High frequency monitoring reveals phytoplankton dynamics
Received
21st June 2004
, Accepted 29th September 2004
First published on 15th November 2004
Abstract
Phytoplankton is an important water quality indicator because of its high species differentiation, growth rates and responsiveness to environmental actuators. The new European Water Framework Directive calls for assessment of the duration, intensity and succession of phytoplankton blooms to determine the ecological status of various types of waters. For common phytoplankton growth rates basic signal processing theory yields a minimum monitoring frequency of once per day, which is much more than applied in standard practice. To assess the nature of this discrepancy we followed the behaviour of about 40 groups of organisms/particles found in the Oude Rijn river by a two-week daily cytometric analysis. Particle counts of the 20 most abundant groups are shown. Their variation rate and magnitude confirm that daily sampling is needed to follow such ecosystems in detail. It is shown that limiting the monitoring to the “coarse line” does not allow a correspondingly decreased sampling frequency. Automated systems may fill the gaps between the microscopical examinations by gathering highly frequent information. The information depth of bulk measurements is poor however, and not used as such. The data shown here demonstrate that modern scanning flow cytometry (SFC) offers an information depth close to the taxonomic level. In the past decade, acquisition and operation costs of these systems have come down considerably, whereas operation is hands free, even in situ and submerged, and data analysis has become more efficient. SFC is used most efficiently complementary to microscopical analyses for mutual validation. In these cases it presents a realistic solution to generate the essential high frequency observations required to assess ecosystem variability.
Introduction
The control and improvement of the ecological condition of surface waters is an important objective of the new European Framework Directive1 for the quality of European waters (WFD). This requires reliable and European-wide comparable methods to determine the ecological status of these waters. The suspended part of the flora, the phytoplankton, is ecologically important as it forms the basis of the food web by their sun light driven conversion of carbon dioxide and water into biomass. In addition, phytoplankton is a very dynamic indicator2 by its large species diversity, high growing rates (doubling times of a day down to one hour) and fast responses to the presence of nutrients, grazing by zooplankton, light-, temperature- and turbulence conditions and pollutants. Phytoplankton therefore forms an important part of the ecological quality rating of waters required by the new European Directive. However, among the various physical, chemical and biological quality elements, phytoplankton still appears to be one of the most difficult to determine. Current general practice is the taking of water samples including various steps such as preservation, concentration, transport and storage in order to allow microscopical determination of species and counting of individuals. The amount of work involved, also by its specialist nature, results in high costs per sample and is very time consuming. Besides the consequently limited number of sampling locations and often degraded counting accuracy, for instance by using a mere “high” or “low” indication of presence, this usually results in a very low sampling frequency. As a result, neither qualitative nor quantitative surveys generally sample the phytoplankton adequately,3,4 and the data obtained is hardly representative for the actual conditions of the water. The question is whether the quality of the monitored data still allows it to be used for determination of the ecological status and how modern technology may possibly be used to advantage.
The music-CD analogy
Counting phytoplankton at discrete moments in time can be seen as digitising a continuously fluctuating state, similar to the music-CD. Besides the numerical accuracy: “the more bits the better”, the other basic principle is that the sampling frequency should be at least double the highest significant frequency in the music in order to obtain good reproduction. If the sampling frequency is lowered too much, an increasingly large part of the signal fluctuations (system dynamics) remains unnoticed, with correspondingly impaired reproduction which may lead to, in cases of ecosystem monitoring, questionable ecological interpretations, if any. Considering that certain species may reach blooming conditions and start disappearing again within a weeks’ time—implying a maximum component of 2.5 to 3 per week in the frequency domain—a minimum sampling frequency of 5 to 6 times per week would be needed to follow this behaviour adequately. This is about ten times the current practice in many cases! The music-CD analogy suggests that almost daily sampling is required to let us clearly hear the violins next to the basses of the phytoplankton orchestra. Whereas noise and false tones are likely to emerge at moderately reduced sampling frequencies, at tenfold reduced sampling frequency it seems that barely any music will remain. How serious is this analogy; in other words are we not exaggerating with daily sampling?
