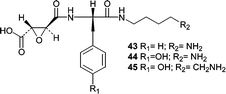
Cathestatins A (43), B (44), and C (45) were isolated from culture of a sponge-derived strain of Microascus longirostris.59 These amino acid-derived metabolites were shown to be irreversible nanomolar inhibitors of cysteine proteases. Although no experimental data is available to support a mechanism of inhibition, it seems logical that the irreversibility is a result of nucleophilic addition of an active site cysteine to the cathestatin epoxide moiety.
6.4 Miscellaneous metabolites from sponge-derived fungi
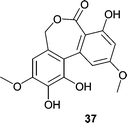
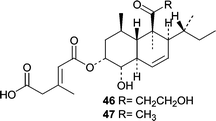
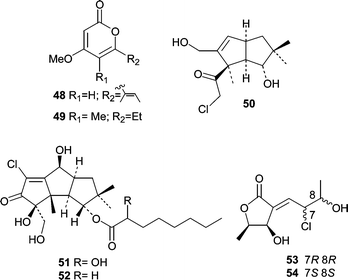
The first report describing a novel metabolite from a sponge-derived fungus was in 1993, and the fungus, Trichoderma harzianum, was isolated from a Mycale cecilia sponge.60T. harzianum, a common soil fungus, was cultured separately in a “salty medium” and in freshwater medium followed by analyses of the ethyl acetate extracts. The polyketide, trichoharzin (46), was isolated from the salt medium and its absolute stereochemistry determined by CD and chemical conversion. The trichoharzin analog, deoxynortrichoharzin (47), was also obtained from a Jaspis sp. sponge-derived fungus, Paecilomyces cf. javanica.61Two reports describing novel metabolites from sponge-derived fungi were published in 1994. The first, from the lab of P. Crews, described demethyl nectriapyrone A (48) and nectriapyrone B (49) from an unidentified fungus obtained from a Stylotella sp. sponge near Taveuni, Fiji.62 The second report63 came from the same group and described chloriolins A (50), B (51), and C (52), chlorinated analogs of the terrestrial fungal coriolin class of sesquiterpenes. They were isolated from an unidentified fungus derived from a Jaspis aff. johnstoni sponge. Abrell et al.64 described chlorinated polyketides from an Aspergillus sp. derived from a Jaspis cf. coriacea sponge. The chlorinated metabolites, chlorocarolides A (53) and B (54) were the first examples of chlorinated polyketides from a marine-derived fungus.
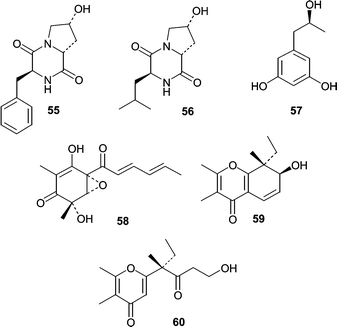
The isolation of yeast from sponges is rare in the natural product literature, but the yeast Aureobasidium pullulans was isolated from an Okinawan sponge. When A. pullulans was grown in a 50% seawater medium, the yeast produced two diketopiperazines (55 and 56) along with the 1,3-dihydroxyphenol derivative orcinotriol (57).65 The absolute stereochemistry of all three metabolites was determined by amino acid analysis of hydrolysates (55 and 56) and by a modified Mosher method for 57. The vertinoid polyketide, epoxysorbicillinol (58) was isolated from the seawater-based fermentation of a Trichoderma longibrachiatum obtained from a Haliclona sp. sponge specimen collected in Indonesia.66 The absolute stereochemistry of epoxysorbicillinol (58) was determined by the exciton interaction observed in the CD spectrum between the enolized 1,3-diketone and the sorbyl unit. Isolates of Drechslera hawaiiensis, from the marine sponge Callyspongia aerizusa, were obtained and cultured to yield four spiciferone derivatives.67 The two novel derivatives included spiciferol A (59), the alcohol congener of spiciferone A and a monocyclic spiciferone derivative (60).
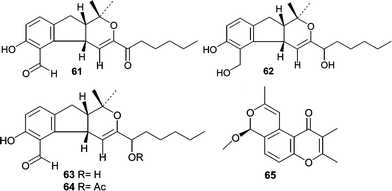
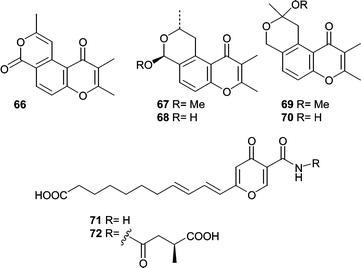
An Aspergillus versicolor, isolated from Xestospongia exigua, was found to be a rich source of novel polyketides. Ethyl acetate extracts of the culture filtrates resulted in the isolation and identification of ten novel metabolites. In one report, the structure elucidation of four novel hydropyranoindeno derivatives, aspergillone (61), aspergillodiol (62), aspergillol (63), and 12-acetyl-aspergillol (64) was described.68 Additionally, six chromone derivatives were isolated and identified, aspergiones A (65), B (66), C (67), D (68), E (69), and F (70).69 Two γ-pyrones, microsphaerones A (71) and B (72), were obtained from fermentation of a Microsphaeropsis sp. obtained from a Mediterranean specimen of the sponge Aplysina aerophoba.70 The absolute stereochemistry of 72 was determined by GC-MS analysis of the hydrolyzed side chain.
6.5 Antimicrobial metabolites from algicolous fungi
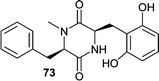
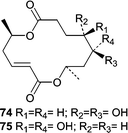
The fungistatic diketopiperazine, mactanamide (73) was isolated from a seawater culture of an undescribed Aspergillus sp. obtained from the surface of a decomposing sample of the brown alga Sargassum sp.71 The absolute stereochemistry was determined by analyzing the hydrolysates. Using a combination of chiral TLC and CD, both amino acids were determined to have D-configuration. Although the absolute stereochemistry was determined correctly the originally published structure showed an L-configuration.A strain of the obligate marine mitosporic fungus, Varicosporina ramulosa, was isolated from a Cytoseira sp. brown alga near Tenerife, Spain. An 80% seawater-based fermentation resulted in the production of a series of macrodiolides including two that were new fungal metabolites, 9,10-dihydro-(6R,11S,12R,14R)-colletodiol (74) and 9,10-dihydro-(6R,11R,12R,14R)-colletodiol (75).55 The report of these two antifungal macrodiolides represents the only chemical investigation from the genus Varicosporina.
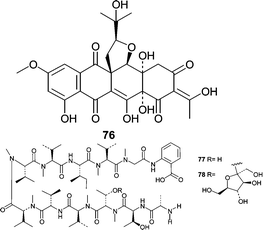
An unidentified fungus (K063) was separated from the Okinawan marine rhodophyte Ceratodictyon spongiosum. The mycelium produced a unique isoprene-containing decaketide, seragikinone A (76).72 Seragikinone A (76) showed weak antifungal activity against C. albicans and moderate antibacterial activity, but did not show cytotoxicity towards L1210 murine leukemia or KB human epidermoid carcinoma cells. Additional studies showed that isolate K063 produced two anthranilic acid containing peptides, dictyonamides A (77) and B (78).73 Dictyonamide A (77) inhibited CDK4 with an IC50 = 16.5 µg mL−1. Cytotoxicity studies were not performed to investigate antineoplastic potential.
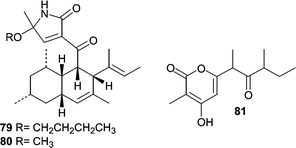
A strain of the obligate marine fungus Ascochyta salicorniae was found to be associated with a marine green alga Ulva sp. Fermentation of A. salicorniae resulted in the production of a diverse array of metabolites including two new unusual tetramic acid derivatives ascosalipyrrolidinones A (79) and B (80) as well as the new pyrone ascosalipyrone (81).74 Ascosalipyrrolidinone A (79) displayed antiplasmodial activity against Plasmodium falciparum, antimicrobial activity, and also inhibited the tyrosine kinase p56lck.
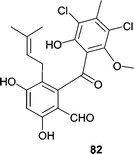
A Pestalotia sp. was obtained from a brown alga Rosenvingea sp. collected in the Bahamas. When the Pestalotia sp. was cultured in the presence of a bacterial antagonist, the fungus produced the potent antibiotic pestalone (82), which showed an MIC = 37 ng mL−1 against MRSA and an MIC = 78 ng mL−1 against vancomycin-resistant Enterococcus faecium (VREF).75 The most interesting observation was that neither the bacterium nor the fungus produced the antibiotic individually suggesting that microbial antagonism could be useful for the discovery of new antibiotics from fungi. Additional examples of bacterial antagonism increasing the production of bioactive secondary metabolites have been reported.76
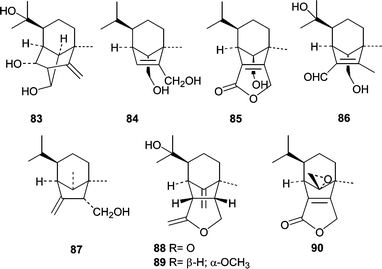
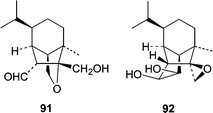
The algicolous fungus Drechslera dematioidea was obtained from the inner tissue of the marine red alga Liagora viscida. Cultivation of D. dematioidea on a solid medium yielded 16 compounds including 10 new sesquiterpenes isosativenetriol (83), drechslerines A (84) and B (85), 9-hydroxyhelminthosporol (86), drechslerines C–G (87–91), and sativene epoxide (92).77 With the exception of drechslerines C (87) and E (90), the sesquiterpenes were screened for activity in antialgal, antimicrobial, and ELISA-based assays. Only drechslerines D (88) and F (91) showed activity. Drechslerines D (88) and F (91) inhibited the growth of P. falciparum with IC50 values less than or equal to 5.1 µg mL−1. D. dematioidea demonstrates the incredible chemical diversity that can be found in algicolous fungi.
