Green chemistry and photochemistry were born at the same time
Angelo
Albini
and
Maurizio
Fagnoni
Department of Organic Chemistry, The University of Pavia, I 27100 Pavia, Italy. E-mail: angelo.albini@unipv.it
First published on 2nd October 2003
Abstract
Where to look for really ‘green’ synthetic methods, under conditions as mild as those nature uses? A hundred years ago, a great scientist, Giacomo Ciamician, confronted the problem. He had no doubt of the answer: it was solar light. The approach and the discoveries by Ciamician are illustrated in connection with present-day green chemistry.
Angelo Albini is currently Professor of Organic Chemistry at the University at Pavia. He is active in the field of organic photochemistry, organic synthesis via radicals and photoinitiated reactions, applied photochemistry (e.g. photodegradation of dyes and plastics, photodegradation of pollutants, phototoxicity of drugs). He is coauthor of two books and ca. 200 research papers. |
Maurizio Fagnoni is currently Research Associate at the University of Pavia. His research concentrates on new synthetic methods, particularly through the mild photochemical generation of active intermediates such as radicals and ions. He is coauthor of ca. 40 research papers. |
Green ContextOne of the more neglected techniques available to green chemistry is photochemistry. The use of light as an energy source, and as an agent of chemical change, can allow very mild reaction conditions, and is certainly a sustainable raw material, at least for the next few tens of millions of years. This paper is an interesting and thought-provoking overview of the work of one of the pioneers in the area, and illustrates nicely the potential which has been around for a century in the field of photochemical synthesis.DJM |
1 Introduction
‘Green’ chemistry is the effort of ‘reducing or eliminating the use or generation of hazardous substances in the design, manufacture and application of chemical products’.1 Its birth is generally connected with the rather recent realization of the damage to the environment caused by the diffusion of polluting man-made materials and to the pollution generated in the process of producing them. Thus, the research for more environment friendly materials and for mild, direct, non-polluting synthetic methods is driven primarily by the moral imperative of avoiding an irreversible damage to the environment and by the economic motivation of avoiding the high cost of recovering polluted air and water.However, behind a research in green chemistry there might be also an intellectual motivation, the desire to show that a chemical process can be carried out in a cleaner, nicer way. Probably many synthetic chemists have felt this nowadays as well as in early times. An unparalleled example of clear-sightedness on this question is offered by the work of Giacomo Ciamician at the beginning of the 20th century.†Work during the last decades of the 19th century had laid the foundations for a large part of present day-used key paths in organic synthesis. In parallel the structure of many classes of primary and secondary metabolites had been recognized. He had himself participated in this effort, being among the founders of the chemistry of pyrrole.6,7 Thus, the basis of organic chemistry had been laid and the synthesis of artificial compounds, e.g. of dyes, was rapidly acquiring industrial significance.
However, he felt some dissatisfaction with this otherwise towering example of ingenuity. Addressing the French Chemical Society on June 8, 1908,8 he made the following remarks.
‘It has often been a reproach to the great successes of modern organic chemistry that victory has been obtained with too great a show of strength. Indeed, one has to admit that such an objection is not deprived of some ground. Using aggressive reagents and high temperatures is almost always unavoidable when carrying out an organic synthesis in the laboratory. Deploying energy would, on the other hand, not be so frustrating for modern organic chemistry, were it not that the living world, in particular plants, gives us the marvelous example of great results obtained, at least from what appears, by using minimal means.
Chemistry in the laboratory differs from chemistry of organisms not in the materials, but in the reagents used. It is thus apparent that the further advancement of biology requires that all of the compounds present in nature can be produced by using only reagents present in nature, rather than agents that do not belong to the living world.’
He had therefore wondered on which grounds may lie the superior ability of nature.
‘From this point of view, one should first consider enzymes. These are the main catalysts of the living world and recent results give us a sense of what we can expect in the future.
However, there is another agent of the highest importance, at least for plants, and which deserves to be studied in detail, and this is light. For plants, light is the source of energy. Through the intervention of chlorophyll, green plants accumulate solar energy and transform it into chemical energy.’
Therefore, he considered whether photochemical reactions could be carried out artificially, and considered also the energy-saving side of the matter.