Observations from a weekly sampling series
Time series of phytoplankton counts at high sampling frequency are scarce. Fig. 1 shows a recent year of a multi year series of phytoplankton counts5 of the Bedford Basin near Darthmouth, Nova Scotia, with a weekly sampling frequency. Noting the logarithmic scale this series shows considerable system fluctuations, which would have been missed out almost entirely when reducing to a sampling frequency of once per month. The “spiky” appearance of the peaks shows that this weekly sampling was still insufficient for an accurate reproduction of the actual system variations and peak values. The Bedford Basin is an estuary of approximately 10 km2 in direct connection with the Atlantic Ocean. This reduces the probability of hydraulic phenomena related to for instance a small water body, such as the fast warming of a pond, or surface water patchiness with local sub-populations floating with the water current. The measuring series shows representative growth and blooming and it appears that with these fast replicating and disappearing species the progression and succession of blooms and the response to system changes cannot be followed adequately using monthly or even bimonthly sampling frequencies. Low sampling frequencies limit the possible data interpretation to the “coarse line”: the annual cycle. However, these data need to be considered with care, because sampling even once a month may be insufficient to follow the coarse line in a reliable manner. To illustrate this, the same summer bloom (from Fig. 1) is shown twice in Fig. 2, one reproduction based on the measured values of each first week of the month and the other reproduction based on the measured values of each second week of the month. Obviously, the sampling frequency plays a key role at all levels.
 |
| | Fig. 1 Original data of weekly flow cytometrical phytoplankton counts of Bedford Basin in 2001 by Dr W. K. W. Li, Bedford Inst. of Oceanography, Nova Scotia, Canada. The information loss caused by reduction to monthly sampling (black dots) is indicated by the hatched area’s. | |
 |
| | Fig. 2 The same summer bloom (from Fig. 1) is shown twice on a linear scale: Black dots: reproduction based on the measured values of each first week of the month; White dots: reproduction based on the measured values of each second week of the month. | |
Options for intensifying the monitoring
The annual cycle may serve at most as a coarse quality indicator. The normative description of the WFD is based upon the assessment of the duration, intensity and succession of phytoplankton blooms. This serves to obtain a classification over five levels from good to bad, depending on the extent of possible deviations of species and their blooming pattern from the “undisturbed” state. Apart from the problem of defining the undisturbed state, it appears that the ecological qualification depends on the dynamic character of the phytoplankton. This in turn requires a monitoring system fast and accurate enough to follow the timing and blooming intensity of the various species. Where microscopical analyses are too expensive to allow a significant increase in sampling frequencies, automated observation systems are the logical next step. Bulk analyses, although relatively easily applicable in automated systems, describe only one aspect of system behaviour. The abundance plotted in Fig. 1 for instance represents the total cell numbers. Although the peaks in this type of measurement are often dominated by the smaller species, we do not know which species they were and we know nothing about the less abundant species. It is this information that is important to assess the biodiversity. The total amount of photosynthetic pigment (chlorophyll a) in the surface water can also be measured automatically as an indication of the amount of phytoplankton. Fig. 3 proves that with this type of entity relatively fast system fluctuations can also be expected that jeopardize the reliability of low frequency measurements. This type of singular analysis also lacks group or species related information, although these may contribute in various ways as shown in Fig. 3.
 |
| | Fig. 3 Fluorescence series from the river “Oude Rijn”, Nieuwerbrug, NL. Horizontal: day in March resp. April 2004. Vertical: total chlorophyll fluorescence of: all species smaller than 20 μm (diamonds); all species larger than 50 μm (triangles); all species together (circles). Measurements with CytoSense. | |
 |
| | Fig. 4 Below: “Nitschia” type colony of 4 symmetrical cells (photo from: Gerhard Drebes, Marines Phytoplankton, 1974) Top: corresponding CytoSense 1D scan consisting of 5 signal profiles. Circles: scattered light captured at near forward angles; diamonds: scatter at sideward angles; dots: red colored fluorescence (emitted by the phytoplankton basic pigment chlorophyll a); black line: orange fluorescence (predominantly from accessory pigments); grey line: green/yellow fluorescence (typically by some ciliates and cysts). | |
Results
This method has been used to execute a short monitoring series from 19 March until 5 April 2004 with (almost) daily sampling from the “Oude Rijn” river. The analysis of the samples was done with a CytoSense benchtop flow cytometer and CytoClus software (CytoBuoy b.v., Nieuwerbrug, The Netherlands). Up to 40 different kinds of organisms and particles could be identified in the measured data. Fig. 5 shows the results for the 20 groups with the highest numbers. Table 1 gives a limited description of those groups. The extent, speed and mutual (in)dependency of the fluctuations in the series is remarkable. This period was characterised by a short period of stormy weather at the beginning and a few sunny days later in the series. The samples were taken at the Nieuwerbrug bridge. This is in the middle of a 10 km long section with sluices at both sides in Woerden and Bodegraven, respectively. With generally low water flow and limited sideways in/out flow, we may assume that hydraulic phenomena as mentioned above were not dominant in this period. Because, as evident from the graphs, temperature and sun did play an important role (explosive growth of many species) we may also expect this type of fluctuation in waters with larger surfaces, especially shallow lakes.
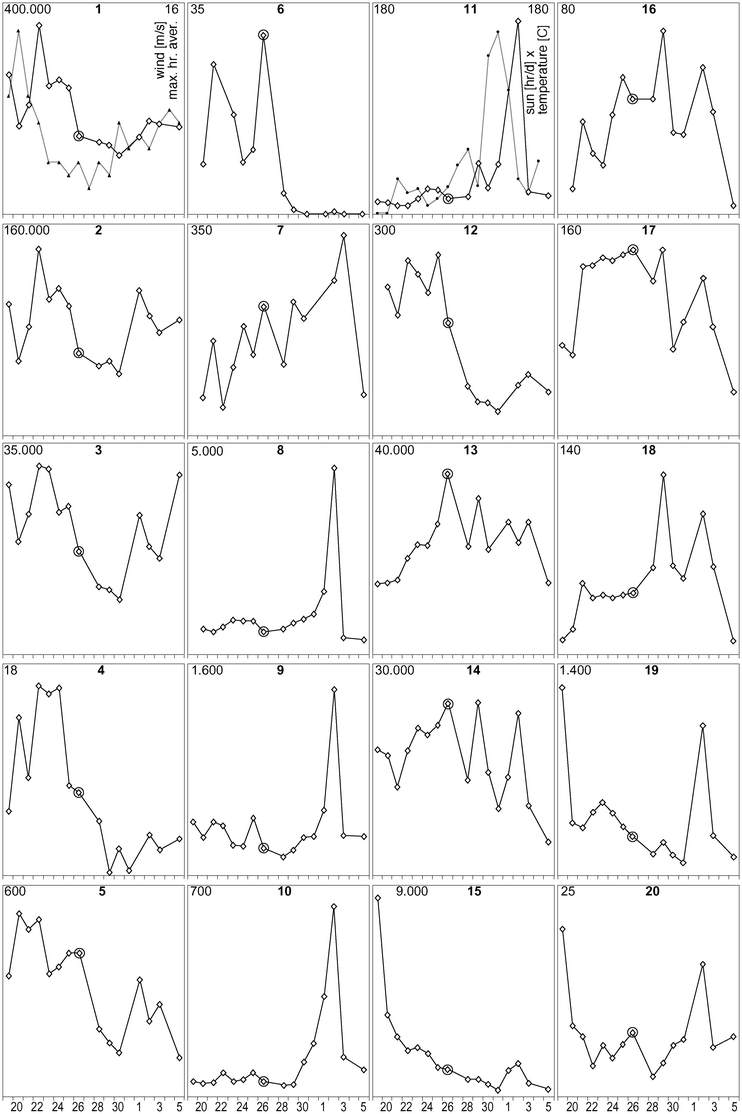 |
| | Fig. 5 CytoSense measuring series from the “Oude Rijn” river, Nieuwerbrug, 19 March–5 April 2004. Vertical: individuals per millilitre (linear scale with the range in upper left corner); Horizontal: day in March/April 2004. Wind and sun/temperature data were added to graph 1 and 11, respectively. | |
Table 1 (Morphological) Description of the 20 groups shown in Fig. 5
| (No taxonomic analyses were available for further identification of these groups.) |
| 1 |
Non fluorescent particles of 1–3 μm (sediment-debris). |
| 2 |
Non fluorescent particles of 3–10 μm (sediment-debris). |
| 3 |
Non fluorescent particles of 10–50 μm (sediment-debris). |
| 4 |
80–140 μm organisms with small local (12–18 μm) orange fluorescent section. |
| 5 |
30–100 μm particles with localized weak to moderate chlorophyll fluorescence. |
| 6 |
Filaments >50 μm, cyano type. |
| 7 |
Picoplankton cyano type. |
| 8 |
ca. 8 μm cells, moderate accessory pigment. |
| 9 |
20–35 μm Cryptomonas type cell. |
| 10 |
50–70 μm Cryptomonas type cell. |
| 11 |
Synura colonies (50–150 μm diam.) |
| 12 |
ca. 7 μm cells, with accessory pigment fluorescence. |
| 13 |
Picoplankton, green. |
| 14 |
Green cells, 3–15 μm. |
| 15 |
ca. 8 μm cells, high chlorophyll fluorescence. |
| 16 |
Closterium-like, symmetrical cells, 50–80 μm. |
| 17 |
Closterium-like, symmetrical cells, 70–100 μm, very thin. |
| 18 |
Spindle shaped, 70–90 μm cell with double chloroplast (30–40 μm). |
| 19 |
15–40 μm cells, high chlorophyll fluorescence. |
| 20 |
Dinobryon colonies, 100–200 μm length. |
From the overview of Fig. 5 it is obvious that no single set of numbers (such as the encircled values if for instance sampling took place on March 26th) yields a realistic indication of the system variations, nor a reliable base line. It shows that measuring frequency and information content have to be combined. As well as many categories, good time series are required to acquire knowledge about these dynamic ecosystems. Another advantage of the high number of measured categories in combination with a daily sampling frequency is the better distinction between ecosystem changes and hydraulic phenomena. Surface water patchiness or plug flow bringing a different water type along the sensor or sampling point would result in abrupt changes in all constituents whereas ecosystem changes generated by growth, grazing or environmental influences are not so fast and do not necessarily affect all constituents simultaneously and equally abruptly. Although these automated in situ instruments may be operated at multiple samplings per hour, a daily sampling with analysis of a sufficiently large number of categories suffices to maintain insight into many phenomena. The sharp drop in cell numbers of many of the species on April 3rd for instance might suggest a hydraulic cause (such as “strange” water flowing through the Oude Rijn from a water inlet) on first impression, but at least 10 of the 20 categories maintain more or less similar concentrations. Statistically this renders such a sudden hydraulic change highly unlikely—the sharp drop is therefore likely to be related to other (environmental) changes.