6.6 Cytotoxic metabolites from algicolous fungi
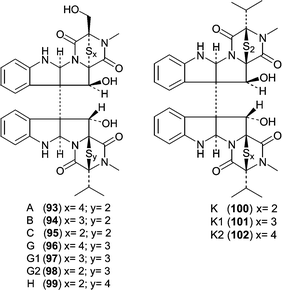
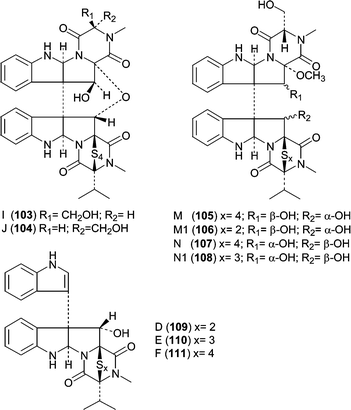
One of the largest classes of cytotoxic fungal metabolites is the leptosin family of dimeric diketopiperazines that contains 19 members, all related to the terrestrial metabolite chaetocin. The producing fungus, Leptosphaeria sp. OUPS-4, was isolated from the marine alga Sargassum tortile. The leptosins can be divided into five subclasses or groups based on structural similarity and biological activity with leptosins A (93), B (94), C (95),78 G (96), G1 (97), G2 (98) and H (99)79 differing only by the number of sulfurs contained within the polythio-bridge. Leptosins K (100), K1 (101) and K2 (102)80 form another group that differs by the stereochemistry of one polythio-bridge and the presence of two valines. The third group is comprised of leptosins I (103) and J (104),81 which contain an additional ether linkage between the two diketopiperazine monomers. The fourth group, leptosins M (105), M1 (106), N (107) and N1 (108)82 lack one polythio-bridge. The final group includes leptosins D (109), E (110) and F (111),78 which are diketopiperazine monomers with an indole appendage.The potency of the leptosins against a P388 leukemia cell line ranged from low ng mL−1 to low µg mL−1 (see Table 6). The activities show that the most detrimental effect on potency arises from the additional ether linkage contained in I (103) and J (104). Leptosins M (105) and M1 (106) are the second least potent analogs, which must be due to the lack of a second polythio bridge in conjunction with an additional methoxyl. Comparison of M (105) and M1 (106) to N (107) and N1(108) demonstrates that the stereochemistry of C11 and C11′ are also important for overall potency. Leptosins A (93) and C (95) showed significant antitumor activity in mice bearing Sarcoma-180 ascites. Mean survival time of the treated animal (T) was compared to that of the control animal (C) expressed as a percent (T/C %) and gave T/C values of 260 and 293 for 93 and 95, respectively. Studies on leptosin M (105) showed that it inhibited (30–70% inhibition) two kinases, PTK and CaMKIII at 10 µg mL−1, but did not inhibit PKA, PKC, or EGFR at 100 µg mL−1.82 Additionally, leptosin M (105) inhibited topoisomerase II (IC50 = 59.1 µM), but does not inhibit topoisomerase I. Since leptosin M (105) exhibits more potent effects on cells versus the targets listed, the activity against these targets does not explain the activity in whole cells unless there is a synergistic effect.
| Leptosin | ED50 µg mL−1 | Leptosin | ED50 µg mL−1 |
|---|---|---|---|
| A (93) | 1.85 × 10−3 | I (103) | 1.13 |
| B (94) | 2.40 × 10−3 | J (104) | 1.25 |
| C (95) | 1.75 × 10−3 | M (105) | 1.05 |
| G (96) | 4.6 × 10−3 | M1 (106) | 1.4 |
| G1 (97) | 4.3 × 10−3 | N (107) | 0.18 |
| G2 (98) | 4.4 × 10−3 | N1 (108) | 0.19 |
| H (99) | 3.0 × 10−3 | D (109) | 8.6 × 10−2 |
| K (100) | 3.8 × 10−3 | E (110) | 4.6 × 10−2 |
| K1 (101) | 2.2 × 10−3 | F (111) | 5.6 × 10−2 |
| K2 (102) | 2.1 × 10−3 |
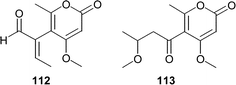
A. Numata's group also described two new lactones, pyrenocines D (112) and E (113), isolated from fermentation of a Penicillium waksmanii separated from the brown alga Sargassum ringgoldianum.83 Although pyrenocine D (112) did not show significant cytotoxicity, pyrenocine E (113) showed moderate cytotoxicity with an ED50 of 1.30 µg mL−1 against P388 leukemia cells.
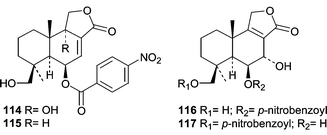
Novel cytotoxic sesquiterpenoid nitrobenzoyl esters (114–117) were isolated from a culture of Aspergillus versicolor isolated from the surface of the Caribbean green alga Penicillus capitatus.84 Compound 114, 9α,14-dihydroxy-6β-p-nitrobenzoylcinnamolide, showed a mean LC50 of 1.1 µg mL−1 in the National Cancer Institute's 60 cell-line panel. Interestingly, compound 114 was also isolated from an Aspergillus insulicola that was obtained from several samples in the Bahamas including a Penicillus sp. green alga.85 Compound 114 was given the name insulicolide A by Rahbaek et al.
A Penicillium sp. (OUPS-79) was obtained from the marine alga Enteromorpha intestinalis and resulted in the production of the unusual amino acid-derived communesins A (118) and B (119).86 Communesins A (118) and B (119) represent a novel carbon skeleton. To date there is only one other compound with a related skeleton, perophoramidine, which was isolated from the ascidian, Perophora nameii.87 Communesin B (119, ED50 0.45 µg mL−1) exhibited potent activity in a P388 lymphocytic leukemia test while communesin A (118) showed only moderate cytotoxicity (ED50 3.5 µg mL−1).
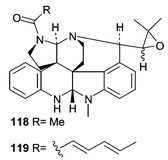
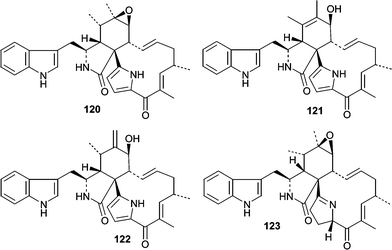
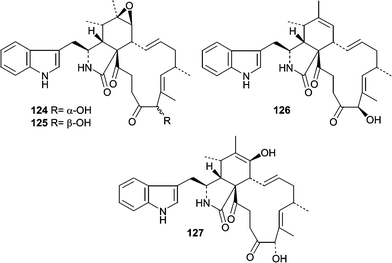
Additional studies on Penicillium isolate OUPS-79 resulted in the isolation of two unrelated classes of cytotoxic compounds, penochalasins A–H (120–127)88,89 and the penostatins (128–136).90–92 Although the penochalasins are described as members of the cytochalasin family of fungal metabolites, they can be more specifically classified as chaetoglobosins due to the presence of a tryptophan moeity rather than phenylalanine.93 Cytochalasins and chaetoglobosins have various effects on cells mainly due to their ability to cap F-actin and have been useful tools for cytoskeletal research.94 Penochalasins A–D (120–123) are particularly unique due to the presence of a pyrrole moiety. The conformations and relative stereostructures of the penochalasins were determined by analysis of coupling constants, molecular modeling, and NOESY spectra. The cytotoxic activities of the penochalasins were investigated in P388 leukemia cells, and penochalasins A–C (120–122) were the most potent. The ED50 values for 120–127 are 0.4, 0.3, 0.5, 3.2, 2.1, 1.8, 1.9, and 2.8 µg mL−1, respectively. These data suggest that the pyrrole present in A–C (120–123) is important for potency while hydrogenation of the pyrrole ring markedly decreased potency based on the differential activity observed between C (122) and D (123). From an SAR standpoint, it would be interesting to see how the pyrrole moiety, present in penochalasins A–C (120–122), affects potency and selectivity toward F-actin.
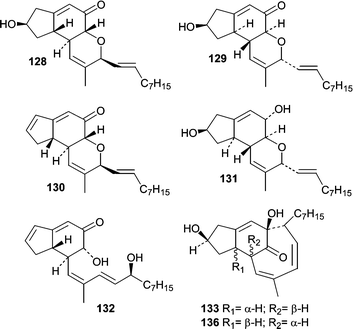
Penostatins A–I (128–136)90–92 represent the third distinct class of new metabolites from Penicillium isolate OUPS-79. Penostatins A–D (128–131) are characterized by a tricyclic core with a pendant nine carbon chain, while F–I (133–136) have undergone additional cyclizations resulting in more elaborate ring systems. In the preliminary report, only the relative stereochemistries were assigned for A–D (128–131),92 but the absolute stereostructures were later determined using a modified Mosher method and CD.90 The activity of the penostatins was evaluated in the P388 lymphocytic leukemia test system, and the cytotoxicity for A–I (128–136) ranged from 0.5 to 11.0 µg mL−1. Penostatin E (132) showed the lowest potency and is the only member that does not contain the tricyclic core. Overall, it is interesting that such a structurally diverse group of metabolites show similar cytotoxic potency. The production of the penostatins, the communesins, and the penochalasins from one Penicillium isolate demonstrates the diversity of the genus. Prior to the penochalasins, no members of the cytochalasins had been reported from a Penicillium sp.
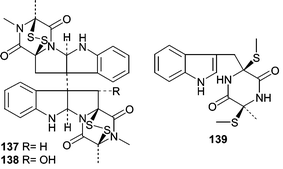
A Penicillium sp. (#CNC-350) was obtained from the Caribbean green alga Avrainvillea longicaulis and cultivated under saline conditions to produce a mycelium extract that showed potent cytotoxicity and yielded two active diketopiperazine dimers, 11,11′-dideoxyverticillin A (137) and 11′-deoxyverticillin A (138) as well as a noncytotoxic diketopiperazine (139).95 Compounds 137 and 138 showed potent cytotoxicity in HCT-116 human colon carcinoma cells with IC50 values of 30 ng mL−1.
6.7 Miscellaneous metabolites from algicolous fungi
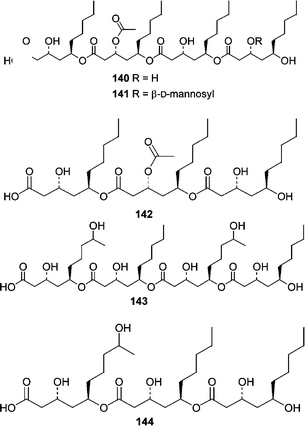
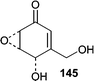
An interesting study of fungi from the marine alga Halymenia dilatata from Palau resulted in the isolation of fungi from two different genera that produced similar chemistry. A Fusarium sp. obtained from the alga produced halymecins A–C (140–142) while an Acremonium sp. isolated from the same alga produced halymecins D (143) and E (144).96 On the basis of their activity profile, the halymecins act primarily as antimicroalgal agents.An Aspergillus parasiticus was isolated from the surface of the marine red alga Carpopeltis cornea. Fermentation resulted in the production of an unstable epoxycylohexenone, parasitenone (145).97 Parasitenone demonstrated radical scavenging abilities.
6.8 Antimicrobial metabolites from fungi obtained from woody substrates
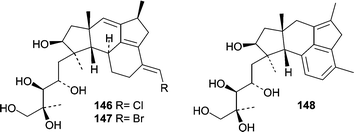
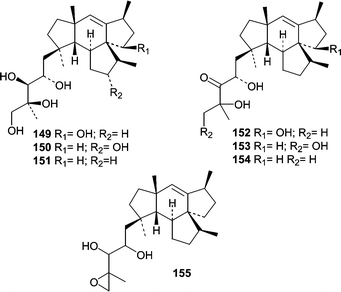
The first antimicrobial natural product from an obligate marine fungus obtained from a woody substrate was reported in 1981.13 The basidiomycete Halocyphina villosa, an inhabitant of intertidal roots and branches in mangroves,9 was isolated from submerged wood by making a spore print. Fermentation of the H. villosa isolate resulted in the production of the previously identified metabolite, siccayne (1).A Fusarium sp. (tentatively F. heterosporum, strain CNC-477) was obtained from a driftwood sample in a mangrove habitat in the Bahamas. A seawater-based fermentation resulted in the production of two related classes of sesterterpenes, neomangicols A–C (146–148)98 and mangicols A–G (149–155).99 The observation that only neomangicol C (148) was isolated when the mycelial extract was chromatographed on silica gel led to the proposal that neomangicol C (148) was a “decomposition” product from neomangicol A (146). The absolute stereochemical assignments for the neomangicols was based on NMR analysis of Mosher esters. On the basis of 13C-acetate labeling studies, a biosynthetic route was proposed for the unusual and unprecedented skeletons, and biosynthesis studies allowed insight into the formation of the neomangicols from mangicol precursors. Neomangicols A (146) and B (147) showed moderate cytotoxicity against a panel of cell lines. Neomangicol A (146) was the most active against an MCF-7 human breast carcinoma (IC50 = 4.9 µM) while neomangicol B (147) was less active (mean IC50 = 27 µM), but also showed activity against B. subtilis at 50 µg/disc. Mangicols A–G (149–155) were screened in the NCI's 60-cell line panel and did not exhibit selectivity, but did show growth inhibition in the low µM range. Additionally, mangicols A (149) and C (151) significantly inhibited phorbol myristate acetate-induced edema.
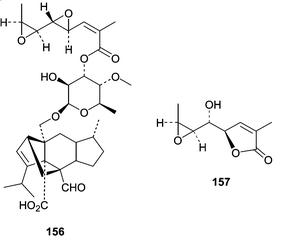
Fruiting bodies of the facultative marine fungus Hypoxylon croceum were obtained from a mangrove estuary driftwood sample. Fermentation of H. croceum resulted in the production of a new member of the sordarin family of antifungal agents, hypoxysordarin (156), and also a new lactone (157).100 Interest in the sordarin family of diterpene glycosides stems from their ability to selectively inhibit fungal protein synthesis by binding to an elongation factor 2 (EF2)-ribosome complex.101 Hypoxysordarin (156) showed lower MICs against several filamentous fungi as compared to sordarin.
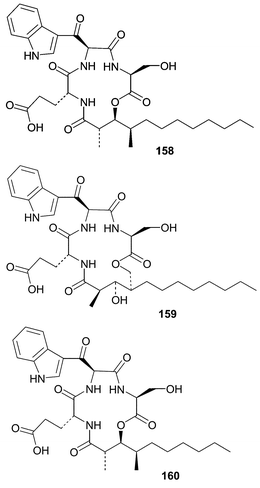
A strain of Hypoxylon oceanicum obtained from mangrove wood produced three lipodepsipeptides, 15G256γ (158), 15G256δ (159) and 15G256ε (160), along with two known macrolactones.102 The depsipeptides were isolated due to their antifungal activity and appear to inhibit fungal cell wall biosynthesis while exhibiting broad spectrum activity against dermatophytic fungal pathogens.103 Fermentation studies showed an interesting trend where increasing the amount of seawater in the media resulted in a temperature dependent decrease in the production of 15G256γ (158).104
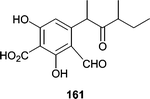
A strain of the marine ascomycete Kirschsteiniothelia maritima was isolated from a submerged wood sample. Upon fermentation, K. maritima produced an aromatic aldehyde, ascochital (161), which showed antibacterial activity and exhibited a fairly potent MIC (500 ng mL−1) against B. subtilis.
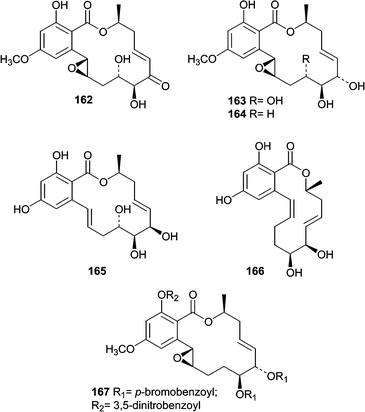
The lignicolous mangrove ascomycete Aigialus parvus was investigated due to its antimalarial activity, and also because no member of the Aigialus genus had previously been chemically investigated.105 Fermentation of A. parvus resulted in isolation and characterization of five new resorcyclic macrolides named aigialomycins A–E (162–166). A bis-p-bromobenzoyl-3,5-dinitrobenzoyl (167) derivative of aigialomycin C (164) was crystallized and anomolous scattering X-ray diffraction methods were used to elaborate the absolute stereostructure. Aigialomycin D (165) showed moderate antimalarial activity against P. falciparum, and also cytotoxicity against human cancer cell lines. Thus, its antimalarial activity is suspected to be a cytotoxic effect.
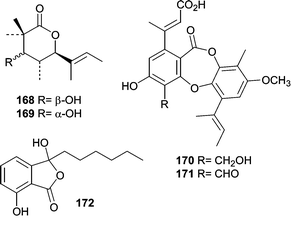
One of the earliest reports on metabolites from mangrove fungi came from J. B. Gloer's research group and focused on the Hawaiian mangrove ascomycete Helicascus kanaloanus (ATCC 18591).106 Two isomeric δ-lactones, helicascolides A (168) and B (169), were obtained from a seawater-based fermentation of H. kanaloanus. A subsequent report from the Gloer research group described the chemical investigation of a Preussia aurantiaca (ATCC 14745) that was originally obtained from a mangrove sediment sample.107 Extracts of culture filtrates were found to exhibit antimicrobial activity against both B. subtilis and S. aureus. The antibiotic activity was mainly attributed to the production of two depsidones, auranticins A (170) and B (171).
An antibacterial metabolite, corollosporine (172) was isolated from a fermentation of Corollospora maritima.108 An isolate of the obligate marine fungus C. maritima was obtained from a driftwood sample collected near Helgoland, Germany.
6.9 Cytotoxic metabolites from fungi isolated from woody substrates
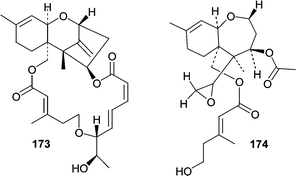
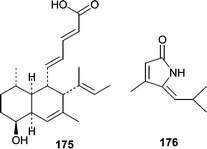
A marine-derived Myrothecium roridum obtained from a submerged woody sample produced a new member of the roridin family, 12,13-deoxyroridin E (173).109 The roridin class of trichothecenes are potently cytotoxic and 173 showed IC50 values of 25 and 15 ng mL−1 in HL-60 and L1210 cell lines, respectively. Roridin E contains an epoxide moiety in place of the exocyclic olefin found in 173 and showed an 80-fold increase in cytotoxic activity. A new deuteromycete, Acremonium neo-caledoniae, obtained from a driftwood sample was characterized both morphologically and by 18S rRNA sequencing.110 The ethyl acetate extract of the culture filtrate exhibited potent cytotoxicity that was attributed to the known metabolites verrucarin A, isororidin A, and the new verrol 4-acetate (174). Verrol 4-acetate (174) showed fairly potent cytotoxic activity against a KB cell line (IC50 = 400 ng mL−1), but did not exhibit antifungal (C. albicans) or antibacterial (S. aureus) activity.Phomopsidin (175), an inhibitor of microtubule assembly, was isolated from a Phomopsis sp. obtained from a submerged mangrove branch.111,112 Phomopsidin (175) inhibited microtubule assembly with an IC50 of 5.7 µM and is similar in potency to colchicine (IC50 = 10 µM) and rhizoxin (IC50 = 4 µM). The absolute stereochemistry, the biosynthesis, and additional studies on the bioactivity of phomopsidin (175) have recently been reported.113
Pulchellalactam (176) was isolated from cultures of the driftwood marine fungus Corollospora pulchella and exhibited inhibitory activity against the CD45 phosphatase.114 CD45 plays central signaling roles by dephosphorylating Src-kinases and therefore represents an attractive drug target.115 However, the cellular effects of pulchellalactam (176) were not described.
6.10 Metabolites from tunicate-derived fungi
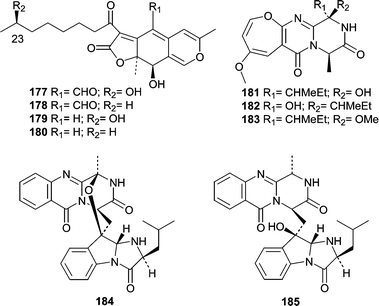
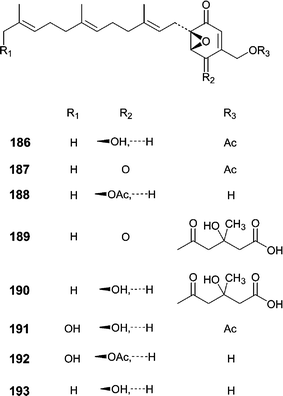
Although the number of reports describing natural products from tunicate-derived fungi is low, a total of four reports describe 20 new metabolites. The first report came in 1997 from Crews' research group and described metabolites produced by a Pithomyces sp. obtained from the Indo-Pacific tunicate Oxycorynia fascicularis.116 Pitholides A–D (177–180) were obtained from a salt water culture and their absolute stereochemistry at position 23 determined by NMR analysis of the R- and S-MPA esters. Additional reports did not appear until 2000 when three different groups described metabolites from tunicate-derived fungi. Two classes of metabolites were produced by an Acremonium sp. obtained from the surface of the ascidian Ecteinascidia turbinata.117 Oxepinamides A–C (181–183) are unusual oxepine containing alkaloids and oxepinamide A (181) showed good antiinflammatory activity in a topical RTX-induced mouse ear edema assay. The structural elucidation of oxepinamide A (181) involved extensive 2D NMR experiments including 1H,15N-HMBC and a 1,1-ADEQUATE. Fumiquinazolines H (184) and I (185) were also isolated from this Acremonium sp. and exhibited weak antifungal activity toward C. albicans.Our research group investigated an Aspergillus niger obtained from an Aplidium sp. tunicate tissue homogenate.118 Bioassay-guided fractionation of the extract yielded eight novel farnesylated epoxycylohexenones, yanuthones A–E (186–190), 1-hydroxy-yanuthone A (191), 1-hydroxy-yanuthone C (192), and 22-deacetyl-yanuthone A (193). Although most of the yanuthones exhibited some antimicrobial activity, yanuthone D (189) was the most active and displayed increased activity against methicillin-resistant S. aureus as compared to methicillin-sensitive S. aureus. Additionally, yanuthone D (189) showed activity against vancomycin-resistant E. faecium.
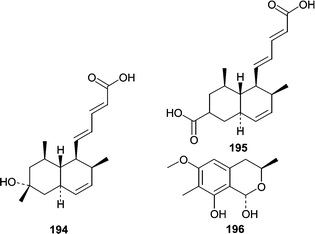
One additional report described studies that compared metabolites from marine isolates of Penicillium sp. to those of terrestrial isolates.119 Of the marine isolates, a P. steckii was obtained from an unidentified tunicate and produced two carboxylic acids, tanzawaic acids E (194) and F (195) as well as the previously undescribed 3,7-dimethyl-1,8-dihydroxy-6-methoxy-isochroman (196). Additionally, terrestrial and marine isolates of P. steckii, P. citrinum, P. sizovae, and P. corylophilum were investigated to see if morphological or chemical differences between marine and terrestrial isolates could be identified. The results showed that, for the Penicillium spp. studied, metabolite production was only dependant on the medium and not on whether the isolate was marine- or terrestrial-derived.
6.11 Metabolites from sediment-derived fungi
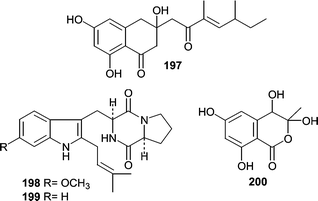
Keissleriella sp. YS4108 was isolated from a sediment sample (−50 m) in the Yellow Sea near Sheyang Port, China.120 Fermentation of the isolate resulted in production of several antifungal metabolites. One of which, polyketide 197 possessed a new carbon skeleton and exhibited MIC values of 40, 20 and 80 µg mL−1 against C. albicans, Tricophyton rubrum and A. niger, respectively.Two diketopiperazine-type cell cycle inhibitors, tryprostatins A (198) and B (199), were isolated from a sea sediment-derived Aspergillus fumigatus.121 Tryprostatin A (198, 50 µg mL−1) and tryprostatin B (199, 12.5 µg mL−1) treated tsFT210 cells caused complete arrest in the G2/M phase of the cell cycle. Tryprostatin A (198) was shown to be a novel inhibitor of microtubule assembly through disruption of the microtubule spindle.122,123 Acetophthalidin (200), from a Penicillium isolate obtained from a sediment sample, arrested tsFT210 cells in G2/M at a concentration of 6.25 µg mL−1, but the cellular target was not described.124
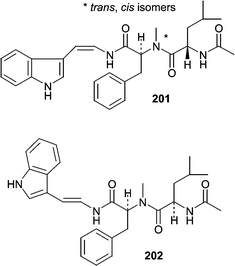
Two isomeric peptides, aspergillamides A (201) and B (202), were isolated from a sediment-derived strain of Aspergillus sp.125 Aspergillamide A (201) exists as a 1 : 1 mixture of trans- and cis-isomers around the indicated amide bond. Aspergillamide A (201) showed moderate cytotoxicity against HCT-116 cells (IC50 = 16 µg mL−1).
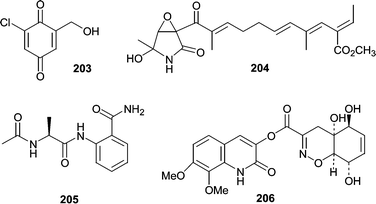
Chlorogentisylquinone (203) was isolated from the marine-derived fungal strain FOM-8108 and was found to be a neutral sphingomyelinase inhibitor.126 A strain of Penicillium sp. (BM1689-P) was obtained from a sea bottom sediment sample of Uchiura Bay. Fermentation resulted in production of a unique lactam, epolactaene (204).127 Originally isolated for its neuritogenic activity in human neuroblastoma cells, epolactaene (204) was later shown to inhibit mammalian DNA polymerases and DNA topoisomerase II.128 Another strain of Penicillium sp. obtained from a sediment sample produced a novel anthranilamide derivative (205).129 The compound was synthesized to confirm the absolute stereochemistry. Penicillazine (206) is a very unusual structure isolated from a Penicillium sp. from the South China Sea.130
6.12 Metabolites from mollusc-derived fungi
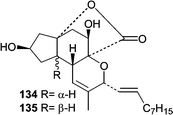
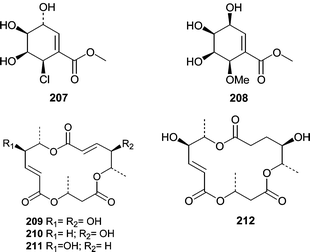
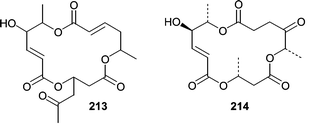
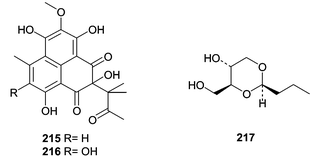
Chemistry has been described from only one fungal isolate from a sea hare. A Periconia byssoides isolate was obtained from the gastrointestinal tract of the sea hare Aplysia kurodai. A culture of the fungus was shown to produce pericosines A (207) and B (208) as well as macrosphelides E–I (209–213)131,132 and macrosphelide L (214).133 Pericosines A (207) and B (208) showed significant cytotoxicity in a P388 lymphocytic leukemia screen exhibiting ED50 values of 0.12 and 4.0 µg mL−1, respectively. Pericosine A (207) also showed in vivo activity in a mouse model, while the macrosphelides were cell adhesion inhibitors.Two Penicillium spp. obtained from the Okinawan marine bivalve Mytilus coruscus were investigated for novel metabolites. Penicillium sp. K036 produced sculezonones A (215) and B (216)134 while Penicillium sp. K029 produced a racemic mixture of coruscol A (217).135 No biological activity was reported for any of these metabolites.
6.13 Metabolites from fungi obtained from coral
A class of DNA-cleaving antitumor antibiotics, spiroxins A–E (218–222), were isolated from an unidentified fungus (LL-37H248) isolated from an unidentified soft coral.136 Spiroxin A (218) showed potent cytotoxicity (IC50 = 0.09 µg mL−1) against a panel of 25 diverse cell lines. Additionally, spiroxin A (218) showed in vivo activity against an ovarian carcinoma in nude mice (59% growth inhibition after 21 days). Spiroxin A (218) appears to act via multiple mechanisms and was shown to nick plasmid DNA under reducing conditions. Furthermore, spiroxin A (218) reacted with thiol-type reducing agents suggesting an oxidative stress mechanism of action involving thiol conjugates.6.14 Metabolites from fungi obtained from non-algal plants and grasses
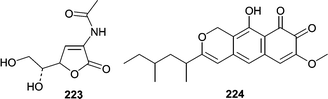
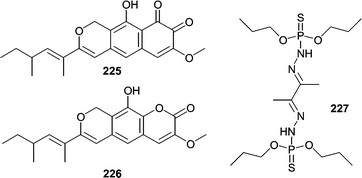
Although marsh grasses and plants have been documented as hosts for several species of fungi, reports of metabolites from plant/grass-derived fungi are low. Several metabolites have been reported from obligate marine species commonly associated with marsh grasses, mainly Leptosphaeria spp. Investigation of cultures of the marine ascomycete L. oraemaris resulted in the identification of leptosphaerin (223).137 In 1989 two closely related metabolites were reported from Leptosphaeria spp. Obionin A (224) was isolated from Leptosphaeria obiones,138 and a closely related metabolite, obioninene (225) was isolated from cultures of L. oraemaris.40 The ortho-quinone present in both compounds was confirmed by reaction with 1,2-phenylenediamine to form quinoxaline derivatives. Obionin A (224) was found to inhibit binding of a dopamine D-1 selective ligand to bovine corpus striatum membrane (IC50 = 2.5 µg mL−1). Thereafter, two analogs related to obionin, leptosphaerolide (226) and leptosphaerodione, which is structurally identical to obioninene (225) were reported from L. oraemaris.139 Another obligate marine fungus, Lignincola laevis, known from marsh grass was shown to produce 7-hydroxyergosterol and a very unusual phosphorohydrazide thioate (227) that was cytotoxic against L1210 cells at 0.25 µg mL−1.140Strains of the fungus, Aspergillus insulicola, were obtained from several samples of plant material collected in the Bahamas.85 The report of insulicolide A (114) represented the first report on the chemical investigation of an A. insulicola.