‘It may also happen that the problem of using solar energy becomes interesting under another respect. When oil will have been all burned in our prodigal industries, it may become necessary, even on social grounds, to come to exploit solar energy.’
2 Discovering photochemical reactions
Ciamician reported in Paris on the work in photochemistry he had been carrying out in the previous ten years. The hypothesis was that the absorption of light was what allowed plants to synthesize chemicals in the cells under much milder conditions than chemists could in a flask. As we now know, it is not exactly like this. Plants use solar energy to accumulate NADPH and ATP and these are used in the actual (thermal) synthesis of chemicals. Ciamician had a feeling of the complexity of the topic and in the last years of his work he devoted pioneering studies to a better understanding of the actual chemistry occurring in cells, in particular to the role of glycosidation and how this affects the metabolism of chemicals in plants.9At any rate, in collaboration with Paul Silber, he set out to examine which chemical reactions are facilitated by, or require, solar light and, as he declared, ‘these studies aimed first of all to rationalize organic chemistry in plants’.‡ He could not achieve this goal, nor was there any indication of which classes of chemicals may be significantly photoreactive. However, an intensive and patient work based on the systematic exposition of all the chemicals he could get hold of to solar light, resulted in the discovery of a range of interesting photochemical processes. These occurred, as he had hoped, under unparalleled mild conditions in what can be considered the first deliberate, and highly successful, effort to establish ‘green’ procedures for chemical synthesis.
A large part of the key photochemical reactions now known was indeed present in his 1908 talk. In the following paragraphs, his classification will be followed (including also his later papers), and it will be attempted to relate his discoveries with the present-day significance of such processes for green synthesis.
2.1 Oxidations and reductions
A large group of reactions comes under this heading and, as he notices, involves straightforward hydrogen transfer, having no parallel in thermal chemistry. A convenient application is as a mild oxidation method, e.g., benzoquinone is quite effective in the selective oxidation of alcohols to aldehydes.10–12 Most usefully, polyalcohols are transformed into sugars identical to the natural products. As he remarks, photoinduced oxidation is a substitute for the use of strong oxidants, such as bromine or nitric acid. A step further, glucose is mildly oxidized to glucosone (Scheme 1). | ||
| Scheme 1 | ||
The reducing side of the reaction is likewise useful. Ciamician and Silber found that, while aliphatic aldehydes and ketones also abstract hydrogen, but give complex mixtures, aromatic ketones (and, with a lower yield, aldehydes) undergo clean bimolecular reduction to pinacols (Scheme 2). In this case not only alcohols, but also alkylaromatics function as reducing agents.13 Further products containing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond undergo reductive dimerization, e.g. alloxane to alloxantin.14
O bond undergo reductive dimerization, e.g. alloxane to alloxantin.14
 | ||
| Scheme 2 | ||
As we now know, all of these reactions exploit the fast hydrogen abstraction by excited carbonyls generating radicals. The principle has been extended in recent literature to poor hydrogen donors, even to alkanes, where photochemistry offers an extremely mild entry for the functionalization of non-activated C–H bonds.15 Further abstracting species, such as inorganic anions (e.g. polyoxometalates) or solid materials (e.g. titanium dioxide powder) have been added to ketones,16–18 with the advantage of being more easily regenerated under the conditions of the experiments and thus having a high turnover number. The radicals generated react with oxygen to give oxidized derivatives or add to electrophilic alkenes to form new C–C bonds (see below).
Nitroaromatics also work as photooxidants. Ciamician and Silber demonstrated the formation of aniline and all of the intermediate products of reduction from the irradiation of nitrobenzene in alcohols,19,20 and further demonstrated the structure of the several photoproducts formed from nitrobenzene and benzaldehyde or its phenylhydrazone.21
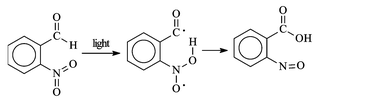
Importantly they found an example of intramolecular redox reaction in the smooth photoconversion of o-nitrobenzaldehyde into o-nitrosobenzoic acid, both in the solid state and in inert solvents (Scheme 3).22 They noted the higher efficiency of this reaction with comparison to the previous ones (actually due not to a higher quantum yield, but to the lower inhibiting effect by oxygen in this intramolecular reaction). They concluded that this is the only reaction ‘comparable in rate with the photographic processes’. Ciamician was pleased to find that another intramolecular reaction of a nitroaromatic was later used by Pfeiffer for preparing a nitrophenylisatogen23 and noted that this bore analogy with the then all important synthesis of indigo and possibly opened the way to the photochemical production of dyestuffs. The field in which intramolecular hydrogen abstraction by nitro group has nowadays found the largest application is actually the use of the o-nitrobenzyl moiety§ as a protecting group removable under exceptionally mild conditions (Scheme 4).25
 | ||
| Scheme 3 | ||
 | ||
| Scheme 4 | ||
2.2 Fragmentations
The irradiation of ketones causes α-cleavage, leading to e.g. methane and acetic acid from an acetone–water mixture.26 This is what is now known as the ‘Norrish I Type’ reaction of ketones. Particular attention was given to the efficient cleavage of cyclic ketones in aqueous media, which is quite effective and gives two types of products. These are open-chain saturated acids and unsaturated aldehydes, e.g. cyclohexanone gives hexanoic acid and 5-hexenal (Scheme 5). | ||
| Scheme 5 | ||
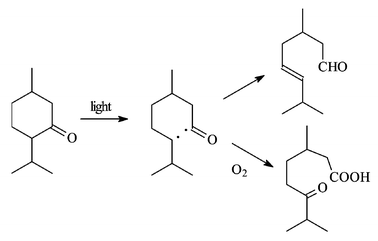
This was also proved by Ciamician and Silber through experiments quite demanding at the time, due to the difficulty of characterizing aliphatic aldehydes.26,27 The reaction has been later rationalized as involving α-cleavage, and the two alternative disproportionations of the biradical, leading either to the unsaturated aldehyde (‘Norrish Type II reaction’) or to the ketene (that undergoes water addition).28 The cleavage to acids has been later used for example for the mild synthesis of secosteroids.29 Furthermore, Ciamician and Silber noted that oxygen had an important effect on the reaction, due, as it is now understood, to trapping of the intermediate radicals. Oxidative cleavage may result under these conditions; e.g. menthone was converted to a ketoacid (whereas it gave 3,7-dimethyl-5-octenal in the absence of oxygen, Scheme 6, see also below).30,31
 | ||
| Scheme 6 | ||
2.3 Autooxidations
Under this category the Authors considered various processes. One is the above α-cleavage of aldehydes and ketones. Under oxygen the radicals are trapped and e.g. acetone gives acetic and formic acids, which may be viewed as a mild version of the permanganate cleavage of ketones (Scheme 7).8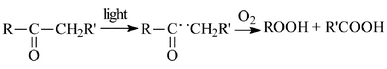 | ||
| Scheme 7 | ||
Furthermore, they noticed the conversion of stilbene to benzaldehyde (Scheme 8, also in the solid state), and drew an analogy between this oxidative cleavage and the results with a more aggressive reagent, ozone.8
 | ||
| Scheme 8 | ||
A large number of useful (also industrially) photoinduced oxygenation reactions have been since developed and recognized as involving the reaction of photogenerated radicals with oxygen (as it happens with ketones), or reactions with activated forms of oxygen, such as singlet oxygen or superoxide anion (as it happens with stilbene).32
Ciamincian and Silber also found that many substrates not themselves liable to auto-oxidation become active in the presence of xylene. As an example alcohols are oxidized to aldehydes (and polyalcohols to sugars) in the presence of p-xylene, reasonably because of the role of adventitious oxidation products from the aromatic in absorbing the light.33
2.4 Polymerizations and condensations
At the time, this title applied to carbon–carbon bond forming reactions in general. In the above mentioned H-abstraction reactions by ketones, cross-coupling products were formed. Thus, isobutyleneglycol was obtained from acetone and methanol (Scheme 9)34 and a similar coupling was obtained with ethers.35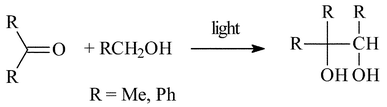 | ||
| Scheme 9 | ||
The reaction was particularly efficient when using aromatic ketones or alcohols, due to the stability of the radicals. Furthermore, cross coupling may be obtained from ketones. 2-Butanone gave 2-butanol and 3,4-dimethyl-1,5-hexandione (Scheme 10).36 As one may have expected from his experience with pyrroles, Ciamician promptly converted this to tetramethylpyrrole and wondered whether this may have a connection with the formation of chlorophyll in plants. Many years later, the final step in the celebrated first synthesis of chlorophyll a was indeed to be a photochemical step, though of a different type (an oxidative cleavage, as in the previous section).37
 | ||
| Scheme 10 | ||
Besides some polymerization reactions of aldehydes, possibly again involving radicals, the Authors classed here a different, and quite important, reaction, viz. 2 + 2 cycloaddition. Examples included the dimerization of cynnamic acid, confirming previous reports that it occurred only in the solid state, as well as the dimerization of stilbene and coumarine, which, they found, occurs also in solution.38,39 The Authors were quite excited by finding an intramolecular example of 2 + 2 cycloaddition in the case of carvone, in what may be considered a prototype for the use of photochemistry for the smooth and direct building of a highly congested molecule30 (Scheme 11, the product structure was correctly proposed, but finally proved later).40
 | ||
| Scheme 11 | ||
Together with the 2 + 2 carbonyl–olefin cycloaddition discovered (likewise with correct product structure proposal, though finally established later) by the other Italian scientist Paternò,41 this reaction has been then largely applied42 and represents an ideal example of directness in organic synthesis, another pillar of modern green chemistry.
2.5 Rearrangements
The Authors mention several cases of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N
C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond isomerization, as well as of syn–anti oxime isomerization.43 These reactions are of obvious importance in applications, e.g. with regard to dye photostability.
N double bond isomerization, as well as of syn–anti oxime isomerization.43 These reactions are of obvious importance in applications, e.g. with regard to dye photostability.
3 The nature of photochemical processes
Most of the reactions that Ciamician and Silber discovered are based on the polymorph reactivity of the triplet state of carbonyls and other nπ* states and on their ability to generate C-centered radicals. This is exactly the same point from which photochemistry started again after World War 2, when 40 years neglect had elapsed (his Alma Mater, Bologna, being one of the places where it flourished). The ability of photochemical reactions of generating highly reactive species with high selectivity and under exceptionally mild (and thus versatile) conditions is what gives significance to this discipline in the field of green chemistry.44 This actually was the target of the quest for synthetic methods as mild as those used by nature that had passionate Ciamician. This concept was still mentioned in the literature for some time after his death,45 but then faded away until present days.As for rationalization, a clear representation of electronically excited states (and still less of their reactivity) was not possible at the time, though Ciamician concurred6 with Plotnikow46 in thinking that light ‘produces a different ionization from that due to electrolytic dissociation; the separation of an ion requires a quantity of light that is determined by the theory of Planck and Einstein’¶
Quenching of the triplet states by oxygen dissolved in the solvents caused the typical induction periods that worried him and led to long irradiation times. Sealing the reaction vessels made the reaction faster after the initial period in which oxygen was consumed. At the moment, attention mainly was at recognizing the reaction occurring, rather than optimizing the yields (and this generally, not only in the case of photochemical reactions). However, Ciamician was highly pleased with the intramolecular reaction of o-nitrobenzaldehyde that, as mentioned above, did not suffer of these limitations. The cleanness of that reaction actually allowed it to be used, some years later, for the first measurement of quantum yield, giving an experimental support to Einstein's predictions (now known as the 2nd law of photochemistry).47
From another perspective, it is the chemistry of carbon-centered radicals that he has been discovering, and there is no need to stress how important in modern chemistry are syntheses based on such versatile intermediates.48 Indeed, photochemical initiation is now giving a contribution for the mild generation of radicals and their addition to electrophilic alkenes.49 In this case, a relation to some of his reactions can be recognized, since the high energy of excited states is exploited for generating radicals by cleaving strong C–H or C–C bond radicals, and such intermediates then are used for the desired bond forming reaction.
4 Exploiting solar energy and creating a photochemical industry
Ciamician had a clear idea of the importance of photosynthesis in the energetic balance of the Earth and the role of chlorophyll in it. In the 1908 lecture he recognized that ‘photochemical phenomena can themselves undergo the influence of catalysts, and it is probably to this class that the imposing phenomenon of assimilation|| has to be ascribed. Chlorophyll can be considered as a photocatalyst, which absorbs red light and causes the decomposition of carbon dioxide and its transformation into sugars’.8 At this time, no artificial photocatalytic reaction was known, but the great example of chlorophyll in nature showed that something of this type must be possible.In a general lecture before the International Congress of Applied Chemistry, held in New York in 1912,50 Ciamician was concerned with the contribution of photochemistry as a source of energy. Following recent contributions by Ramsay and Engler, he evaluated the available amount of coal and that mined yearly. He remarked that this was nothing else than a form of fossil solar energy.50,51 He compared available coal with the much larger amount of solar energy daily received by the Earth, and found that this was the only form of energy that could offer a lasting supply to industrialization (except, he noted, for the then barely suspected enormous energy involved in ‘atomic disintegration’). Yet, only a minimal fraction of that energy was stored by plants (still, an amount much larger than that daily production of coal). Advancement should then be in two directions.
The first one was a more advanced agriculture, specialized towards different aims, viz. both for food and for industrial supply, at this time the typical example being rapidly growing trees affording pulp for the paper industry. He thought however that plants could be adapted for the production of a harvest that, dried by the sun, could be converted ‘entirely to gaseous fuel, taking care during this operation to fix ammonia’,50 to be reused for inorganic fertilizers that thus would have been used in a closed cycle, minimizing waste (clearly the idea of ‘green gasoline’ had not to wait for the present decades). Furthermore, many industries elaborated agricultural products (cotton, starch, sugar, etc.) and of course would take advantage of an advanced agriculture.
However, he envisaged as even more important the second topic, the development of solar photochemistry on an industrial scale. He considered that plants produced an enormous amount of secondary metabolites, ultimately exploiting solar energy for the conversion. Chemists were learning how to produce a variety of compounds through artificial reactions and he was hoping the progress of science would have made these available also through photochemical reactions in the laboratory. A small example was offered by the photoreactions that he had been finding in those years.
Furthermore, the then growing fine chemicals industry (particularly for dyes) had experienced trouble in finding starting materials that were available in sufficient amounts and at convenient prices from the only source available for bulk chemicals, i.e. coal tar (as an example, naphthalene had to be chosen for making indigo, since toluene was not available in sufficient amounts). But what would happen if prices sored or supplies decreased? On the contrary, if a convenient panel of photochemical reactions could be developed, this would never suffer any shortcoming of the starting material, since non-exhaustible light would convert easily available raw materials into the desired compounds. He thought that there must be a way to transform water and carbon dioxide into methane and oxygen by the action of light in the presence of an appropriate catalyst, and similarly for other simple molecules such as ozone, sulfur trioxide or ammonia. As for fine chemicals, his own work had indeed shown that oxidation–reduction processes as well as carbon–carbon forming reactions, which he envisaged as an alternative to base catalyzed reactions such as aldolic condensation, were indeed possible. He did not confront the question of the efficiency of photochemical reactions (which at any rate could not be measured at the time), possibly because he felt that the flux of solar energy was so large as to make the point unimportant.
He thought that industry should not wait for a possible shortage of fossil fuel, but begin immediately and take advantage of photochemistry. With no prejudice against the development of advanced agriculture on suitable land, on arid lands a photochemical industry ‘without smoke and smokestacks’ would flourish. A ‘forest of glass tubes’ would rise and produce chemicals more abundantly than nature, ‘for nature is not in a hurry and mankind is’, through clean processes that would not harm the environment. This, he hoped, would lead the way for mankind to a ‘quieter civilization based on the utilization of solar energy’, where progress and happiness should not find the drawback that had characterized the ‘black and nervous civilization based on coal’.50
5 Conclusions
Ciamician pursued his research plan at a time where chemical industry was on the verge of the dramatic development that would make man-made materials predominant over natural ones in the course of the 20th century. Still in these early days, the supply of fossil fuel seemed next to inexhaustible and what seemed important was the extraordinary advancement in chemical synthesis, not on any drawbacks in the methods used. It is appalling how this great chemist foresaw the need that scientists should find alternative methods that were economic and non-polluting for giving mankind the chemicals it needed for a more prosperous life without exhausting natural resources or degrading the environment. These are the principles of present day green chemistry. Very little has been done in exploiting solar light (an in general really alternative paths for green chemistry) at the industrial level.52 However, academic research has opened many paths and, as Ciamician pointed out, ‘it has been established that modern industry is affiliated intimately with pure science; the progress of one determines necessarily that of the other’.50 His proposal, photochemistry, remains one of the basic alternatives to be considered for a really green synthesis and the progress of science now suggests how to use it.References
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; P. Tundo, P. Anastas, D. stC. Black, J. Breen, T. Collins, S. Memoli, J. Miyamoto, M. Polyakoff and W. Tumas, Pure Appl. Chem., 2000, 72, 1207 Search PubMed.
- W. McPherson, J. Am. Chem. Soc. (Proc.), 1922, 44, 101 Search PubMed.
- R. Plancher, Gazz. Chim. Ital., 1924, 54, 1.
- R. Nasini, J. Chem. Soc., 1926, 996 Search PubMed.
- N. D. Heindel and M. A. Pfau, J. Chem. Ed., 1965, 42, 383 CAS.
- G. Ciamician and M. Dennestedt, Ber. Dtsch. Chem. Ges., 1881, 14, 1153 Search PubMed.
- G. Ciamician, Ber. Dtsch. Chem. Ges., 1904, 37, 4200.
- G. Ciamician, Bull. Soc. Chim. Fr. [4], 1908, 3, i Search PubMed . (translated from the French by the present authors).
- G. Ciamician and C. Ravenna, Bull. Soc. Chim. Biol., 1923, 5, 59 Search PubMed.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1900, 33, 2911.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1901, 34, 1530.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1911, 44, 1280.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1910, 43, 1536 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1903, 36, 1575.
- A. Fokin and P. R. Schreiner, Chem. Rev., 2002, 102, 1551 CrossRef CAS.
- C. L. Hill, Synlett, 1995, 127 CrossRef CAS.
- A. Maldotti, A. Molinari and R. Amadelli, Chem. Rev., 2002, 102, 3811 CrossRef CAS.
- L. Cermenati, D. Dondi, M. Fagnoni and A. Albini, Tetrahedron, 2003, 59, 6409 CrossRef CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1905, 38, 3813.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1906, 39, 4343.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1905, 38, 1176.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1901, 34, 2040 CAS.
- P. Pfeiffer and E. Kramer, Ber. Dtsch. Chem. Ges., 1913, 46, 3655.
- F. Sachs and S. Hilpert, Ber. Dtsch. Chem. Ges., 1904, 37, 3425.
- C. G. Bochet, J. Chem. Soc., Perkin 1, 2002, 125 RSC.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1907, 40, 2415 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1908, 41, 1071 CAS.
- G. Quinkert, B. Wegemund and E. Blanke, Tetrahedron Lett., 1962, 221 CrossRef CAS.
- D. Arigoni, D. H. R. Barton, R. Bernasconi, C. Djerassi, J. S. Mills and R. E. Wolff, J. Chem. Soc., 1960, 1900 RSC.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1908, 41, 1928 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1909, 42, 1510 CAS.
- C. S. Foote, Photochem. Photobiol., 1991, 54, 659 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1913, 46, 3894.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1910, 43, 945 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1911, 44, 1554 CAS.
- G. Ciamician and P. Silber, Atti Acc. Lincei, Cl. Sc. Nat., 1912, 21, 547 Search PubMed.
- R. B. Woodward, G. L. Closs, E. Le Goff, W. A. Ayer, H. Dutler, W. Leimganter, J. M. Beaton, J. Hannah, W. Lwowski, F. Bickelhapt, F. P. Hanck, J. Sauer, R. Bonnet, S. Ito, Z. Valentia, P. Buchschacher, H. Langeman and H. Volz, J. Am. Chem. Soc., 1960, 82, 3800 CrossRef CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1902, 35, 4128 CAS.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1909, 42, 1386 CAS.
- G. Büchi and I. M. Goldman, J. Am. Chem. Soc., 1957, 79, 4741 CrossRef CAS.
- E. Paternò and G. Chieffi, Gazz. Chim. Ital., 1909, 39(I), 341.
- G. Kaupp, Houben-Weyl Methoden Organischen Chemie, Thieme, Stuggart, 1975, Vol. IV 5a, p. 278 Search PubMed.
- G. Ciamician and P. Silber, Ber. Dtsch. Chem. Ges., 1903, 36, 4266.
- A. Albini, M. Fagnoni and M. Mella, Pure Appl. Chem., 2000, 72, 1321 Search PubMed.
- E. Oliveri-Mandalà, Chim. Ind. (Milan), 1938, 20, 535 Search PubMed.
- J. Plotnikow, 1st Mendeleieff Congress, St. Petersburg, 1907–1908, p. 117, quoted in J. Plotnikow, Allgemeine Photochemie, de Gruyter, Berlin, 2nd edn., 1936, p. 236 Search PubMed.
- E. J. Bowen, H. Hartley, W. D. Scott and H. G. Watts, J. Chem. Soc., 1924, 1218 RSC.
- P. Renaud and M. Sibi, Radicals in Organic Synthesis, Wiley, Weinheim, 2001 Search PubMed.
- A. M. Cardarelli, M. Fagnoni, M. Mella and A. Albini, J. Org. Chem., 2001, 66, 7320 CrossRef CAS.
- G. Ciamician, Science, 1912, 36, 385 CAS.
- compare G. Porter, in J. D. Coyle, R. R. Hill and D. R. Roberts (Eds), Light, Chemical Change and Life, Open University Press, Milton Keynes, 1982, p. 338 Search PubMed.
- compare D. Martinez Plaza and S. Malato Rodriguez, Industrial Application of Solar Energy, Ciemat, Madrid, 2000 Search PubMed.
Footnotes |
| † Giacomo Ciamician was born in Trieste in 1857 from a family of Armenian origin. He studied chemistry at the University of Vienna and after graduation (1880 at Gießen) joined the very active group led by Cannizzaro in Rome. Here he pursued the study of the animal gelatin distillate begun in Vienna. This led to important contributions to the chemistry of pyrrole and other five-membered nitrogen heterocycles, and to the fundamental intuition of the similarity in the chemical behavior with phenols. In the same group worked the German chemist Paul Silber, who was to follow him when he accepted calls first to Padua (1887) and then to Bologna (1889) and was a life-long collaborator. In Bologna he extended his work on pyrrole, on several alkaloid families and on flavones, and then gave full impetus to the work on photochemistry, begun in 1886 in Rome. In this work, carried out by exposing for days or months sealed flasks of solutions to solar light on the roof of the Institute, Ciamician and Silber showed an extraordinary experimental skill in the identification of the sometimes labile photoproducts. In the final part of his work he studied chemical reactions in plants and the biological role of alkaloids. An internationally well known personality, he was proposed for the Nobel Prize (with the support of Emil Fischer) and strived throughout his life for cooperation among different disciplines, in particular of chemistry with physics on one hand and with biology on the other. Whoever, Italian3 or foreigner,2 had the opportunity of attending his lectures, was deeply impressed by his clear and logical presentations and by the enthusiasm he poured into them. Key accounts of his life and work may be found in refs. 2–5. |
| ‡ The first paper of the photochemical series was published in 1900,10 but he mentions that he had begun work in the field 14 years earlier. |
| § The intramolecular reactivity of o-nitrobenzyl alcohols has been reported in 1904 by Sachs.24 |
| ¶ Ciamician was among the first scientists to use systematically spectroscopy and a physical chemistry approach in both research and teaching organic chemistry. He, though seriously ill, was present at a lecture by Einstein in Bologna in 1922.3 |
| || This indicated the ‘assimilation’ of carbon dioxide and water yielding carbohydrates. |
| This journal is © The Royal Society of Chemistry 2004 |