Discussion
SFC scans show less detail as compared to microscopy. Their discriminative potential depends on the number of independent optical profiles measured from each particle, and the parameterizing of these profiles: how efficiently the discriminatory features are extracted and expressed numerically. Boddy et al.10 presented Artificial Neural Network based analyses of 72 cultured species in a mixed sample with an overall identification rate of at least 70% using standard flow cytometric data basing on 7 optical parameters. Wilkins et al.11 had shown a 92% success rate for 34 somewhat more distinct species measured with an 11 parameter flow cytometer. The scanning flow cytometry presented here yields 30 parameters, of which we have used only 14 so far and distinguished typically between 30 and 50 groups of particles in natural samples of fresh and marine waters. Considering that the existing parameters were not yet exploited fully in these results, and additional parameters as well as optical observables are available for implementation, it is safe to assume that in the order of 100 groups of organisms can be distinguished with SFC if present in one sample of water. One could argue that this is by far insufficient to discriminate between the thousands of species of phytoplankton known in the world. However, thousands of species rarely occur simultaneously in one sample, in fact most microscopical species lists are below 100 species. Therefore in principle the majority of the species occurring in natural samples can be discriminated as separate groups and sometimes even as subgroups of single species in various life cycle or colony forming stages. It can occur, particularly with the smaller (<10 μm) single cell species, that two taxonomically different species are so similar in size, shape, intracellular structure and pigment content and type, that these species yield undistinguishable optical fingerprints and cannot be discriminated. In such cases the data group is not classified uniquely, but may represent more than one ‘candidate’ species. Overall, the measured categories are close to the species level, which constitutes an acceptable basis to draw ecological conclusions, as full species differentiation is not absolutely required. To identify the discriminated groups in terms of species names they are matched with available ‘template profiles’. Libraries of such templates can be made very easily by analysing monocultures. However, not all species can be cultured, particularly the culturing of colony forming species is cumbersome, and the morphology of natural species may differ from available cultures. Constructing the libraries from natural samples is possible however and it can be done most effectively using a laboratory based SFC instrument with physical sorting and/or “in-flow-imaging” capabilities to directly visualize the particles representing a selected profile. Even without these more expensive instruments, natural sample data from basic benchtop and in situ SFC instruments can be validated and libraries created using microscopical validation. With both the morphological information and the concentration of each group in the SFC data, an accurate microscopical analysis can be conclusive of the species representing these groups. Using high frequency SFC to complement low frequency microscopy is therefore ideal: the SFC classification of the groups found is validated by the microscopy and the variability of these groups is monitored by the SFC.
The data shown here only represent the basic possibilities of this type of equipment.12 As shown in various validations,13,14 flow cytometric analyses can improve the reliability of time series by reducing or eliminating errors related to mechanical and chemical sample treatment and counting statistics. Fig. 6 shows an example from a trial13 of counting of Rhodomonas sp. cells in natural North Sea samples by flow cytometry (OPA instrument—predecessor of CytoSense) and standard microscopy (Utermohl method). In the first year, large discrepancies between the flow cytometer and microscope results were observed. Then it was decided to change the microscopy protocol by using glutaraldehyde instead of lugol for fixation and including fluorescence observations. The flow cytometry was not changed. The next year showed a much better match, underlining the need for a critical evaluation of the traditional methods with the advent of new technologies. Generally, the flow cytometer counts were found to be accurate within approx. 10%.
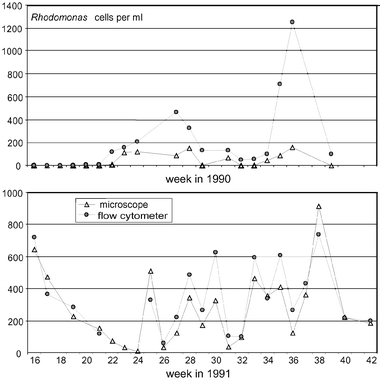 |
| | Fig. 6 Cell counts of Rhodomonas sp. in North Sea samples by flow cytometry (OPA instrument, predecessor of CytoSense) and microscopy. 1990 (top): microscopy according to traditional Utermohl method after lugol fixation; 1991 (below): fluorescence microscopy after glutaraldehyde fixation. | |
The SFC optical profiles acquired for each particle contain interesting quantitative information such as light scattering, chlorophyll fluorescence, cell size and colony size etc. This information can be combined into group information for each required classification level: from individual particles to identified species, groups, down to the whole sample (as shown basically in Fig. 3). Summation of this quantitative data over all particles analyzed in the whole sample yields information comparable to some “bulk” analyses such as abundance, chlorophyll a, and turbidity. Chlorophyll a for instance is often used in current monitoring practice as an indicator for the biomass of the phytoplankton. Whereas the determination of the relative biomass contributions of various groups and species is cumbersome with microscopy, it is a straightforward output of flow cytometric analyses.15 This can be of particular interest to assess specific indicators for deteriorated water quality: the harmful algae. These often include species with the highest population dynamics causing harm to the ecosystem or nuisance during blooming conditions. With positive indicators the presence or absence may be the most important parameter. The concentration may be less important than the detection threshold. The freshwater Desmids are a well described and relatively easy to recognize group of algae, increasingly used as positive water quality indicators. With their relatively stable shape and size they are particularly suited for fast recognition with the 1D flow cytometer scans.
This type of cytometric instrumentation evolved in a series of Dutch and European research and development projects. In the last decade further development resulted in lower investment and sustaining costs, combined with a higher level of instrument automation and more efficient data analysis. Currently available instruments allow application on fixed measuring points and moored buoy’s, as mobile units on shipboard or as submerged instruments.16,17 They generate highly frequent measuring series that may be used to improve the interpretation potential of low frequency microscopy analyses and allow a more accurate determination of the ecological condition of the phytoplankton in surface waters. The high analysis speed (a few minutes per sample) and the immediate availability of the measuring results may be used to dynamically adjust the sampling regime for microscopy based on real ecosystem changes. This may yield information on phenomena that would have been missed by a fixed sampling regime and may add functionality to the monitoring infrastructure aimed at surveillance and early warning. Besides addressing temporal variability, the assessment of spatial phytoplankton variability can also be improved owing to the high number of samples that can be processed. A very efficient strategy to combine high horizontal spatial coverage with high temporal frequency is to deploy a benchtop instrument on a regularly moving platform such as a ferry ship, sampling autonomously from a water supply hose. Using a lab based instrument is possible, but requires the collection of samples and the associated logistics. With a moored in situ instrument, a sample is taken at a standard 0.5 m depth at a fixed point. In the case of flowing waters horizontal patchiness will show up in the data, and correlating this with hydraulic data may yield insight into these patterns. Vertical profiles can be sampled on fixed locations, for instance by interfacing a benchtop or moored instrument with an undulating pump and hose system. An option for deep vertical casts is to use a submerged instrument operated from a ship’s winch. The ultimate solution to get any required horizontal and vertical spatial coverage is to deploy a submersible instrument in an autonomous underwater vehicle such as the UK Autosub. After an overview of ecosystem variability is obtained by high frequency spatial and temporal mapping, a sensible reduction can be made towards the most efficient combination of laboratory, mobile and/or fixed point in situ SFC instruments at representative locations and their dovetailing with microscopy.
Conclusions
Intensifying phytoplankton monitoring with the quantitative and reproducible analyses described here is a necessary and realistic option for the ecological monitoring of phytoplankton, particularly for situations requiring a high level of information. These include the determination of reference levels, at bio-hydraulic key locations, monitoring of important bathing areas and intake locations for large drinking water plants and in the case of project monitoring: the assessment of the effects of recovery measures. SFC is used most efficiently to complement microscopical analyses for mutual validation. The quantitative and versatile character of the data accommodates better comparisons between different types of water, more straightforward dovetailing to other observation platforms such as remote sensing, as well as more accurate identification of short and long-term trends and a basis for standardisation of phytoplankton monitoring in Europe.
Acknowledgements
The measurement data used for Figs. 1 and 2 was kindly provided by Dr W. K. W. Li, Bedford Inst. of Oceanography, Nova Scotia, Canada. The local water authorities “Hoogheemraadschap Stichtse Rijnlanden”, Houten NL, assisted us with information on water flow in the “Oude Rijn” river. We thank Prof. Dr J. Ringelberg and H. L. Hoogveld, NIOO – Center for Limnology, Dept. of Microbial Ecology, Nieuwersluis NL, for their valuable contributions to the text.
References
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy, Off. J. Eur. Communities EN, 22.12.2000 L 327/1.
- S. Nehring, ICES J. Mar. Sci., 1998, 55, 818–823 CrossRef.
- Th. J. Smayda, ICES J. Mar. Sci., 1998, 55, 562–573 CrossRef.
- J. W. Baretta, J. G. Baretta-Bekker and P. Ruardij, ICES J. Mar. Sci., 1998, 55, 756–766 CrossRef.
- W. K. W. Li and P. M. Dickie, Cytometry, 2001, 44, 236–246 CrossRef CAS.
- P. F. Culverhouse, R. Williams, B. Reguera, V. Herry and S. González-Gil, Mar. Ecol. Prog. Ser., 2003, 247, 17–25 Search PubMed.
- L. Boddy, C. W. Morris, M. F. Wilkins, G. A. Tarran and P. H. Burkill, Cytometry, 1994, 15, 283–293 CAS.
- J. W. Hofstraat, M. E. J. de Vreeze, W. J. M. van Zeijl, L. Peperzak, J. C. H. Peeters and H. W. Balfoort, J. Fluoresc., 1991, 1(4), 249–265.
-
G. B. J. Dubelaar, R. R. Venekamp and P. L. Gerritzen, Handsfree counting and classification of living cells and colonies, Proceedings of the 6th Congress on Marine Sciences, Havana, 1–5 December 2003, in press Search PubMed.
- L. Boddy, C. W. Morris, M. F. Wilkins, L. Al-Haddad, G. A. Tarran, R. R. Jonker and P. H. Burkill, Mar. Ecol. Prog. Ser., 2000, 195, 47–59 Search PubMed.
- M. F. Wilkins, L. Boddy, C. W. Morris and R. R. Jonker, Appl. Environ. Microbiol., 1999, 65, 4404–4410 CAS.
- M. Reckermann and F. Colijn, Sci. Mar., 2000, 64(2), 2000 Search PubMed.
-
W. J. M. van Zeijl, M. Rademaker, J. W. Hofstraat and W. A. J. de Waal, Analyse van algenmonsters met de Optical Plancton Analyser. Rapport DGW-93.052, RIKZ, DG, Rijkswaterstaat, The Netherlands Ministry of Public Works and Transportation (report in Dutch), The Hague, 1993 Search PubMed.
-
J. W. Hofstraat, W. J. M. van Zeijl, J. C. H. Peeters, L. Peperzak and G. B. J. Dubelaar, in Flow cytometry and other optical methods for characterization and quantification of phytoplankton in seawater, ed. H.O. Nielsen, Environment and Pollution Measurements Sensors and Systems, Int. Soc. Opt. Eng, S.P.I.E. Proceedings 1269, Bellingham, WA, 1990, pp. 116–133 Search PubMed.
- R. R. Jonker, J. T. M. Meulmans, G. B. J. Dubelaar and J. Ringelberg, Water Sci. Technol., 1995, 32(4), 177–182 CrossRef.
- G. B. J. Dubelaar and P. L. Gerritzen, Sci. Mar., 2000, 64(2), 255–265 Search PubMed.
-
A. Cunningham, G. B. J. Dubelaar and P. L. Gerritzen, in Submersible flow cytometer for marine particle analysis: design and initial trials, ed. S. G. Ackleson and J. Marra, SPIE, ASL&O, IOCCG Proc. Ocean Optics XV, Monaco, 2000, p. 1026 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2004 |
Click here to see how this site uses Cookies. View our privacy policy here. 





