Thermodynamic and spectroscopic study on the binding of cationic Zn(ii) and Co(ii) tetrapyridinoporphyrazines to calf thymus DNA: the role of the central metal in binding parameters
Abstract
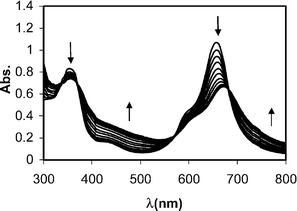
Binding of water-soluble N,N′,N″,N‴-tetramethyltetra-3,4-pyridinoporphyrazinatozinc(II) {[Zn(3,4-tmtppa)]4+} and N,N′,N″,N‴-tetramethyltetra-3,4-pyridinoporphyrazinatocobalt(II) {[Co(3,4-tmtppa)]4+} with calf thymus DNA has been investigated in 1 mM phosphate buffer and 5 mM NaCl at low ionic strength by UV-vis, fluorescence, and circular dichroism (CD) spectroscopic techniques, and also by the viscometric method. The appearance of a dramatic hypochromicity without any shift in the Q-band maximum of Zn(tmtppa) in the presence of DNA and a positive ellipticity in the visible CD of this porphyrazine complexed with DNA are definitive evidence of outside binding of this complex. It can be assumed that [Zn(3,4-tmtppa)]4+ in water is axially ligated by one H2O molecule. Therefore, this complex is inhibited from intercalation and binds externally. The interaction of [Co(3,4-tmppa)]4+ with DNA causes a decrease of the absorbance in the Q-band region of porphyrazine and a large red-shift. The visible CD of Co(tmtppa) complexed with DNA manifests a pattern of bisignate CD spectra, which possibly lead us to the coexistence of intercalation and outside binding modes. The binding constants were determined from the changes in the Q-band maximum of the porphyrazine spectra using SQUAD software. The values of K were (8.9 ± 0.3) × 104 and (2.3 ± 0.0) × 105 M−1 for [Co(3,4-tmtppa)]4+ and [Zn(3,4-tmtppa)]4+, respectively, at 27 °C. The higher affinity of the zinc complex towards DNA with respect to the cobalt complex was attributed to the coordination interaction as the major contributing factor, which enables superior interaction of the zinc complex with the DNA duplex. The thermodynamic parameters (ΔG°, ΔH°, ΔS°) were calculated from the van't Hoff equation at various temperatures. The enthalpy and entropy changes were determined to be: +39.6 ± 3.0 kJ mol−1 and +226.7 ± 10.5 J mol−1 K−1 for [Co(3,4-tmtppa)]4+ and +44.0 ± 3.3 kJ mol−1 and +249.6 ± 11.3 J mol−1 K−1 for [Zn(3,4-tmtppa)]4+. The positive and large values of the entropy and enthalpy suggest that both hydrophobic and electrostatic interactions may play an important role in the stabilization of the complex. The influence of the ionic strength was investigated. It was concluded that the apparent binding constants decrease with increasing [Na+] as predicted. Titration of DNA with Zn(tmtppa) produces moderate decreases in the solution-reduced viscosity (SRV), indicative of outside binding. The increase in the viscosity of DNA in the presence of the cobalt complex is related to the lengthening of the DNA helix due to the intercalation. The quenching of the DNA–ethidium bromide complex by the above-mentioned porphyrazines was investigated. The values of the quenching constants (KSV) and the rate constants of the quenching (kq) were determined by the Stern–Volmer equation.

 Please wait while we load your content...
Please wait while we load your content...