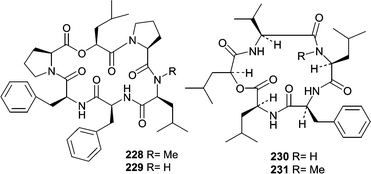
Fermentation of a Scytaladium sp. obtained from decaying plant material (−3m Exuma Islands, Bahamas) produced two cyclic hexapeptides, exumolides A (228) and B (229).141 Exumolides A (228) and B (229) were found to exhibit cytostatic activity against the unicellular marine chlorophyte Dunaliella sp., which could imply an ecological role for these metabolites.
Another report from Fenical's research group described sansalvamide (230) from a Fusarium sp. obtained from the surface of the seagrass Holodule wrightii.142 Sansalvamide (230) showed a mean IC50 of 27.4 µg mL−1 against the NCI's 60-cell line panel, with some selectivity against a colon cancer cell line COLO 205 (IC50 = 3.5 µg mL−1) and a melanoma cell line SK-MEL-2 (IC50 = 5.9 µg mL−1). Additional studies showed that sansalvamide (230) possessed antiviral activity and was shown to selectively inhibit topoisomerase from the poxvirus Molluscum contagiosum virus (MCV). N-methylsansalvamide (231) was subsequently isolated from a different strain of Fusarium obtained from the Caribbean green alga Avrainvillea sp.143 Fatty acid methyl ester (FAME) analysis showed that each Fusarium sp. had a different similarity index, but they were not confirmed to be different species. N-methylsansalvamide (231) showed growth inhibition with a GI50 of 8.3 µM against NCI's cell line panel, while sansalvamide (230) had a GI50 of 3.6 µM.
6.15 Metabolites from fungi isolated from fish
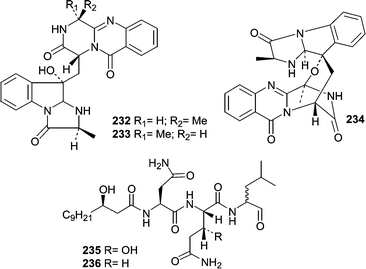
A strain of Aspergillus fumigatus was isolated from the gastrointestinal tract of the marine fish, Pseudolabrus japonicus.144 A seawater fermentation of the fungus resulted in the production of the cytotoxic modified diketopiperazines, fumiquinazolines A–C (232–234).A Penicillium fellutanum was isolated from the gastrointestinal tract of the marine fish Apogon endecataenia.145 The lipopeptides, fellutamides A (235) and B (236) were isolated from the mycelium of P. fellutanum and found to be cytotoxic against several cell lines, but showed the most activity against P388 murine leukemia exhibiting IC50 values of 0.2 and 0.1 µg mL−1, respectively.
6.16 Metabolites from miscellaneous substrates
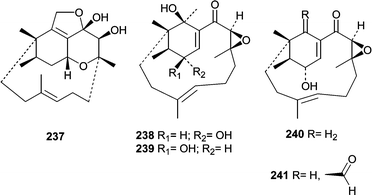
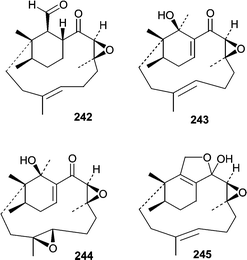
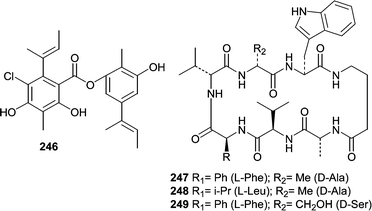
A report by Sugano et al.146 describing phomactin A (237) marked the beginning of a series of reports describing platelet activating factor (PAF) antagonists produced by a Phoma sp. obtained from a shell of the crab Chionoecetes opilio. Subsequently, phomactins B (238), B1 (239), B2 (240), C (241), D (242)147 and E–G (243–245)148 were identified and investigated for PAF antagonistic activity. Of the series, phomactin D (242) was the most potent and inhibited PAF binding to its receptor with an IC50 of 0.12 µM while inhibiting platelet aggregation with an IC50 of 0.80 µM. Additional structure activity relationships were investigated after synthetic modifications to produce a larger series of phomactin congeners.149Isolates of Emericella unguis, the teleomorph of Aspergillus unguis, were obtained from a mollusc and a medusa from Venezuela. The fungus was shown to produce the depside guisinol (246),150 and a group of cyclic peptides, unguisins A–C (247–249).151,152 Guisinol (246) showed antibacterial activity against S. aureus, but did not inhibit the growth of Vibrio parahaemolyticus. The unguisins were the first peptides reported to contain a γ-aminobutyric acid. Unguisin A (247) and B (248) production was stimulated by the addition of L-phenylalanine and L-leucine. Furthermore, the addition of L-leucine resulted in the production of unguisin D, which contained a leucine in place of valine-2. Unguisin A (247) and B (248) displayed moderate activity against S. aureus.
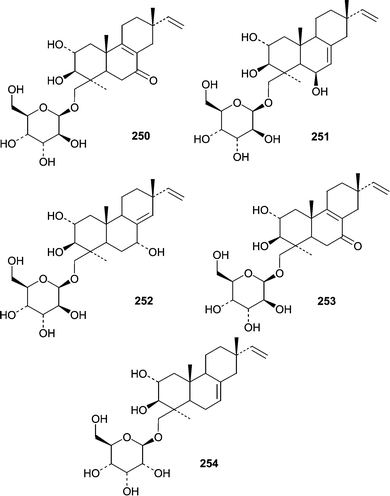
The holothurian Eupentacta fraudatrix was the source of an isolate of Acremonium stratisporum that produced a series of diterpene glycosides. One report described the isolation of virescenosides M (250) and N (251)153 from A. stratisporum. A second report described virescenosides O (252), P (253) and Q (254).154 All five virescenosides showed cytotoxic activity against Ehrlich carcinoma cells (IC50 = 10–100 µM). Additionally, M (250), N (251) and P (253) showed cytotoxic effects on developing sea urchin eggs.
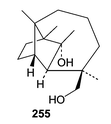
Kallichroma tethys (isolate no. 6175) from the Portsmouth Culture Collection grown in an enriched seawater medium yielded isoculmorin (255).155 The closely related sesquiterpene, culmorin, has been implicated in competition between lignicolous marine fungi. The biological activity of isoculmorin (255) may therefore be of ecological interest.
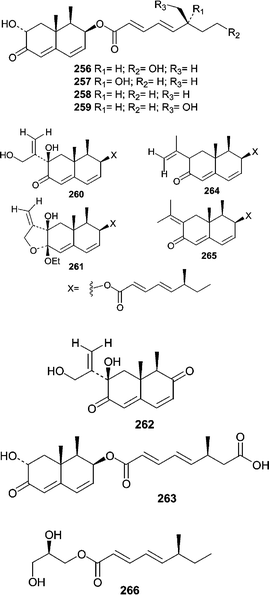
A diverse group of trinor-eremophilanes, eremophilanes, and one glyceryl ester were obtained from the obligate marine deuteromycete Dendryphiella salina, which is a common algal saprobe (lives on dead or decaying material). Of the series, dendryphiellin A (256) was reported first and its unprecedented structure demonstrated the potential of marine fungi as a source of unique chemistry.156 Prior to the report of dendryphiellin A (256), there was no precedent for either fungal trinor-eremophilanes or the branched C9 carboxylic acids that comprise the dendryphiellin A (256) side chain. Subsequently, three trinor-eremophilanes, dendryphiellins B (257), C (258), and D (259), were reported along with eremophilanes, dendryphiellins E (260), F (261), and G (262).157 Dendryphiellins E (260) and G (262) exist in equilibrium with their corresponding hemiacetals while F (261) is thought to be a product of isolation since E (260) forms F (261) in the presence of EtOH and trace amounts of acetic acid in hexanes. Additionally, the free acid side chains of A (256) and B (257) were isolated from extracts of D. salina. Synthesis of the free acid allowed stereochemical assignments of the methyl group on the side chain. Dendryphiellins A1 (263), E1 (264), and E2 (265) were subsequently reported along with the glyceryl ester of the dendryphiellin side chain, glyceryl dendryphiellate A (266).158
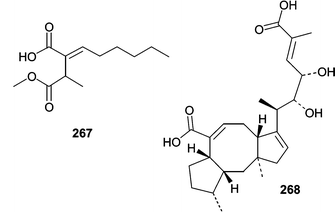
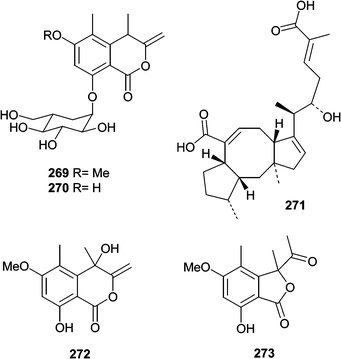
The succinic acid derivative (267) and halorosellinic acid (268) were isolated from the culture broth of the obligate marine fungus Halorosellinia oceanica (BCC 5149).159 Halorosellinic acid (268) showed only weak antimycobacterial activity with an MIC value of 200 µg mL−1. Further studies showed that H. oceanica produced five additional new metabolites. Two new isocoumarin derivatives, halorosellins A (269) and B (270), were isolated along with 17-dehydroxyhalorosellinic acid (271), the isochromanone (272), and the phenyl lactone (273).160 Only compound 273 was active, exhibiting an MIC value of 200 µg mL−1 against Mycobacterium tuberculosis.
7 Conclusions
From the literature review, several conclusions can be drawn. Overall, most metabolites are produced by Aspergilli and Penicillia. Although members of each genus are prolific sources of metabolites, they are somewhat ubiquitous and most are not unique species. One explanation for the high number of compounds reported from these two genera is that both Aspergillus and Penicillium spp. are salt tolerant, fast growing species and are easily obtained from many substrates. Additionally, many Aspergillus and Penicillium spp. are known to produce extracts with a wide variety of activities. Decreasing the number of ubiquitous species isolated could represent a valid method to increase the probability of producing novel chemistry.Some of the most unique structures, the dendryphiellins for example, came from obligate marine species. Since very few obligate species have been investigated, the marine environment still represents a virtually untapped resource for both fungal and chemical diversity. Relatively little is known about the relationships between fungi and their hosts especially with respect to chemical ecology. Nonetheless, endophyte studies on marine grasses and mangroves clearly show that these habitats represent rich sources for both obligate and facultative fungi.31,161,162 Overall, both endophytic fungi from marine habitats and obligate marine fungi are one of the least studied groups of fungi and therefore represent great potential for the discovery of new pharmacologically active metabolites.
8 Acknowledgements
The authors would like to thank Mary Kay Harper for editorial expertise and comments on this manuscript. Additionally, the authors would like to thank Valerie S. Bernan and Jeffrey E. Janso for sharing their expertise and knowledge over the past five years. Finally, the authors dedicate this manuscript to Professor D. John Faulkner, a wonderful mentor and dear friend.9 References
- W. Fenical, Trends Biotechnol., 1997, 15, 339 CrossRef CAS.
- V. S. Bernan, M. Greenstein and W. M. Maiese, Adv. Appl. Microbiol., 1997, 43, 57 Search PubMed.
- U. Holler, A. D. Wright, G. F. Matthee, G. M. Konig, S. Draeger, H. Aust and B. Schulz, Mycol. Res., 2000, 104, 1354 Search PubMed.
- P. R. Jensen and W. Fenical, Marine microorganisms and drug discovery: current status and future potential, in Drugs from the Sea, Karger Publishers: Basel, 2000, 6 Search PubMed.
- P. R. Jensen and W. Fenical, Secondary metabolites from marine fungi, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press: Hong Kong, 2002 Search PubMed.
- K. Liberra and U. Lindequist, Pharmazie, 1995, 50, 583 Search PubMed.
- F. Pietra, Nat. Prod. Rep., 1997, 14, 453 RSC.
- J.-F. Verbist, C. Sallenave and Y.-F. Pouchus, Stud. Nat. Prod. Chem., 2000, 24, 979 Search PubMed.
- J. Kohlmeyer and E. Kohlmeyer, Marine Mycology: The Higher Fungi, Academic Press: New York, 1979 Search PubMed.
- C. Hart, Mod. Drug Discovery, 1999, 2, 20 Search PubMed.
- H. W. Florey, E. Chain, N. G. Heatley, M. A. Jennings, A. G. Sanders, E. P. Abraham, and M. E. Florey, Antibiotics, Oxford University Press: Oxford, 1949 Search PubMed.
- E. P. Abraham and P. B. Loder, Cephalosporin C, in Cephalosporins and Penicillins: Chemistry and Biology, ed. E. H. Flynn, Academic Press: New York, 1972 Search PubMed.
- J. Kupka, T. Anke, W. Steglich and L. Zechlin, J. Antibiot., 1981, 34, 298 CAS.
- D. L. Hawksworth, P. M. Kirk, B. C. Sutton, and D. N. Pegler, in Ainsworth and Bisby's Dictionary of Fungi, CAB International: New York, 1995 Search PubMed.
- P. M. Kirk, P. F. Cannon, J. C. David and J. C. Stalpers, Ainsworth and Bisby's Dictionary of Fungi, CAB International: New York, 2001 Search PubMed.
- M. J. Carlile, S. C. Watkinson, and G. W. Gooday, The Fungi, Academic Press: New York, 2001 Search PubMed.
- F. Driver and R. J. Milner, PCR applications to the taxonomy of entomopathogenic fungi, in Applications of PCR to Mycology, ed. P. D. Bridge, D. K. Arora, C. A. Reddy and R. P. Elander, CAB International: New York, 1998 Search PubMed.
- P. D. Bridge and D. K. Arora, Interpretation of PCR methods for species definition, in Applications of PCR to Mycology, ed. P. D. Bridge, D. K. Arora, C. A. Reddy and R. P. Elander, CAB International: New York, 1998 Search PubMed.
- S. Takamatsu, PCR applications in fungal phylogeny, in Applications of PCR to Mycology, ed. P. D. Bridge, D. K. Arora, C. A. Reddy and R. P. Elander, CAB International: New York, 1998 Search PubMed.
- T. L. Kiesling, M. R. Diaz, A. Statzell-Tallman, and J. W. Fell, Field identification of yeasts with DNA hybridization macroarrays, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press: Hong Kong, 2002 Search PubMed.
- D. S. Hibbett, Trans. Mycol. Soc. Jpn., 1992, 33, 533 Search PubMed.
- M. Jimenez, S. Rodriguez, J. J. Mateo, J. V. Gil and R. Mateo, Syst. Appl. Microbiol., 2000, 23, 546 Search PubMed.
- S. W. Peterson, Phylogenetic relationships in Aspergillus based on rDNA sequence analysis, in Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification, ed. R. A. Sampson and J. I. Pitt, Harwood Academic Publishers: Singapore, 2000 Search PubMed.
- S. W. Peterson, Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences, in Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification, ed. R. A. Sampson and J. I. Pitt, Harwood Academic Publishers: Singapore, 2000 Search PubMed.
- T. J. White, T. Bruns, S. Lee, and J. Taylor, Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in PCR Protocols: A guide to methods and applications, ed. M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, Academic Press: San Diego, 1990 Search PubMed.
- V. Edel, Polymerase chain reaction in mycology: an overview, in Applications of PCR to Mycology, ed. P. D. Bridge, D. K. Arora, C. A. Reddy and R. P. Elander, CAB International: New York, 1998 Search PubMed.
- J. C. Frisvad and U. Thrane, J. Chromatogr., 1987, 404, 195 CrossRef CAS.
- J. C. Frisvad, Arch. Environ. Contam. Toxicol., 1989, 18, 452 CAS.
- Chemical Fungal Taxonomy, ed. J. C. Frisvad, P. D. Bridge and D. K. Arora, Marcel Dekker: New York, 1998 Search PubMed.
- A. Nakagiri, Halophytophthoraspecies from tropical and subtropical mangroves: A review of their characteristics, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press, Hong Kong, 2002 Search PubMed.
- E. M. Leano, Ecology of straminipiles from mangrove habitats, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press: Hong Kong, 2002 Search PubMed.
- G. W. Smith, L. D. Ives, I. A. Nagelkerken and K. B. Ritchie, Nature, 1996, 383, 487 CrossRef CAS.
- H. D. Tresner and J. A. Hayes, Appl. Microbiol., 1971, 22, 210 Search PubMed.
- D. J. Alderman and J. L. Polglase, Are fungal diseases significant in the marine environment, in The Biology of Marine Fungi, ed. S. T. Moss, Cambridge University Press: Cambridge, 1986 Search PubMed.
- D. Porter, Mycoses of marine organisms: An overview of pathogenic fungi, in The Biology of Marine Fungi, ed. S. T. Moss, Cambridge University Press: Cambridge, 1986 Search PubMed.
- D. L. Kingham and L. V. Evans, The Pelvetia-Mycosphaerella interrelationship, in The Biology of Marine Fungi, ed. S. T. Moss, Cambridge University Press: Cambridge, 1986 Search PubMed.
- C. J. Bentis, L. Kaufman and S. Golubic, Biol. Bull., 2000, 198, 254 Search PubMed.
- J. Kohlmeyer and B. Vokmann-Kohlmeyer, Can. J. Chem., 1990, 68, 1554.
- R. Masuma, Y. Yamaguchi, M. Noumi, S. Omura and N. Michio, Mycoscience, 2001, 42, 455 Search PubMed.
- J. D. Miller and M. E. Savard, Proc. N. S. Inst. Sc., 1989, 39, 51 Search PubMed.
- D. H. Jennings, Biol. Rev., 1983, 58, 423 CAS.
- L. M. Abrell, B. Borgeson and P. Crews, Tetrahedron Lett., 1996, 37, 8983 CrossRef CAS.
- G.-Y.-S. Wang, L. M. Abrell, A. Avelar and B. M. Borgeson, Tetrahedron, 1998, 54, 7335 CrossRef CAS.
- U. Holler, G. M. Konig and A. D. Wright, J. Nat. Prod., 1999, 62, 114 CrossRef.
- R. Jadulco, P. Proksch, V. Wray, Sudarsono, A. Berg and U. Graefe, J. Nat. Prod., 2001, 64, 527 CrossRef CAS.
- C. J. Smith, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 2000, 63, 142 CrossRef CAS.
- J. Malmstrom, C. Christophersen, A. F. Barrero, J. E. Oltra, J. Justicia and A. Rosales, J. Nat. Prod., 2002, 65, 364 CrossRef.
- R. A. Edrada, M. Heubes, G. Brauers, V. Wray, A. Berg, U. Grafe, M. Wohlfarth, J. Muhlbacher, K. Schaumann, S. Sudarsono, G. Bringmann and P. Proksch, J. Nat. Prod., 2002, 65, 1598 CrossRef CAS.
- A. Numata, T. Amagata, K. Minoura and T. Ito, Tetrahedron Lett., 1997, 38, 5675 CrossRef CAS.
- T. Amagata, M. Doi, T. Ohta, K. Minoura and A. Numata, J. Chem. Soc., Perkin Trans. 1, 1998, 3585 RSC.
- T. Amagata, K. Minoura and A. Numata, Tetrahedron Lett., 1998, 39, 3773 CrossRef CAS.
- T. Amagata, M. Doi, M. Tohgo, K. Minoura and A. Numata, Chem. Commun., 1999, 1321 RSC.
- M. Varoglu and P. Crews, J. Nat. Prod., 2000, 63, 41 CrossRef CAS.
- Y. Usami, T. Ikura, T. Amagata and A. Numata, Tetrahedron: Asymmetry, 2000, 11, 3711 CrossRef CAS.
- U. Holler, G. Konig and A. D. Wright, Eur. J. Org. Chem., 1999, 2949 CrossRef CAS.
- D. R. Goldberg, T. Butz, M. G. Cardozo, R. J. Eckner, A. Hammach, J. Huang, S. Jakes, S. Kapadia, M. Kashem, S. Lukas, T. M. Morwick, M. Panzenbeck, U. Patel, S. Pav, G. W. Peet, J. D. Peterson, A. S. Prokopowicz 3rd, R. J. Snow, R. Sellati, H. Takahashi, J. Tan, M. A. Tschantz, X. J. Wang, Y. Wang, J. Wolak, P. Xiong and N. Moss, J. Med. Chem., 2003, 46, 1337 CrossRef CAS.
- J. Shin and W. Fenical, Phytochemistry, 1987, 26, 3347 CrossRef CAS.
- G. Brauers, R. A. Edrada, R. Ebel, P. Proksch, V. Wray, A. Berg, U. Grafe, C. Schachtele, F. Totzke, G. Finkenzeller, D. Marme, J. Kraus, M. Munchbach, M. Michel, G. Bringmann and K. Schaumann, J. Nat. Prod., 2000, 63, 739 CrossRef CAS.
- C. M. Yu, J. M. Curtis, J. A. Walter, J. L. Wright, S. W. Ayer, J. Kaleta, L. Querengesser and Z. R. Fathi-Afshar, J. Antibiot., 1996, 49, 395 CAS.
- M. Kobayashi, H. Uehara, K. Matsunami, S. Aoki and I. Kitagawa, Tetrahedron Lett., 1993, 34, 7925 CrossRef CAS.
- L. Rahbaek, S. Sperry, J. E. Piper and P. Crews, J. Nat. Prod., 1998, 61, 1571 CrossRef CAS.
- L. M. Abrell, X. Cheng and P. Crews, Tetrahedron Lett., 1994, 35, 9159 CrossRef CAS.
- X. Cheng, M. Varoglu, L. Abrell, P. Crews, E. Lobkovsky and J. Clardy, J. Org. Chem., 1994, 59, 6344 CrossRef CAS.
- L. M. Abrell, B. Borgeson and P. Crews, Tetrahedron Lett., 1996, 37, 2331 CrossRef CAS.
- H. Shigemori, M. Tenma, K. Shimazaki and J. Kobayashi, J. Nat. Prod., 1998, 61, 696 CrossRef CAS.
- S. Sperry, G. J. Samuels and P. Crews, J. Org. Chem., 1998, 63, 10011 CrossRef CAS.
- R. A. Edrada, V. Wray, A. Berg, U. Grafe, Sudarsono, G. Brauers and P. Proksch, Z. Naturforsch. C: Biosci., 2000, 55, 218 Search PubMed.
- W. H. Lin, J. Li, H. Z. Fu and P. Proksch, Chin. Chem. Lett., 2001, 12, 435 CAS.
- W. H. Lin, H. Z. Fu, J. Li and P. Proksch, Chin. Chem. Lett., 2001, 12, 235 CAS.
- C. Y. Wang, B. G. Wang, G. Brauers, H. S. Guan, P. Proksch and R. Ebel, J. Nat. Prod., 2002, 65, 772 CrossRef CAS.
- P. Lorenz, P. R. Jensen and W. Fenical, Nat. Prod. Lett., 1998, 12, 55 Search PubMed.
- H. Shigemori, K. Komatsu, Y. Mikami and J. i. Kobayashi, Tetrahedron, 1999, 55, 14925 CrossRef CAS.
- K. Komatsu, H. Shigemori and J. Kobayashi, J. Org. Chem., 2001, 66, 6189 CrossRef CAS.
- C. Osterhage, R. Kaminsky, G. M. Koenig and A. D. Wright, J. Org. Chem., 2000, 65, 6412 CrossRef CAS.
- M. Cueto, P. R. Jensen, C. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Nat. Prod., 2001, 64, 1444 CrossRef CAS.
- J. G. Burgess, E. M. Jordan, M. Bregu, A. Mearns-Spragg and K. G. Boyd, J. Biotechnol., 1999, 70, 27 CrossRef CAS.
- C. Osterhage, G. M. Konig, U. Holler and A. D. Wright, J. Nat. Prod., 2002, 65, 306 CrossRef.
- C. Takahashi, A. Numata, Y. Ito, E. Matsumura, H. Araki, H. Iwaki and K. Kushida, J. Chem. Soc., Perkin Trans. 1, 1994, 1859 RSC.
- C. Takahashi, Y. Takai, Y. Kimura, A. Numata, N. Shigematsu and H. Tanaka, Phytochemistry, 1995, 38, 155 CrossRef CAS.
- C. Takahashi, K. Minoura, K. Yamada, A. Numata, K. Kushida, T. Shingu, S. Hagishita, H. Nakai, T. Sato and H. Harada, Tetrahedron, 1995, 51, 3483 CrossRef CAS.
- C. Takahashi, A. Numata, E. Matsumura, K. Minoura, H. Eto, T. Shingu, T. Ito and T. Hasegawa, J. Antibiot., 1994, 47, 1242 CAS.
- T. Yamada, C. Iwamoto, N. Yamagaki, T. Yamanouchi, K. Minoura, T. Yamori, Y. Uehara, T. Andoh, K. Umemura and A. Numata, Tetrahedron, 2002, 58, 479 CrossRef CAS.
- T. Amagata and K. Minoura and A. Numata, J. Antibiot., 1998, 51, 432 CAS.
- G. N. Belofsky, P. R. Jensen, M. K. Renner and W. Fenical, Tetrahedron, 1998, 54, 1715 CrossRef CAS.
- L. Rahbaek, C. Christophersen, J. Friswald, H. S. Bengaard, S. Larsen and B. R. Rassing, J. Nat. Prod., 1997, 60, 811 CrossRef.
- A. Numata, C. Takahashi, Y. Ito, T. Takada, K. Kawai, Y. Usami, E. Matsumura, M. Imachi, T. Ito and T. Hasegawa, Tetrahedron Lett., 1993, 34, 2355 CrossRef CAS.
- S. M. Verbitski, C. L. Mayne, R. A. Davis, G. P. Concepcion and C. M. Ireland, J. Org. Chem., 2002, 67, 7124 CrossRef CAS.
- C. Iwamoto, T. Yamada, Y. Ito, K. Minoura and A. Numata, Tetrahedron, 2001, 57, 2997 CrossRef CAS.
- A. Numata, C. Takahashi, Y. Ito, K. Minoura, T. Yamada, C. Matsuda and K. Nomoto, J. Chem. Soc., Perkin Trans. 1, 1996, 239 RSC.
- C. Iwamoto, K. Minoura, T. Oka, T. Ohta, S. Hagishita and A. Numata, Tetrahedron, 1999, 55, 14353 CrossRef CAS.
- C. Iwamoto, K. Minoura, S. Hagishita, K. Nomoto and A. Numata, J. Chem. Soc., Perkin Trans. 1, 1998, 449 RSC.
- C. Takahashi, A. Numata, T. Yamada, K. Minoura, S. Enomoto, K. Konishi, M. Nakai, C. Matsuda and K. Nomoto, Tetrahedron Lett., 1996, 37, 655 CrossRef CAS.
- R. J. Cole and R. H. Cox, The Cytochalasins, in The Handbook of Toxic Fungal Metabolites, Academic Press: New York, 1981 Search PubMed.
- H. Maruta, H. He, A. Tikoo and M. Nur-e-Kamal, Ann. N. Y. Acad. Sci., 1999, 886, 48 CAS.
- B. W. Son, P. R. Jensen, C. A. Kauffman and W. Fenical, Nat. Prod. Lett., 1999, 13, 213 Search PubMed.
- C. Chen, N. Imamura, M. Nishijima, K. Adachi, M. Sakai and H. Sano, J. Antibiot., 1996, 49, 998 CAS.
- B. W. Son, J. S. Choi, J. C. Kim, K. W. Nam, D. S. Kim, H. Y. Chung, J. S. Kang and H. D. Choi, J. Nat. Prod., 2002, 65, 794 CrossRef CAS.
- M. K. Renner, P. R. Jensen and W. Fenical, J. Org. Chem., 1998, 63, 8346 CrossRef CAS.
- M. K. Renner, P. R. Jensen and W. Fenical, J. Org. Chem., 2000, 65, 4843 CrossRef CAS.
- M. Daferner, S. Mensch, T. Anke and O. Sterner, Z. Naturforsch. C: Biosci., 1999, 54, 474 Search PubMed.
- M. C. Justice, M. J. Hsu, B. Tse, T. Ku, J. Balkovec, D. Schmatz and J. Nielsen, J. Biol. Chem., 1998, 273, 3148 CrossRef CAS.
- G. Schlingmann, L. Milne, D. R. Williams and G. T. Carter, J. Antibiot., 1998, 51, 303 CAS.
- D. Albaugh, G. Albert, P. Bradford, V. Cotter, J. Froyd, J. Gaughran, D. R. Kirsch, M. Lai, A. Rehnig, E. Sieverding and S. Silverman, J. Antibiot., 1998, 51, 317 CAS.
- D. Abbanat, M. Leighton, W. Maiese, E. B. Jones, C. Pearce and M. Greenstein, J. Antibiot., 1998, 51, 296 CAS.
- M. Isaka, C. Suyarnsestakorn, M. Tanticharoen, P. Kongsaeree and Y. Thebtaranonth, J. Org. Chem., 2002, 67, 1561 CrossRef CAS.
- G. K. Poch and J. B. Gloer, J. Nat. Prod., 1989, 52, 257 CrossRef CAS.
- G. K. Poch and J. B. Gloer, J. Nat. Prod., 1991, 54, 213 CrossRef CAS.
- K. Liberra, R. Jansen and U. Lindequist, Pharmazie, 1998, 53, 578 Search PubMed.
- M. Namikoshi, K. Akano, S. Meguro, I. Kasuga, Y. Mine, T. Takahashi and H. Kobayashi, J. Nat. Prod., 2001, 64, 396 CrossRef CAS.
- D. Laurent, G. Guella, M. F. Roquebert, F. Farinole, I. Mancini and F. Pietra, Planta Med., 2000, 66, 63 CrossRef CAS.
- T. Yoshimoto, M. Namikoshi, H. Kobayashi and T. Hosoya, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1997, 39, 637 Search PubMed.
- M. Namikoshi, H. Kobayashi, T. Yoshimoto, S. Meguro and K. Akano, Chem. Pharm. Bull., 2000, 48, 1452 CAS.
- H. Kobayashi, S. Meguro, T. Yoshimoto and M. Namikoshi, Tetrahedron, 2003, 59, 455 CrossRef CAS.
- K. A. Alvi, A. Casey and B. G. Nair, J. Antibiot., 1998, 51, 515 CAS.
- J. Irie-Sasaki, T. Sasaki and J. M. Penninger, Curr. Top. Med. Chem., 2003, 3, 783 Search PubMed.
- G.-Y.-S. Wang, B. M. Borgeson and P. Crews, Tetrahedron Lett., 1997, 38, 8449 CrossRef CAS.
- G. N. Belofsky, M. Anguera, P. R. Jensen, W. Fenical and M. Kock, Eur. J. Chem., 2000, 6, 1355 Search PubMed.
- T. S. Bugni, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, R. M. VanWagoner and C. M. Ireland, J. Org. Chem., 2000, 65, 7195 CrossRef CAS.
- J. Malmstrom, C. Christophersen and J. C. Frisvad, Phytochemistry, 2000, 54, 301 CrossRef CAS.
- C. H. Liu, J. C. Meng, W. X. Zou, L. L. Huang, H. Q. Tang and R. X. Tan, Planta Med., 2002, 68, 363 CrossRef CAS.
- C. B. Cui, H. Kakeya, G. Okada, R. Onose, M. Ubukata, I. Takahashi, K. Isono and H. Osada, J. Antibiot., 1995, 48, 1382 CAS.
- M. Kondoh, T. Usui, T. Mayumi and H. Osada, J. Antibiot., 1998, 51, 801 CAS.
- T. Usui, M. Kondoh, C. B. Cui, T. Mayumi and H. Osada, Biochem. J., 1998, 333, 543 CAS.
- C. B. Cui, M. Ubukata, H. Kakeya, R. Onose, G. Okada, I. Takahashi, K. Isono and H. Osada, J. Antibiot., 1996, 49, 216 CAS.
- S. G. Toske, P. R. Jensen, C. A. Kauffman and W. Fenical, Tetrahedron, 1998, 54, 13459 CrossRef CAS.
- R. Uchida, H. Tomoda, M. Arai and S. Omura, J. Antibiot., 2001, 54, 882 CAS.
- H. Kakeya, C. Onozawa, M. Sato, K. Arai and H. Osada, J. Med. Chem., 1997, 40, 391 CrossRef CAS.
- Y. Mizushina, S. Kobayashi, K. Kuramochi, S. Nagata, F. Sugawara and K. Sakaguchi, Biochem. Biophys. Res. Commun., 2000, 273, 784 CrossRef CAS.
- H. Onuki, H. Miyashige, H. Hasegawa and S. Yamashita, J. Antibiot., 1998, 51, 442 CAS.
- Y. Lin, Z. Shao, G. Jiang, S. Zhou, J. Cai, L. L. P. Vrijmoed and E. B. Gareth Jones, Tetrahedron, 2000, 56, 9607 CrossRef CAS.
- A. Numata, M. Iritani, T. Yamada, K. Minoura, E. Matsumura, T. Yamori and T. Tsuruo, Tetrahedron Lett., 1997, 38, 8215 CrossRef CAS.
- T. Yamada, M. Iritani, M. Doi, K. Minoura, T. Ito and A. Numata, J. Chem. Soc., Perkin Trans. 1, 2001, 3046 RSC.
- T. Yamada, M. Iritani, K. Minoura, A. Numata, Y. Kobayashi and Y.-G. Wang, J. Antibiot., 2002, 55, 147 CAS.
- K. Komatsu, H. Shigemori, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2000, 63, 408 CrossRef CAS.
- T. Kagata, H. Shigemori, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2000, 63, 886 CrossRef CAS.
- L. A. McDonald, D. R. Abbanat, L. R. Barbieri, V. S. Bernan, C. M. Discafani, M. Greenstein, K. Janota, J. D. Korshalla, P. Lassota, M. Tischler and G. T. Carter, Tetrahedron Lett., 1999, 40, 2489 CrossRef CAS.
- G. A. Schiehser, J. D. White, G. K. Matsumoto, J. O. Pezzanite and J. Clardy, Tetrahedron Lett., 1986, 27, 5587 CrossRef CAS.
- G. K. Poch and J. B. Gloer, Tetrahedron Lett., 1989, 30, 3483 CrossRef CAS.
- A. Guerriero, M. D'Ambrosio, V. Cuomo and F. Pietra, Helv. Chim. Acta, 1991, 74, 1445 CAS.
- S. P. Abraham, T. D. Hoang, M. Alam and E. B. Gareth Jones, Pure Appl. Chem., 1994, 66, 2391 CAS.
- K. M. Jenkins, M. K. Renner, P. R. Jensen and W. Fenical, Tetrahedron Lett., 1998, 39, 2463 CrossRef CAS.
- G. N. Belofsky, P. R. Jensen and W. Fenical, Tetrahedron Lett., 1999, 40, 2913 CrossRef CAS.
- M. Cueto, P. R. Jensen and W. Fenical, Phytochemistry, 2000, 55, 223 CrossRef CAS.
- A. Numata, C. Takahashi, T. Matsushita, T. Miyamoto, K. Kawai, Y. Usami, E. Matsumura, M. Inoue, H. Ohishi and T. Shingu, Tetrahedron Lett., 1992, 33, 1621 CrossRef CAS.
- H. Shigemori, S. Wakuri, K. Yazawa, T. Nakamura, T. Sasaki and J. Kobayashi, Tetrahedron, 1991, 47, 8529 CrossRef CAS.
- M. Sugano, A. Sato, Y. Iijima, T. Oshima, K. Furuya, H. Kuwano, T. Hata and H. Hanzawa, J. Am. Chem. Soc., 1991, 113, 5463 CrossRef CAS.
- M. Sugano, A. Sato, Y. Iijima, T. Oshima, K. Furuya, H. Kuwano, H. Haruyama, K. Yoda, T. Hata and H. Hanzawa, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1991, 33, 699 Search PubMed.
- M. Sugano, A. Sato, Y. Iijima, K. Furuya, H. Kuwano and T. Hata, J. Antibiot., 1995, 48, 1188 CAS.
- M. Sugano, A. Sato, K. Saito, S. Takaishi, Y. Matsushita and Y. Iijima, J. Med. Chem., 1996, 39, 5281 CrossRef CAS.
- J. Nielsen, P. H. Nielsen and J. C. Frisvad, Phytochemistry, 1998, 50, 263 CrossRef CAS.
- J. Malmstrom, J. Nat. Prod., 1999, 62, 787 CrossRef CAS.
- J. Malmstrom, A. Ryager, U. Anthoni and P. H. Nielsen, Phytochemistry, 2002, 60, 869 CrossRef CAS.
- S. S. Afiyatullov, T. A. Kuznetsova, V. V. Isakov, M. V. Pivkin, N. G. Prokof'eva and G. B. Elyakov, J. Nat. Prod., 2000, 63, 848 CrossRef CAS.
- S. Afiyatullov, A. I. Kalinovsky, T. A. Kuznetsova, V. V. Isakov, M. V. Pivkin, P. S. Dmitrenok and G. B. Elyakov, J. Nat. Prod., 2002, 65, 641 CrossRef CAS.
- M. Alam, E. B. Jones, M. B. Hossain and D. van der Helm, J. Nat. Prod., 1996, 59, 454 CrossRef CAS.
- A. Guerriero, M. D'Ambrosio, V. Cuomo, F. Vanzanella and F. Pietra, Helv. Chim. Acta, 1988, 71, 57 CAS.
- A. Guerriero, M. D'Ambrosio, V. Cuomo, F. Vanzanella and F. Pietra, Helv. Chim. Acta, 1989, 72, 438 CrossRef CAS.
- A. Guerriero, V. Cuomo, F. Vanzanella and F. Pietra, Helv. Chim. Acta, 1990, 73, 2090 CAS.
- M. Chinworrungsee, P. Kittakoop, M. Isaka, A. Rungrod, M. Tanticharoen and Y. Thebtaranonth, Bioorg. Med. Chem. Lett., 2001, 11, 1965 CrossRef CAS.
- M. Chinworrungsee, P. Kittakoop, M. Isaka, R. Chanphen, M. Tanticharoen and Y. Thebtaranonth, J. Chem. Soc., Perkin Trans. 1, 2002, 2473 RSC.
- M. Barata, Fungi on the halophyte Spartina maritima in salt marshes, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press: Hong Kong, 2002 Search PubMed.
- V. Kumaresan, T. S. Suryanarayanan and J. A. Johnson, Ecology of mangrove endophytes, in Fungi in Marine Environments, ed. K. D. Hyde, Fungal Diversity Press: Hong Kong, 2002 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |