Ultrafast temporary charge transfer in pyrrolidinyl–benzonitrile and pyrrolyl–benzonitrile in the gas phase
Werner
Fuß
*a,
Wolfgang
Rettig
b,
Wolfram E.
Schmid
a,
Sergei A.
Trushin
ac and
Tomoyuki
Yatsuhashi
ad
aMax-Planck-Institut für Quantenoptik, D-85741 Garching, Germany
bHumboldt-Universität zu Berlin, Institut für Chemie, Brook-Taylor-Str. 2, D-12489 Berlin, Germany
cB.I. Stepanov Institute of Physics, Belarus Academy of Sciences, 220602, Minsk, Belarus
dDepartment of Chemistry, Graduate School of Science, Osaka City University, 3-3-138 Sugimoto, Sumiyoshi, 558-8585, Osaka, Japan
First published on 13th April 2004
Abstract
4-(N-pyrrolidinyl)benzonitrile and 4-(N-pyrrolyl)benzonitrile were excited by an ultrashort pulse at 270 nm to their La (S2) states and then probed by ionization at long wavelengths. Parent and fragment ion signals show components with time constants <100 fs which we attribute to ultrafast relaxation to the Lb (S1) state. From this short time we infer a conical intersection between the Lb and La surfaces. The wave packet can branch there, one part going temporarily to a strongly displaced state. Its shift was concluded from an anisotropy observed only there. The only excited state known to have a large displacement is the charge-transfer (CT) state. The positive anisotropy indicates that the CT state belongs to the 2A species for both molecules. For pyrrolylbenzonitrile, this is in contrast to previous assignments. The anisotropy, and a coherent oscillation observed in pyrrolidinylbenzonitrile, support the idea that the amino-group twist is an important component of the CT reaction coordinate.
1. Introduction
On UV excitation in solvents of suitable polarity, many donor–acceptor substituted aromatics such as 4-dimethylaminobenzonitrile (DMABN) show dual fluorescence, originating from an Lb-like and a charge-transfer (CT) state (reviews1–4). The pathway for charge transfer is still controversial (see e.g.refs. 5–7.), although the amino-group twist is most popular as the reaction coordinate. Gas-phase investigations with femtosecond time resolution have resulted in new insights, revealing the key role of a conical intersection (CI),8–10 a feature only beginning to be considered in CT reactions. Furthermore, coherent oscillations observed with this method gave evidence of the relaxation direction and thus also provides information about components of the reaction coordinate.9,10 The method involves exciting such a molecule by a weak femtosecond UV pump pulse (in our case at 270 nm) and then probing it by ionization by an intense IR pulse, measuring the ion yields mass-selectively as a function of pump–probe delay. Using various probe wavelengths (810, 1300 and 2100 nm), we investigated in this work 4-(N-pyrrolidinyl)–benzonitrile (PYRBN) and 4-(N-pyrrolyl)–benzonitrile (PBN). The donor group in the former is a saturated amino group, whereas in the latter it is the aromatic pyrrole.According to spectroscopy in cold molecular beams (PYRBN,11–13 PBN;14 see also the work on pyrrolylbenzene15), the longest-wavelength absorption leads to the Lb state (1B2 in the idealized symmetry C2v). The transition is weak and polarized perpendicularly to the long molecular axis. This band is insensitive to para-substituents (since the latter are in a node of the Lb wavefunction) and the medium (due to the small transition moment and little change of dipole moment). A much stronger absorption, parallel-polarized and partially covering the weaker one in solution (solution spectra of PYRBN:,16 of PBN:17–19) leads to the La state (symmetry 2A1). The excitation wavelength is near the absorption maximum of this band.
As will be further discussed in sec. 4, in the compounds with a saturated amino group the La state involves promotion of an electron from the HOMO to the LUMO, the former being π-antibonding and the latter weakly π-bonding between the amino group and the benzene ring (see e.g.refs. 20 and 21). As to conclude from the fluorescence polarization, the CT state also belongs to the A species in these cases;22 the La and CT states are thus just two locations on the same 2A surface.1,4,8,21,23,24 This surface is lowered from La to CT by interaction with a higher π-antibonding nA state on excursion along certain coordinates, a candidate for which is the amino-group twist.8,21 Along the same coordinates, the lower Lb state rises and crosses the 2A surface in a symmetry-allowed conical intersection (CI). After initial excitation to La, a wave packet can thus rapidly reach the CI and branch there to the CT and Lb states. The temporary population in CT is then depleted towards Lbvia a path leading around the lower cone of the CI (see sec. 4). We found this scheme to apply for DMABN and PIPBN8–10 and present evidence that it is also suitable for PYRBN. Surprisingly, it seems also to apply for PBN. This was not expected, because quantum chemical calculations predicted a 2B symmetry for the CT state, involving excitation from another pyrrole orbital to the LUMO.20,25 On the other hand, another calculation26 only reported on a CT state of 2A type. In pyrrolylbenzene two CT states, one with B and the other with A symmetry, were found very close-by.27
The pyrrole ring is slightly twisted versus the benzene ring in S0 and Lb of pyrrolylbenzene15 and probably similarly in PBN (symmetry group C2). Both rings are expected to be planar. In PYRBN, however, the amino N is slightly pyramidal, and the two rings are not twisted against each other (symmetry group Cs) according to a DFT calculation.28 This was also inferred from photoelectron spectra.16
(Such spectra were reported for PBN, too.17,29) A previous prediction of a slight twist and puckering of the pyrrolidino ring by molecular mechanics12 is probably less reliable than the DFT calculation.28 The kinetics and fluorescence of PYRBN30–36 and PBN17,18,29,37 were previously investigated in solution. It was found that the Lb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CT charge-transfer reaction required a solvent of at least medium polarity (to lower the CT state) for PYRBN, whereas PBN shows this reaction already in alkanes.
CT charge-transfer reaction required a solvent of at least medium polarity (to lower the CT state) for PYRBN, whereas PBN shows this reaction already in alkanes.
2. Experimental
The preparation of PYRBN and PBN is described in refs. 17, 29 and 33. The two species were investigated in the gas phase in the set-up (details described in ref. 38) heated to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C to avoid adsorption; the substances themselves were at 20
°C to avoid adsorption; the substances themselves were at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The molecules were pumped by a frequency-tripled pulse of a Ti:sapphire laser system (270 nm, 75 fs, 109 W cm−2) and then probed by nonresonant dissociative ionization at the fundamental wavelength (810 nm, 50 fs, 1013 W cm−2); in further experiments, probing was done by the output of a parametric generator (Topas) at 1300 and 2100 nm (40 fs, 1013 W cm−2). The two beams had linear polarizations with an angle of 55°
(“magic angle”) between each other in order to avoid time dependences induced by molecular rotation. In some experiments at 810 nm we also compared the signals at parallel and perpendicular polarizations. More experimental details are described in refs. 8 and 10.
°C. The molecules were pumped by a frequency-tripled pulse of a Ti:sapphire laser system (270 nm, 75 fs, 109 W cm−2) and then probed by nonresonant dissociative ionization at the fundamental wavelength (810 nm, 50 fs, 1013 W cm−2); in further experiments, probing was done by the output of a parametric generator (Topas) at 1300 and 2100 nm (40 fs, 1013 W cm−2). The two beams had linear polarizations with an angle of 55°
(“magic angle”) between each other in order to avoid time dependences induced by molecular rotation. In some experiments at 810 nm we also compared the signals at parallel and perpendicular polarizations. More experimental details are described in refs. 8 and 10.
The shape of the signals did not depend on the pump intensity (varied by a factor of about three). This proves that they are produced by a single pump photon. Varying the probe intensity by a similar factor did not change the time constants, although the later parts of the signals (coming from lower molecular states) were raised at higher probe intensities.
3. Results
The UV spectra of PYRBN and PBN (not shown), measured in the gas phase at 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, look very similar to that of DMABN.39 The strong structureless La band has a maximum at 273 (PYRBN) and 270 nm (PBN)
(4.54 and 4.59 eV). A weak precursor at longer wavelengths is certainly the Lb band for both molecules; according to the supersonic-beam spectra, their 00 bands are at 32
°C, look very similar to that of DMABN.39 The strong structureless La band has a maximum at 273 (PYRBN) and 270 nm (PBN)
(4.54 and 4.59 eV). A weak precursor at longer wavelengths is certainly the Lb band for both molecules; according to the supersonic-beam spectra, their 00 bands are at 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01811–13 and 34
01811–13 and 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424 cm−114
(3.97 and 4.26 eV), respectively. Hence the initial excitation at 270 nm places the molecules at an energy of 0.57 and 0.33 eV above the Lb minimum.
424 cm−114
(3.97 and 4.26 eV), respectively. Hence the initial excitation at 270 nm places the molecules at an energy of 0.57 and 0.33 eV above the Lb minimum.
The ion signals investigated with time resolution were the parent ion (M+) and the most intense fragment (about 10% of M+), which was M+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for PYRBN and M+
1 for PYRBN and M+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 for PBN. Time dependent signals at different probe wavelengths are shown for the first few picoseconds in Figs. 1 and 2 for PYRBN and 3 for PBN. Similarly as found in PIPBN10,40 and DMABN,9 PYRBN exhibits a periodic modulation of the signals between about 0.5 and 3 ps which is extracted (see below) and Fourier-transformed in Fig. 2 from the data for the parent ion with 1.3 μm probing. By contrast, although the PBN signals show some irregularities, an oscillation could not unambiguously be extracted from them. The time evolution was measured up to 600 ps (not shown); in this part, the signals simply decayed doubly exponentially.
28 for PBN. Time dependent signals at different probe wavelengths are shown for the first few picoseconds in Figs. 1 and 2 for PYRBN and 3 for PBN. Similarly as found in PIPBN10,40 and DMABN,9 PYRBN exhibits a periodic modulation of the signals between about 0.5 and 3 ps which is extracted (see below) and Fourier-transformed in Fig. 2 from the data for the parent ion with 1.3 μm probing. By contrast, although the PBN signals show some irregularities, an oscillation could not unambiguously be extracted from them. The time evolution was measured up to 600 ps (not shown); in this part, the signals simply decayed doubly exponentially.
 | ||
| Fig. 1 The time-dependent PYRBN parent (mass 172 u) and fragment (171 u) ion signals (normalized to the maxima), measured at the indicated probe wavelengths at the magic angle between pump and probe polarizations. The parent is actually about 10 times stronger than the fragment. The solid lines represent simulations of the nonperiodic parts (see text). | ||
 | ||
Fig. 2 Upper panel: The PYRBN parent ion signal with probing at 1310 nm. The thin solid lines show the simulated contributions 172σ1L1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172σ2L2,172σ3L3 and 172σ4L4
(see text) from locations L1–L4 to the nonperiodic part of the signal. Subtracting their sum (the sums are shown in Fig. 1) from the data leaves the oscillatory residue at the bottom of the panel, which is simulated by function (1). The bold solid line is the sum of nonperiodic and periodic parts of the simulation. Lower panel: Fourier transform of the bottom trace of the upper panel. 172σ2L2,172σ3L3 and 172σ4L4
(see text) from locations L1–L4 to the nonperiodic part of the signal. Subtracting their sum (the sums are shown in Fig. 1) from the data leaves the oscillatory residue at the bottom of the panel, which is simulated by function (1). The bold solid line is the sum of nonperiodic and periodic parts of the simulation. Lower panel: Fourier transform of the bottom trace of the upper panel. | ||
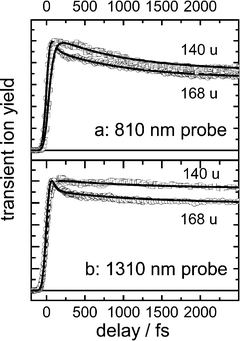 | ||
| Fig. 3 The time-dependent PBN parent (mass 168 u) and fragment (140 u) ion signals (normalized to the maxima), measured at two probe wavelengths at the magic angle between pump and probe polarizations. The parent is actually about 10 times stronger than the fragment. The solid lines represent simulations of the nonperiodic parts (see text). | ||
In a first step, we simulated the nonperiodic part of the signals by a sum of exponentials with time constants τi (Table 1), convoluted with pump and probe pulses. Subtracting this part from the PYRBN data left the oscillating part (Fig. 2), whose Fourier transformation (Fig. 2 bottom) gave the wavenumber and period τosc also reported in Table 1. This part could also be simulated by a damped oscillation of the type
exp(−t/τdeph)cos(2πt/τosc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) φ) φ) | (1) |
| Exponential parts of PYRBN | ||||||
|---|---|---|---|---|---|---|
| i | 1 | 2 | 3 | 4 | 5 | Anisotropy r |
| τ i | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 fs 5 fs |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 fs 10 fs |
540![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 fs 50 fs |
160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ps 10 ps |
1100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ps 100 ps |
|
| 172 σ i (810 nm) | 1 | 0.243 | 0.235 | 0.262 | 0.157 | 0 |
| 171 σ i (810 nm) | 1 | 0.188 | 0.175 | 0.225 | 0.135 | 0.2 |
| 172 σ i (1300 nm) | 1 | 0.40 | 0.144 | 0.128 | ||
| 171 σ i (1300 nm) | 0 | 1 | 0.674 | 0.550 | ||
| 172 σ i (2100 nm) | 1 | 0.277 | 0.025 | 0.018 | ||
| Exponential parts of PBN | ||||||
|---|---|---|---|---|---|---|
| i | 1 | 2 | 3 | 4 | 5 | Anisotropy r |
| τ i | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 fs 5 fs |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 fs 10 fs |
910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 fs 90 fs |
104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ps 10 ps |
352![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 ps 21 ps |
|
| 168 σ i (810 nm) | 1 | 0.680 | 0.373 | 0.389 | 0.123 | 0 |
| 140 σ i (810 nm) | 0 | 0.890 | 1 | 0.940 | 0.205 | 0.3 |
| 168 σ i (1300 nm) | 1 | 0.488 | 0.447 | 0.335 | ||
| 140 σ i (1300 nm) | 0 | 1 | 0.673 | 0.496 | 0.162 | |
For a given molecule, the set of time constants is the same, independent of probe conditions. Only the coefficients of the exponentials depend on the probe wavelength and the ion mass observed. This is interpreted by population Li flowing consecutively from one observation window Li to the next. This flow is described by rate equations, whose solution is a sum of exponentials for each population (the same, independent of probe conditions). Each Li has a mass spectrum of its own, and their dependence on the probing conditions help to distinguish the signals from the different Li; the spectra are represented by the relative ionization cross sections mσi, i.e. the probabilities to produce an ion of mass m from window Li. They are simulation parameters and are also given in the table. The data are redundant for deducing the τi and the populations Li. Fig. 2 shows by the thin lines the simulation components (mσi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li), whose sums for all i add up to the signals for mass m. The very short τ1 is needed to reproduce the steep rise of the parent signals. τ2 causes, for instance, the delayed rise of the fragment as compared to the parent, clearly visible at 1300 nm. τ3 represents the decay of the initial spike, which is evident in particular at the longer probe wavelengths. The flat part of the signals extending from <1 ps to beyond the range shown is due to contributions from L3 and L4, the former decaying in the same time τ3 as the latter rises. The decays in the range up to 600 ps (τ4, τ5) are not shown.
Li), whose sums for all i add up to the signals for mass m. The very short τ1 is needed to reproduce the steep rise of the parent signals. τ2 causes, for instance, the delayed rise of the fragment as compared to the parent, clearly visible at 1300 nm. τ3 represents the decay of the initial spike, which is evident in particular at the longer probe wavelengths. The flat part of the signals extending from <1 ps to beyond the range shown is due to contributions from L3 and L4, the former decaying in the same time τ3 as the latter rises. The decays in the range up to 600 ps (τ4, τ5) are not shown.
Fig. 4 shows the anisotropies r calculated for the two molecules from the signals at 810 nm (other wavelengths not investigated) with the probe polarization parallel and perpendicular to that of the pump:
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (S∥ (S∥![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S⊥)/(S∥ S⊥)/(S∥![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2S⊥). 2S⊥). | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 for the parent ion, whereas for the fragment r begins with positive values and decays in the picosecond range. The asymptotic value, which is difficult to determine, is set to 0 in the figure.
0 for the parent ion, whereas for the fragment r begins with positive values and decays in the picosecond range. The asymptotic value, which is difficult to determine, is set to 0 in the figure.
 | ||
Fig. 4 Time dependence of the anisotropy r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (S∥ (S∥![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S⊥)/(S∥ S⊥)/(S∥![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2S⊥) of parent and fragment signals. (a) PYRBN, (b) PBN. The probe laser was at 810 nm. For the parent, r is practically zero. 2S⊥) of parent and fragment signals. (a) PYRBN, (b) PBN. The probe laser was at 810 nm. For the parent, r is practically zero. | ||
4. Discussion
4.1. Relaxation path for PYRBN
The PYRBN signals are very similar to those for DMABN and PIPBN.8,10 Because also the molecules are very similar, we can directly take over the assignments. Consequently we also use the same scheme of potential surfaces and relaxation paths (Fig. 5). Similarly as in DMABN, an evidence for transient population of the CT state is based on the anisotropy. This can be seen as follows: The ionization itself is isotropic, which is confirmed by the zero-anisotropy observed for the parent ion. Photodissociation of the parent ion by absorption of an additional probe photon can, however, give rise to an anisotropy of the fragment ions, which will be positive if there is a resonant ionic transition at 810 nm (1.53 eV) polarized parallel to the pump laser, that is parallel to the long axis of the molecule. After vertical ionization from a state with near-equilibrium geometry, there is no such resonance, as to judge from the energy difference of photoelectron bands.16 Therefore a substantial distortion is necessary in the neutral molecule to reach a geometry with such a resonance in the ion; the only low-lying excited state with strong displacement is the CT state. This assignment implies that the anisotropy decay of Fig. 4 contains a contribution from this internal relaxation (time constant τ3) and is not purely due to rotation; evidence for such a double origin was presented for DMABN in ref. 9. As already discussed previously,8 the temporary population of the CT state implies that low-quantum yield CT emission should be detectable from the gas phase and in nonpolar solvents; the latter has indeed been observed in DMABN.41,42 It is interesting in this context that a recent work on spectroscopy of similar molecules in cold supersonic beams also suggested population of the CT state, if the excess energy is sufficient.43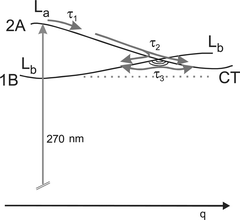 | ||
| Fig. 5 Potential energy surfaces and relaxation paths. The reaction coordinate q changes direction along the path, but probably everywhere contains the amino-group twist as an important component. The second coordinate spanning the branching space of the conical intersection (CI) must have b symmetry and probably contains the amino-group pyramidalization as a component. The τ3 equilibration follows a path around the lower cone. This diagram is used for both PYRBN and PIPBN. | ||
It is instructive to have a closer look to the ionic resonance. Energy differences of ion states are just equal to orbital energy differences in the neutral molecules. Orbitals and their energies are easy to understand by the help of their nodal pattern. The HOMO of PYRBN has the symmetry species b1 and is π-antibonding between donor and acceptor moieties (Fig. 6). The ground state of the parent ion (2B1) has a hole in this orbital (single occupancy). The HOMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a node containing the amino group; it has the species a2. An ionic 2A2
1 has a node containing the amino group; it has the species a2. An ionic 2A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2B1 transition would be perpendicularly polarized, which would disagree with our parallel anisotropy in observation window L3
(or the strongly displaced state claimed to correspond to CT). HOMO
2B1 transition would be perpendicularly polarized, which would disagree with our parallel anisotropy in observation window L3
(or the strongly displaced state claimed to correspond to CT). HOMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 provides the right symmetry (b1), since an ionic 2B1
2 provides the right symmetry (b1), since an ionic 2B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2B1 transition would be parallel; it is π-bonding between amino donor and the benzonitrile parts. A displacement coordinate drastically weakening this π interaction may approach the two b1 orbitals sufficiently, so that a resonant transition in the ion will be possible, as indicated in Fig. 6. There are not many coordinates with this property. A twist of the amino group was predicted to do it,16,20 and Figs. 5 and 6 were therefore drawn assuming that the twist is an important (but not the only, see below) component of the reaction coordinate. A strong pyramidalization of the amino group would probably be less effective, and planarization would do the opposite.
2B1 transition would be parallel; it is π-bonding between amino donor and the benzonitrile parts. A displacement coordinate drastically weakening this π interaction may approach the two b1 orbitals sufficiently, so that a resonant transition in the ion will be possible, as indicated in Fig. 6. There are not many coordinates with this property. A twist of the amino group was predicted to do it,16,20 and Figs. 5 and 6 were therefore drawn assuming that the twist is an important (but not the only, see below) component of the reaction coordinate. A strong pyramidalization of the amino group would probably be less effective, and planarization would do the opposite.
 | ||
Fig. 6 Bottom: π orbitals and energies of neutral PYRBN (a) and PBN (b)
versus twist angle θ as calculated by Parusel.20 Top: Ionic states resulting from a hole in the corresponding orbitals; for instance, the ground state (2B1) of the ion PYRBN+ has a hole in the HOMO (b1 orbital). Ionic excitation energies are equal to orbital energy differences. The suggested resonance with the probe laser is indicated. Symmetry species in C2v are only given for zero twist angle. For θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90°, see ref. 20. The indicated ionization energies (in eV) from the different occupied orbitals were taken from photoelectron spectra.16 90°, see ref. 20. The indicated ionization energies (in eV) from the different occupied orbitals were taken from photoelectron spectra.16 | ||
So, as in PIPBN10 and DMABN,8,9 we assign for PYRBN the times below 100 fs (τ1, τ2) to departure from the Franck–Condon region and further relaxation through a conical intersection (CI) to both the Lb and CT minima (Fig. 5). The conclusion that the path leads through a CI can again be obtained from the shortness of τ2. The next time constant (τ3) corresponds to Lb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔
↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CT equilibration around the lower cone of the CI. As before, the much longer τ4 is ascribed to intersystem crossing, probably to Tn, which then can rapidly equilibrate with T1. From there, the molecule decays within τ5 to the ground state, from which ionization was too weak to be detected. (Any excited state would be detectable.) This process may be accompanied by decomposition such as H elimination, as suggested in PIPBN.9
CT equilibration around the lower cone of the CI. As before, the much longer τ4 is ascribed to intersystem crossing, probably to Tn, which then can rapidly equilibrate with T1. From there, the molecule decays within τ5 to the ground state, from which ionization was too weak to be detected. (Any excited state would be detectable.) This process may be accompanied by decomposition such as H elimination, as suggested in PIPBN.9
4.2. The oscillation in PYRBN
The observed oscillation period is 530 fs. Its damping (dephasing) time is 1.4 ps. The only excited state with sufficiently long lifetime is Lb (or the observation window L4, representing the Lb-CT equilibrium. This equilibrium is expected to lie on the Lb side, because the CT state is higher in energy). So the oscillation must be a vibration in the Lb well. In fact, converting the period τosc to wavenumbers ν by ν![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/cτosc, one receives 64 cm−1, very close to a value (69 cm−1) observed in supersonic-beam spectra of the Lb state of PYRBN.11–13
1/cτosc, one receives 64 cm−1, very close to a value (69 cm−1) observed in supersonic-beam spectra of the Lb state of PYRBN.11–13
There are not many vibrations with such a low wavenumber in this molecule. According to semiempirical PM3 calculations for S0, there are only three below 100 cm−1: (1) arching (42 cm−1), where the CN and pyrrolidine groups bend in phase out of plane versus the benzene ring, (2) amino group twist (54 cm−1) and (3) another arching (85 cm−1), where the CN and pyrrolidino-N bend in phase out of plane, whereas the C2H4 end group moves out of phase. Because the electronic interaction of the benzene with its substituents is only minor in the Lb state, these wavenumbers are not expected to be very different in this excited state. Hence, the observed oscillation should be assigned to the lower-frequency arching or to the twist vibrations. Arching involves the inversion motion of the amino nitrogen.
However, both vibrations deserve a little more scrutiny, taking the symmetry of PYRBN into account: According to DFT calculations,28 in the ground state, the twist angle θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and the wagging angle (angle between the benzene and pyrrolidine planes) is only ω
0 and the wagging angle (angle between the benzene and pyrrolidine planes) is only ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8°. Any barrier at ω
8°. Any barrier at ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 will be smaller than in DMABN (where ω
0 will be smaller than in DMABN (where ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15° in S044), where it has recently been reevaluated and found to be <100 cm−1 in S0 and <50 cm−1 in Lb.45
(As mentioned in the Introduction, a molecular-mechanics calculation predicted θ
15° in S044), where it has recently been reevaluated and found to be <100 cm−1 in S0 and <50 cm−1 in Lb.45
(As mentioned in the Introduction, a molecular-mechanics calculation predicted θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15° in S0.12 This would imply also a minor barrier at θ
15° in S0.12 This would imply also a minor barrier at θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0°. It would not much change the following considerations.) The Lb potential must be very similar, since the 00 transition is by far stronger than the adjacent bands in the vibrational structure.11–13 Therefore both types of vibration will occur between two turning points that are symmetry-equivalent. Also the ion is expected, due to the partial double bond between the amino group and the benzene ring, to have its potential minimum at θ
0°. It would not much change the following considerations.) The Lb potential must be very similar, since the 00 transition is by far stronger than the adjacent bands in the vibrational structure.11–13 Therefore both types of vibration will occur between two turning points that are symmetry-equivalent. Also the ion is expected, due to the partial double bond between the amino group and the benzene ring, to have its potential minimum at θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and ω
0 and ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, not displaced versus that of Lb. A vibration (belonging to an antisymmetric species) in such a symmetric potential will therefore give rise to signal maxima twice in each period. (This is the equivalent to the spectroscopic selection rule Δv
0, not displaced versus that of Lb. A vibration (belonging to an antisymmetric species) in such a symmetric potential will therefore give rise to signal maxima twice in each period. (This is the equivalent to the spectroscopic selection rule Δv![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for antisymmetric vibrations in electronic transitions. It will also apply for the 69-cm−1 structure in the PYRBN supersonic beam spectrum,11–13 if our assignment is correct.) Therefore we should divide by two to convert the observed period to wavenumbers:
2 for antisymmetric vibrations in electronic transitions. It will also apply for the 69-cm−1 structure in the PYRBN supersonic beam spectrum,11–13 if our assignment is correct.) Therefore we should divide by two to convert the observed period to wavenumbers:
ν![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2cτosc)−1 (2cτosc)−1 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 cm−1 for the vibration. At present we cannot decide between the two possible assignments to twist or arching. In DMABN we observed both types of oscillations,9 whereas in PIPBN we attributed a strong modulation to the arching vibration.9 Its assignment was based on a substituent mass effect observed for it. In PIPBN, the neutral and ion potentials are displaced against each other (due to the nonplanar piperidine ring), so that the factor of two in (3) does not apply, although it does apply in PYRBN for symmetry reasons: The potential is symmetric to ω
32 cm−1 for the vibration. At present we cannot decide between the two possible assignments to twist or arching. In DMABN we observed both types of oscillations,9 whereas in PIPBN we attributed a strong modulation to the arching vibration.9 Its assignment was based on a substituent mass effect observed for it. In PIPBN, the neutral and ion potentials are displaced against each other (due to the nonplanar piperidine ring), so that the factor of two in (3) does not apply, although it does apply in PYRBN for symmetry reasons: The potential is symmetric to ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 in this molecule. (We assume that the vibration takes place above any barrier.). The wavenumber thus calculated for PIPBN was 31.4 cm−1, very close to that calculated by (3) for PYRBN. Therefore we tend to the assignment as arching (involving amino group inversion). The other vibration might be excited, too, even if it is not observed because possibly the Lb and ion potentials are parallel in this direction, so that this vibration would not be Franck–Condon active and would not give rise to a signal modulation. The wavenumber observed by us (0.5
0 in this molecule. (We assume that the vibration takes place above any barrier.). The wavenumber thus calculated for PIPBN was 31.4 cm−1, very close to that calculated by (3) for PYRBN. Therefore we tend to the assignment as arching (involving amino group inversion). The other vibration might be excited, too, even if it is not observed because possibly the Lb and ion potentials are parallel in this direction, so that this vibration would not be Franck–Condon active and would not give rise to a signal modulation. The wavenumber observed by us (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 cm−1) deviates from that in the cold supersonic jet (0.5
64 cm−1) deviates from that in the cold supersonic jet (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 cm−1) by much more than the experimental error (0.5 cm−1, see Table 1). A plausible interpretation assumes that levels with v
69 cm−1) by much more than the experimental error (0.5 cm−1, see Table 1). A plausible interpretation assumes that levels with v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≫
≫![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are excited in our experiment, and the wavenumber difference is due to an anharmonic shift.
1 are excited in our experiment, and the wavenumber difference is due to an anharmonic shift.
Also the La potential will be symmetric to θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and ω
0 and ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, again due to the partial double bond. Therefore these vibrations will not (or nearly not) be Franck–Condon active in the pump transition. They are thus not expected to be excited by the pump laser. Instead, they will be stimulated by acceleration on the last slope on entering the Lb well from the CI. The direction of this slope therefore contains either θ or ω as an important component. Both can be justified (and would be supported by an assignment): It was argued above (see also Fig. 5) and previously8–10 that a CI is probably displaced from the Lb minimum towards a direction with θ as a component. On the other hand, a b-type vibration such as the inversion (ω) could be stimulated by the 2A
0, again due to the partial double bond. Therefore these vibrations will not (or nearly not) be Franck–Condon active in the pump transition. They are thus not expected to be excited by the pump laser. Instead, they will be stimulated by acceleration on the last slope on entering the Lb well from the CI. The direction of this slope therefore contains either θ or ω as an important component. Both can be justified (and would be supported by an assignment): It was argued above (see also Fig. 5) and previously8–10 that a CI is probably displaced from the Lb minimum towards a direction with θ as a component. On the other hand, a b-type vibration such as the inversion (ω) could be stimulated by the 2A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1B relaxation in the CI, which needs a symmetry-breaking distortion.9 It is remarkable that such oscillations can be understood as described, by considering a pathway with intermediate excursion of the wave packet via a conical intersection (as also recommended in ref. 46). A previous model47 would only consider electronic matrix elements and Franck–Condon factors between La and Lb; the latter factors are
1B relaxation in the CI, which needs a symmetry-breaking distortion.9 It is remarkable that such oscillations can be understood as described, by considering a pathway with intermediate excursion of the wave packet via a conical intersection (as also recommended in ref. 46). A previous model47 would only consider electronic matrix elements and Franck–Condon factors between La and Lb; the latter factors are![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, because the potentials are not shifted against each other in the θ and ω directions.
0, because the potentials are not shifted against each other in the θ and ω directions.
4.3. Relaxation path for pyrrolylbenzonitrile
The kinetic behavior of PBN is very similar to that of PYRBN: There seems to be the same number of steps or observation windows, and the time constants differ by not more than a factor of 2. This similarity is not expected, because PBN has 4 more π electrons than PYRBN, so that the level scheme could be different. In fact, the lowest-energy CT state was predicted to belong to the 1B species20,25 (in contrast to the 2A CT state of DMABN or PYRBN; see also the reviews.2,4Ref. 17 found evidence that two different CT states play a role in PBN, depending on the solvent). This state involves a transition from the HOMO of a2 symmetry (localized on the carbons of pyrrole, node containing the nitrogen) to the b1 LUMO of the benzonitrile moiety. The orbital energies thus calculated20 are shown as functions of the twist angle θ in Fig. 6.In this scheme, ionization from the CT state would leave a hole in the a2 orbital, giving an ion ground state 2A2. The positive anisotropy found (Fig. 4) indicates a resonance in the ion parallel to the molecular axis. We thus would need a 2A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2A2 transition in the ion or the corresponding transition between a pair of a2 orbitals. It is obvious from Fig. 6 that the available orbital pair does not approach each other along the twist, and it seems hard to imagine any other coordinate, relevant to a charge transfer reaction, that could do so: Both orbitals have a node containing the substituents. On the other hand, the pair of b1 orbitals just does the required energy approach along the twist coordinate. If we postulate that the CT state observed by us involves excitation of a b1 electron (HOMO
2A2 transition in the ion or the corresponding transition between a pair of a2 orbitals. It is obvious from Fig. 6 that the available orbital pair does not approach each other along the twist, and it seems hard to imagine any other coordinate, relevant to a charge transfer reaction, that could do so: Both orbitals have a node containing the substituents. On the other hand, the pair of b1 orbitals just does the required energy approach along the twist coordinate. If we postulate that the CT state observed by us involves excitation of a b1 electron (HOMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in Fig. 6b), certainly to the LUMO (b1) so that a 2A state would result, then the positive anisotropy would be understood by an ionic 2B1
1 in Fig. 6b), certainly to the LUMO (b1) so that a 2A state would result, then the positive anisotropy would be understood by an ionic 2B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2B1 transition in much the same way as for PYRBN (Fig. 6a). This postulate is very much encouraged by a CASPT2 calculation which predicts practically degenerate CT states of 2B and 2A symmetry in pyrrolylbenzene at perpendicular geometry.27 We suppose that attaching a para-cyano substituent (resulting in PBN) will lower the 2A state more than the 2B state. In fact, another CASPT2 calculation reports for PBN at low energy only a 2A state with CT character.26 We therefore interpret our anisotropy data as a support that the lowest CT state of PBN belongs to the 2A species and is thus on the same potential energy surface as the initially excited La state. This implies that the same scheme for the surfaces and relaxation paths can be used as for PYRBN (Fig. 5).
2B1 transition in much the same way as for PYRBN (Fig. 6a). This postulate is very much encouraged by a CASPT2 calculation which predicts practically degenerate CT states of 2B and 2A symmetry in pyrrolylbenzene at perpendicular geometry.27 We suppose that attaching a para-cyano substituent (resulting in PBN) will lower the 2A state more than the 2B state. In fact, another CASPT2 calculation reports for PBN at low energy only a 2A state with CT character.26 We therefore interpret our anisotropy data as a support that the lowest CT state of PBN belongs to the 2A species and is thus on the same potential energy surface as the initially excited La state. This implies that the same scheme for the surfaces and relaxation paths can be used as for PYRBN (Fig. 5).
In contrast to PYRBN, no oscillations were observed in PBN (although some irregularities in the signals might indicate that there are some). It may be that the Lb potential and that of the ion are better parallel in PBN than in PYRBN, so an existing vibration would cause less signal modulation in PBN, because it would not be Franck–Condon active in ionization.
5. Concluding remarks
It was surprising that the same potential energy scheme and relaxation paths (Fig. 5) could be used for PYRBN and PBN. Apparently the states arising from the additional π electrons in PBN are higher in energy, including also a CT state of B symmetry. It is also remarkable how little the early times τ1–τ3 differ between the molecules DMABN,8 PIPBN,10 PYRBN and PBN investigated by our method. The largest variation was found in τ3, which was 1.0, 1.9, 0.53 and 0.91 ps for the four compounds, respectively. τ3−1 is the sum of the forward (Lb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CT) and backward (CT
CT) and backward (CT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lb) rates in the Lb/CT equilibration, with the rate for the exothermic backward reaction dominating. The CT
Lb) rates in the Lb/CT equilibration, with the rate for the exothermic backward reaction dominating. The CT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lb reaction is less exothermic in the gas phase for PBN than for PYRBN. This can be concluded from the fact that CT fluorescence is already observed in nonpolar environments for PBN, whereas at least medium polar solvents are needed for PYRBN. Hence, the longer τ3 for the former is not surprising. The same can be said about the pair PIPBN and DMABN. It seems also natural that reverse differences of rates were found in solution for the forward (Lb
Lb reaction is less exothermic in the gas phase for PBN than for PYRBN. This can be concluded from the fact that CT fluorescence is already observed in nonpolar environments for PBN, whereas at least medium polar solvents are needed for PYRBN. Hence, the longer τ3 for the former is not surprising. The same can be said about the pair PIPBN and DMABN. It seems also natural that reverse differences of rates were found in solution for the forward (Lb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CT) reactions, whose reaction enthalpy is the negative of that of the CT
CT) reactions, whose reaction enthalpy is the negative of that of the CT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lb reaction.
Lb reaction.
It may be worth repeating8–10 a comment on the amino-group twist as a component of the reaction coordinate. Whereas this twist gives rise to observable consequences such as the anisotropy (in this work) or a coherent oscillation (this work and refs. 9 and 10) and many others,1–4 it will not be the only component: It seems natural that the electron transferred in the CT state to the benzonitrile moiety causes CC bond lengthening there. A CASPT2 calculation for PBN in fact found such effects.26 Because bond stretching is energetically more effective than twisting, such components can give rise to steep slopes, which may explain our very fast initial relaxations (τ1,τ2).
Acknowledgements
T.Y. thanks the Alexander von Humboldt foundation for a research fellowship and S.A.T. thanks the VW Foundation for a financial support within project I/76 283.References
- E. Lippert, W. Rettig, V. Bonačić-Koutecký, F. Heisel and J. A. Miehé, Adv. Chem. Phys., 1987, 68, 1 CAS.
- W. Rettig, Angew. Chem., Int. Ed. Engl., 1986, 25, 971 CrossRef.
- W. Rettig, Top. Curr. Chem., 1994, 169, 254.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef.
- W. Rettig, B. Bliss and K. Dirnberger, Chem. Phys. Lett., 1999, 305, 8 CrossRef CAS.
- K. A. Zachariasse, Chem. Phys. Lett., 2000, 320, 8 CrossRef CAS.
- W. Rettig and B. Zietz, Chem. Phys. Lett., 2000, 317, 187 CrossRef CAS.
- W. Fuß, K. K. Pushpa, W. Rettig, W. E. Schmid and S. A. Trushin, Photochem. Photobiol. Sci., 2002, 1, 255 RSC.
- S. A. Trushin, T. Yatsuhashi, W. Fuß and W. E. Schmid, Chem. Phys. Lett., 2003, 376, 282 CrossRef CAS.
- T. Yatsuhashi, S. A. Trushin, W. Fuß, W. Rettig, W. E. Schmid and S. Zilberg, Chem. Phys., 2004, 296, 1 CrossRef CAS.
- C. Dedonder-Lardeux, C. Jouvet, S. Martrenchard, D. Solgadi, J. McCombie, B. D. Howells, T. F. Palmer, A. Subaric-Leitis, C. Monte, W. Rettig and P. Zimmermann, Chem. Phys., 1995, 191, 271 CrossRef CAS.
- B. D. Howells, J. McCombie, T. F. Palmer, J. P. Simons and A. Walters, J. Chem. Soc., Faraday Trans., 1992, 88, 2587 RSC.
- B. D. Howells, J. McCombie, T. F. Palmer, J. P. Simons and A. Walters, J. Chem. Soc., Faraday Trans., 1992, 88, 2595 RSC.
- L. Belau, Y. Haas and W. Rettig, Chem. Phys. Lett., 2002, 364, 157 CrossRef CAS.
- K. Okuyama, Y. Numata, S. Odawara and I. Suzuka, J. Chem. Phys., 1998, 109, 7185 CrossRef CAS.
- W. Rettig and R. Gleiter, J. Phys. Chem., 1985, 89, 4676 CrossRef CAS.
- W. Rettig and F. Marschner, New J. Chem., 1990, 14, 819 Search PubMed.
- C. Cornelissen-Gude and W. Rettig, J. Phys. Chem. A, 1998, 102, 7754 CrossRef CAS.
- T. Yoshihara, V. A. Galievsky, S. I. Druzhinin, S. Saha and K. A. Zachariasse, Photochem. Photobiol. Sci., 2003, 2, 342 RSC.
- A. B. J. Parusel, Phys. Chem. Chem. Phys., 2000, 2, 5545 RSC.
- L. Serrano-Andrés, M. Merchán, B. O. Roos and R. Lindh, J. Am. Chem. Soc., 1995, 117, 3189 CrossRef CAS.
- W. Rettig and S. Lutze, Chem. Phys. Lett., 2001, 341, 263 CrossRef CAS.
- A. L. Sobolewski, W. Sudholt and W. Domcke, J. Phys. Chem. A, 1998, 102, 2716 CrossRef CAS.
- W. Sudholt, A. L. Sobolewski and W. Domcke, Chem. Phys., 1999, 240, 9 CrossRef CAS.
- W. Rettig, J. Mol. Struct., 1982, 84, 303 CrossRef CAS.
- S. Zilberg and Y. Haas, J. Phys. Chem. A, 2002, 106, 1 CrossRef CAS.
- B. Proppe, M. Merchán and L. Serrano-Andrés, J. Phys. Chem. A, 2000, 104, 1608 CrossRef CAS.
- A. B. J. Parusel, Chem. Phys. Lett., 2001, 340, 531 CrossRef CAS.
- W. Rettig and F. Marschner, Nouv. J. Chim., 1983, 7, 425 Search PubMed.
- W. Rettig and G. Wermuth, J. Photochem., 1985, 28, 351 CrossRef CAS.
- R. Günther and D. Oelkrug, J. Phys. Chem., 1993, 97, 8512 CrossRef.
- D. Braun and W. Rettig, Chem. Phys. Lett., 1997, 268, 110 CrossRef CAS.
- W. Rettig, J. Lumin., 1980, 26, 21 CrossRef CAS.
- K. A. Zachariasse, M. Grobys, T. von der Haar, A. Hebecker, Y. V. Il'ichev, O. Morawski, I. Rückert and W. Kühnle, J. Photochem. Photobiol. A, 1997, 105, 373 CrossRef.
- K. A. Zachariasse, M. Grobys, T. von der Haar, A. Hebecker, Y. V. Il'ichev, Y.-B. Jiang, O. Morawski and W. Kühnle, J. Photochem. Photobiol. A, 1996, 102, 59 CrossRef CAS.
- T. von der Haar, A. Hebecker, Y. V. Il'ichev, Y.-B. Jiang, W. Kühnle and K. A. Zachariasse, Recl. Trav. Chim. Pays-Bas, 1995, 114, 430.
- M. Van der Auweraer, Z. R. Grabowski and W. Rettig, J. Phys.Chem., 1991, 95, 2083 CrossRef CAS.
- W. Fuß, W. E. Schmid and S. A. Trushin, J. Chem. Phys., 2000, 112, 8347 CrossRef CAS.
- R. Daum, S. Druzhinin, D. Ernst, L. Rupp, J. Schroeder and K. A. Zachariasse, Chem. Phys. Lett., 2001, 341, 272 CrossRef CAS.
- S. A. Trushin, W. Fuß, W. E. Schmid, T. Yatsuhashi, presentation at Conference on Femtochemistry 6, Paris, 2003.
- W. M. Kwok, C. Ma, D. Phillips, P. Matousek, A. W. Parker and M. Towrie, J. Phys. Chem. A, 2000, 104, 4188 CrossRef CAS.
- G. Wermuth, Z. Naturforsch., A, 1983, 38, 641.
- S. Jiang and D. H. Levy, J. Phys. Chem. A, 2002, 106, 8590 CrossRef CAS.
- O. Kajimoto, H. Yokoyama, Y. Ooshima and Y. Endo, Chem. Phys. Lett., 1991, 179, 455 CrossRef CAS.
- H. Saigusa, N. Miyakoshi, C. Mukai, T. Fukagawa, S. Kohtani and R. Nakagaki, J. Chem. Phys., 2003, 119, 5414 CrossRef CAS.
- W. Fuß, S. Lochbrunner, A. M. Müller, T. Schikarski, W. E. Schmid and S. A. Trushin, Chem. Phys., 1998, 232, 161 CrossRef.
- M. Bixon and J. Jortner, J. Chem. Phys., 1997, 107, 1470 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |

