Wetting and hydration of insoluble soot particles in the upper troposphere
N. M.
Persiantseva
a,
O. B.
Popovicheva
*a and
N. K.
Shonija
b
aInstitute of Nuclear Physics, Moscow State University, 119 992, Moscow, Russia. E-mail: polga@mics.msu.su; Fax: +7-95-939 49 56; Tel: +7-95-939 49 54
bChemical Department, Moscow State University, 119 992, Moscow, Russia
First published on 11th November 2004
Abstract
Wettability and hydration are determined for aircraft combustor and laboratory-made soots which are used as surrogates for the insoluble part of aircraft-generated black carbon particles in the upper troposphere (UT). The measured water/ice contact angles on the soot surfaces are in the range 60–80°. Factors influencing the soot wetting show a tremendous dependence on the surface chemical composition and microstructure. Wetting characteristics of soots are directly related to its hydrophilicity. The inverse Kelvin effect is considered as a mechanism of ice nucleation which is facilitated by the soot agglomerated structure with interparticle cavities in which condensation takes place on the insoluble surface with a high water contact angle. Estimations of the critical supersaturations needed for the ice condensation growth of particles are provided to determine which of the wetting characteristics are required for cirrus cloud formation in ice saturated regions of the UT.
1. Introduction
Black carbon (BC) particles are found to be one of main constituents of the background aerosols in the upper troposphere (UT).1,2 Recent accounts of soot particle measurements in the UT have reported their mean number density to be in the order of 0.1 cm−3, being in covariance with aircraft traffic.3 Observations in the aircraft near-field confirm that the insoluble aerosols of jet exhausts consist mainly of BC particles.4,5Formation of visible contrails in the UT is the most obvious effect of soot emission into the atmosphere,6 but aircraft may also impact the climate indirectly if exhaust particles act as ice nuclei (IN) and alter natural cirrus clouds. Remote sensing observations and theoretical studies of the earth’s climate prove that clouds have a strong influence on the radiative energy balance. In this context, ice nucleating aerosols have special significance because they may allow ice nucleation at lower supersaturations than those required for homogeneous freezing, resulting in an increase in the cirrus cloud coverage and changing its microphysics and optical properties.7 Analysis of ice nucleating aerosols taken from cirrus clouds showed that they are dominated by carbonaceous particles.8 However, there is considerable uncertainty regarding the quantitative estimate of the ice-nucleating ability of soot aerosols in the UT because (1) from in situ measurements it is difficult to obtain unambiguous evidence that soot particles are directly involved in the cloud formation9 and (2) surface physical and chemical requirements for IN are complex and poorly understood. Moreover, laboratory studies of soots produced by different combustion sources show a surprising variation in their water nucleation properties.10–15
Incorporation of soot aerosols into cloud particles depends on the wettability of their surface.16 However, the literature on the wettability of carbonaceous compounds yields a consistent picture of hydrophobicity.17–19 It means a nonzero water droplet contact angle, θ, which is traditionally associated with a poor ability to adsorb water and a low rate of heterogeneous nucleation. This is why there is a widespread assumption that fresh aircraft-emitted soot particles should be hydrophobic and poor substrates for nucleating water embryos until they have undergone a chemical activation through the accumulation of soluble species, such as sulfuric acid.20,21 As a result, ice nucleation in the exhaust plume is assumed to be due to freezing of sulfate coated soot particles.21,22 Following this suggestion, a mechanism for ice growth on insoluble soot particles in cirrus clouds is described by heterogeneous freezing nucleation.7
However, according to the fundamental accepted mechanism of water adsorption on carbonaceous adsorbents23–25 the initial water adsorption on soot is via oxygen-containing polar groups which act as active sites. Primary adsorbed water molecules may then act as secondary sites for further water molecule adsorption to build up hydrogen bonded clusters on the soot surface. Recent measurements of the water adsorption on soot produced by burning sulfur-free propane–butane fuel in an aircraft combustor have shown26 that due to chemical heterogeneity and microporous structure, such soot aerosols can acquire a substantial fraction of a water monolayer even under conditions found in the young plume.27 Also, kerosene flame soot produced by burning aviation kerosene in a lamp burner demonstrated the relatively high extent of its hydrophilicity.28 Quasi-elastic neutron scattering (QENS) and neutron diffraction (ND) studies combined with adsorption/texture analysis have showed that kerosene flame soot exhibits a significant freezing potential for ice nucleation in the soot mesopores.28,29 These data are in agreement with the results of Diehl and Mitra11 that kerosene burner exhaust particles may act as ice nuclei at temperatures lower than 250 K. This finding cannot be easily explained by the effect of soluble compounds on the soot surface16 because of the greatly reduced water soluble mass fraction obtained in the case of burning aviation kerosene under laboratory conditions.13
However, BC particles coated with sulfuric acid were not detected in large ice residual particles from contrails30 and sulfur was not observed in ice residual particles of cirrus clouds and interstitial aerosols.4 The analysis of contrail threshold conditions at different fuel sulfur contents (FSC) suggested that soot particles may take up water even at zero FSC.21 This means that there is a sulfur-independent pathway for IN formation on insoluble soot particles which are probably the most widely-distributed in the upper troposphere.
This paper examines the wettability and hydrophilicity of aircraft combustor and kerosene flame soots, which are suggested as the soot surrogates of insoluble carbonaceous particles in the UT, to determine a possible pathway of IN formation under tropospheric conditions. We report measurements of water/ice contact angles on soots and factors influencing the soot wetting. The isotherms of the water adsorption provide data on the amount of water adsorbing to and filling the interparticle mesopores. An irregular soot agglomerated structure is suggested to amplify ice nucleation due to condensation into soot interparticle cavities. The inverse Kelvin effect allows estimations of the critical supersaturations needed for ice condensation growth in the UT.
2. Experimental
The combustion chamber of a gas-turbine engine has been used to produce soot particles by burning a gaseous propane–butane (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture, for details see ref. 26. Typical combustion conditions simulated the cruise ones at an average air–fuel equivalence ratio near 4 and a flame temperature in the range 1500–1800 K. Soot samples were collected from the exhaust on an air-cooled stainless-steel probe at a distance of 12 cm from the combustor exit to minimize the time of contact with reactive exhaust gases. We will call this soot “combustor soot”.
1) mixture, for details see ref. 26. Typical combustion conditions simulated the cruise ones at an average air–fuel equivalence ratio near 4 and a flame temperature in the range 1500–1800 K. Soot samples were collected from the exhaust on an air-cooled stainless-steel probe at a distance of 12 cm from the combustor exit to minimize the time of contact with reactive exhaust gases. We will call this soot “combustor soot”.
Kerosene flame soot was produced in an oil lamp by burning aviation fuel of german production. We will call this soot “kerosene soot”. Additionally, we produced and examined properties of kerosene flame soots obtained by burning the aviation fuels known as TC1 and T6 (“TC1 soot” and “T6 soot”, respectively). One of the important differences in the composition of these kerosenes is the content of sulfur: in TC1 and T6 kerosenes it is not more than 0.11 and 0.05 wt%, respectively. Since the simplest approach to producing laboratory soot using different fuels is popular among researchers31,32 we also examined properties of soot obtained by burning the fuel for kerosene stoves and lanterns (“lamp soot”). All flame soots were collected at a distance of 15–20 cm above the flame on a glass support and were stored under vacuum until the laboratory examination.
The specific surface areas of soots were determined by a single point BET technique using N2 adsorption as described by Popovicheva et al.26 The total water soluble mass fraction of soots was determined after ultrasonic treatment in deionized water, filtration and evaporation. The amount of sulfur was determined by ion chromatography allowing determination of the sulfate ion (SO42−) mass in water extracted from the soot surface.
The electrical conductivity of soots is a useful macroscopic characteristic for comparative analysis because it is governed by both the soot microstructure and chemical composition.33 The specific conductivity was measured by compacting the soot powder in a compression setup similar to the “thumb screw” device described by Gregg and Pope.34 50 mg of the fluffy powder was poured into a mould and compacted at low speed. The experimental method consists of the simultaneous measurement of the volume of compacted soot and the soot conductivity. The electrical conductivity was measured by an LCR-meter E7-12 using a 1 MHz signal with an amplitude of 1 V. Experiments for each soot sample were carried out in triplicate and characterized by a reproducibility of 10%. During compaction the soot powder was pressed into a pellet of 0.08 cm height and 0.8 cm diameter with a polished mirror-like surface. The highest soot density reached was 0.5 g cm−3 at a compacting pressure of 106 N m−2. This corresponds to a soot porosity of approximately 0.75.
The classic technique of sessile drop measurement18 was used to determine the contact angle of the water droplet on the soot pellet and to analyze changes in the wettability which may accompany changes in the surface microstructure and composition of soots. Prior to the determinations, water droplets were calibrated to the small radius of 0.13 cm. The measurements of the geometrical size and contact angle of the water droplet on the soot pellet were performed using a Canon digital photocamera. Two light-guides were used for lighting the droplet and transferring its image to the optical system of the photocamera. The geometrical sizes and contact angles of droplets were quite reproducible within the accuracy of 0.003 cm and four degrees, respectively. The criterion for sufficient polishing of the pellet surface was the absence of any significant variation of contact angles for a particular position of a water droplet on the soot surface.
Soot pellets obtained as a result of compaction were porous and enabled water to diffuse into the soot bulk at room temperature. To nullify the effect of changing water droplet geometry during the measurement time, we performed the experiments at T = 275 K because at this temperature the rate of water diffusion was too small to see any change in droplets within the experimental accuracy.
Different kinds of pretreatment allowed us to determine the factors influencing the soot wetting. For this purpose kerosene soot was (1) outgassed at 2 × 10−3 Torr for 18 h and (2) outgassed and heated at 200 °C for 25 h. To remove organic carbon, TC1 soot was baked at 300 °C in air for 30 min as was suggested by Ohta and Okita.35
To measure the ice contact angle, water droplets were cooled to 238 K. Within 5 min, droplets froze. The appearance of ice was monitored by observation of their optical images. Ice contact angles were measured at 253, 243 and 238 K.
The amount of water adsorbed on soot was measured as a function of the relative humidity in a controlled desiccator by a gravimetrical method. Because the chemical nature of the soot surface is changed significantly after the outgassing and heating (see below) we limited the soot sample pretreatment for water adsorption measurements to drying in air at a water vapor pressure of not more than 2 × 10−5 Torr. Such conditions of pretreatment are necessary to clear the soot surface of preadsorbed water and impurities which may be accumulated from the ambient atmosphere. The level of cleaning was controlled by repeatedly weighing the soot sample until a constant weight was reached. Because of the very slow water adsorption kinetics,26 the amount of water adsorbed on the soot sample was measured after one day of exposure at each given value of the relative humidity. The accuracy of water adsorption measurements was 3–5%.
3. Results and discussion
3.1. Wettability of soots
A typical picture of a water droplet on a kerosene soot pellet is shown in Fig. 1. All laboratory-made soots produced by aviation kerosene burning in the oil lamp demonstrated hydrophobic features since the contact angles were found to be 80, 78 and 70° for T6, kerosene and TC1 soots, respectively. The different composition of aviation fuels gave a 10° difference in the contact angles between TC1 and T6 soots. Lamp soot demonstrated the smallest contact angle, θ ≈ 59°, which may be due to the purification of lamp kerosene for its domestic useage. Aircraft combustor soot appeared to be most wettable in comparison with aviation kerosene soot surrogates, with θ ≈ 63°. | ||
| Fig. 1 Image of a water droplet on kerosene soot pellet. | ||
The shape of frozen water droplets on all soot pellets was found to be independent of temperature. The contact angles of liquid and frozen droplets were essentially the same. For this reason, we will use the same value for the ice contact angle as presented above for the water contact angle on a given soot sample.
To clarify which factors influence the wettability of soot, different treatments of soots were tried. To distinguish between the impact of the surface chemical composition and the role of the soot microstructure we measured the soot electrical conductivity by compacting the soot powder. Fig. 2 shows the electrical conductivity, σ, of compacts of kerosene and TC1 soots as a function of the soot compacted number density, ρ. Similar dependencies σ(ρ) for both soots are observed indicating the similarities in their morphology. A slow increase of σ under compaction up to 0.1 g cm−3 is consistent with charge carriers which tunnel micropores between graphite microcrystallites as observed by transmission electron microscopy in combustor and kerosene soot.26,28 Water and organic molecules filling the micropores may play an important role in the soot conductivity by creating bridges for the charge transport. The rise in the conductivity is observed above 0.1 g cm−3 (see Fig. 2) because interparticle contacts increase by compacting the soot powder.
 | ||
| Fig. 2 Electrical conductivities of (1) untreated kerosene soot, (2) kerosene soot outgassed at 2 × 10−3 Torr for 18 h, (3) untreated TC1 soot; (4) TC1 soot heated at 300 °C for 30 min; and (5) kerosene soot outgassed and heated at 200 °C. Contact angles are indicated. | ||
The outgassing of kerosene soot at 2 × 10−3 Torr for 18 h leads to a decrease in its conductivity of one order of magnitude but does not change the soot microstructure because the typical shape of the σ(ρ) dependence remains (see Fig. 2). A decrease in the conductivity means the depletion of the charge carriers, probably volatile compounds, which provide the conductivity between microcrystallites. The outgassing facilitates the wettability and decreases the contact angle to 65°.
It is noted that heating leads to same qualitative results as vacuum treatment. The heating of TC1 soot at 300 °C for 30 min in air gave a loss of 1 wt% of the soot mass including 0.6 wt% of water. Fig. 2 shows the conductivity and the contact angle for TC1 soot after the heating procedure. We see a decrease in the conductivity which is accompanied by a drop in θ of 17°. Consequently, the reason for original kerosene soot hydrophobicity is the existence of volatile organics and water in soot micropores.
Fig. 2 proves that both the outgassing at 2 × 10−3 Torr for 18 h and the heating at 200 °C of kerosene soot dramatically influence both the surface composition and the soot microstructure because the steep rise in the conductivity is already observed at ρ > 0.06 g cm−3. Such “hard” treatment leads to the restructuring of the soot particle morphology and a dramatic decrease in the contact angle by 35° in comparison with the original soot. Thus, more compacted and less porous soot agglomerates are more wettable.
3.2 Soot hydration
Hydration properties of BC aerosols are of increasing importance because they provide direct information about the amount of water on the soot surface and a pathway of its wetting. The water adsorption on black carbon is determined most of all by the chemical nature of the surfaces. Being the combustion product of hydrocarbon fuels, soot particles consist mostly of carbon with some oxygen (≈5% in combustor soot and ≈7.7% in kerosene soot) as well as small amounts of metal and mineral impurities.36 Some organics, including oxidized ones may condense on the soot surface during soot formation which may also impact on its hygroscopicity.37Fig. 3 shows the isotherms of water adsorption at T = 295 K on combustor, kerosene, lamp and TC1 soots as a function of the relative pressure p/p0, where p0 is the saturated vapor pressure of water. For the correct comparison, absolute adsorption isotherms calculated per unit of surface area are presented. Measured values of the surface areas for combustor, kerosene, lamp and TC1 soots were 54, 43, 47, and 60 m2 g−1, respectively. Adsorption equivalent to one statistical monolayer of water, am, calculated assuming an effective molecular cross-section area for the water molecule of 0.105 nm2, is indicated in Fig. 3. A loose-packed monolayer of continuous adsorbed water is not formed on soot surfaces until the highest pressures due to the strong adsorbate–adsorbate interaction in the water–soot system, however am was proposed by Carrott 38 as a reference to characterize the level of the soot polarity in comparison with amount of water adsorbed at p/p0 = 0.5.
 | ||
| Fig. 3 Isotherms of water adsorption versus the relative pressure for (1) TC1 soot, (2) kerosene soot, (3) lamp soot, (4) combustor soot at T = 298 K, (5) Insert: kerosene soot after heating and outgassing (taken from ref. 28). Dashed line with open symbols is the isotherm for TC1 soot at 240 K. | ||
TC1 and kerosene soots were found to be more hydrophobic than other soots. Their isotherms are initially convex to the pressure axis and characterized by negligible adsorption in the initial region. Moreover, we observed a small loss of sample weight during the adsorption process up to p/p0 ≈ 0.15. Such behavior may be explained by the replacement of hydrophobic organic molecules by water molecules during the adsorption process as was observed for the water–organics–soot system by Gubkina et al.39 Such specific behavior is dramatically different from the water adsorption observed by vacuum gravimetry for kerosene soot heated at 423 K and outgassed at 10−5 Torr for 10 h.28 For comparison the isotherms of treated and untreated kerosene soots are shown in Fig. 3 (insert). One sees a steep rise in the water adsorption on treated soot at the lowest pressures due to filling of micropores containing the oxidized active sites.28 It means that for untreated (only dried) kerosene soot the adsorption of water molecules to these active sites is limited, probably because the micropores are occupied by organics. Only after replacement of this organic fraction is significant water adsorption observed, isotherms for kerosene and TC1 soot reach a ‘plateau’ close to a statistical monolayer am at p/p0 > 0.5. Such shape of isotherms corresponds to surfaces of intermediate polarity according to the classification of Carrott.38
The coverage of lamp soot is also close to am at intermediate pressures but its isotherm is concave at low pressures. It is clear that lamp soot contains more active sites accessible for water adsorption. Combustor soot appeared to be the most hydrophilic amongst other soots because its isotherm is characterized by the most amount of water adsorbed at all pressures. At low pressures it is highly concave and at p/p0 ≈ 0.5 it reaches approximately two statistical monolayers. This finding is roughly correlated with the low growth of engine soot particles observed by Gysel et al. at low FSC.40
Combustor soot as well as lamp soot were found to be the most wettable. Fig. 3 shows that they may absorb significant amounts of water at p/p0 ≤ 0.3. So, relatively high adsorption at low pressures is correlated with the soot wettability.
At the highest relative pressures the increase in the amount of water adsorbed is connected with capillary condensation into the large mesopores and macropores. Irregular agglomerates of soot have mesopores between the primary particles which may be filled by water due to the inverse Kelvin effect. Since the water contact angles on all soots in our study were found to be less than 90°, mean curvature of the water meniscus inside interparticle cavities will start with negative values for small filling angles (see Fig. 4 and more detailed description in Appendix). As the surrounding vapor pressure becomes larger, the mean curvature increases reaching the final equilibrium curvature in correspondence with the relative humidity in the ambient atmosphere.
 | ||
| Fig. 4 Pendular ring of condensed water between two contacting primary soot particles. Ψ is the filling angle. | ||
Significant water adsorption was found to take place at low temperatures. The isotherm on TC1 soot at T = 240 K is shown in Fig. 3, it is higher than the one at room temperature for all pressures. The temperature dependence of water adsorption was studied in previous work on combustor soot which was outgassed and heated,27 and a decrease in the water adsorption with temperature was observed.
4. Application: ice nucleation in UT
Measurements of the water soluble mass fraction (WSF) in our atmospheric soot surrogates showed the WSF to be only ≈ 0.3 wt%, and only 1.5 × 10−3 wt% was due to SO42− ion mass. This shows strong correlation with the results of Hallett et al.13 for the reduced water soluble mass fraction of soot particles resulting from open burning of the aviation kerosene. This is why laboratory-made kerosene and sulfur-free combustor soots are believed to represent the insoluble part of aircraft-generated soot in the UT. For such BC particles the mechanism of ice nucleation assumes the condensation process is related to the specific hydration and morphology features.The Fletcher theory41 generally assumes particles to be spherical implying that they present a positively curved surface to the surrounding vapor. Enhancement of the heterogeneous nucleation rate on the diesel soot in comparison with that predicted by the Fletcher theory has been found by Chen et al.17 According to the Fletcher theory, these results can be explained by the perfect wetting of the diesel soot surface, in spite of the fact that the water contact angle obtained on this soot was ≅40°. To resolve this problem it is enough to mention that the different kinds of hydrocarbon combustion soots as well as soot aerosols encountered in the UT originated as the typical chain agglomerates of small coalesced primary particles of 20–50 nm.2,3,17,28 Favored condensation in the interparticle cavities of soot agglomerates may lead to a lower critical supersaturation in comparison with the Fletcher theory.42
The Appendix presents the calculations of the mean curvature of the water meniscus between two primary soot particles. Dimensionless mean curvatures (HR) calculated using eqn. (4) and (5) for contact angles of 59, 63, 70 and 80° corresponding to lamp, combustor, TC1, and T6 soots, respectively, are shown in Fig. 5. At significant filling of the interparticle cavity, the size of the water meniscus increases. With increasing relative humidity the curvature becomes positive and finally reaches its maximum, HRmax, as shown in Fig. 5. This point corresponds to an unstable state43 and means that any further increase in the water vapor pressure results in the unconstrained condensation growth of particles.42 The ability of soot to form nuclei is given by the critical supersaturation, corresponding to the maximum, HRmax, needed to activate the particle, i.e. convert it into a freely growing drop. The specific surface properties and temperature would determine the ability of soot to act as ice nuclei or to form supercooled water droplets.
 | ||
| Fig. 5 Dimensionless mean curvature versus filling angle for contact angles of θ = 59, 63, 70, and 80°. | ||
QENS studies of water dynamics on kerosene soot28 showed that at UT conditions, T = 220 K, nearly 25% of adsorbed water exists as liquid-like water in supermicropores of 2 nm and 35% of adsorbed water transforms into ice in soot mesopores and on the external surface. This means that if the mean size of the interparticle cavities between primary soot particles is near 2 nm, ice nucleation at supersaturation conditions will start from a small amount of liquid water present in soot interparticle regions. When the filling of the mesopores is significant, the curvature is large and the effect of confined water becomes negligible, water freezes. Since we did not find any difference in contact angles for water and ice droplets, the curvature of the meniscus at this point should not be changed. Then, critical saturations with respect to ice, Si, may be calculated using eqn. (7). Values for Si are presented in Table 1 for measured contact angles of 59, 63, 70 and 80° and radii of primary soot particles of 20 nm and 40 nm.
| Contact angle | HR max | Saturation ratio SiT = 220 K | Saturation ratio SwT = 235 K | |
|---|---|---|---|---|
| Radius 20 nm | Radius 40 nm | Radius 20 nm | ||
| 59 | 0.98 | 1.053 | 1.026 | 1.037 |
| 63 | 1.12 | 1.062 | 1.030 | 1.042 |
| 70 | 1.35 | 1.075 | 1.036 | 1.051 |
| 80 | 3.02 | 1.175 | 1.083 | 1.114 |
The data obtained for the typical background UT temperature near 220 K show that if the critical ice supersaturation in the UT, si = Si − 1, reaches ≈5%, then more hydrophilic insoluble soot particles, characterized by θ ≈ 60°, may act as cirrus cloud nuclei. At a supersaturation ≈16% most hydrophobic BC particles with θ ≈ 80° will be activated.
In situ UT observations show a large supersaturation range in which heterogeneous nuclei could lead to cirrus formation before atmospheric particles freeze homogeneously.44 Tropospheric water vapor measurements show that supersaturation with respect to ice is common in the UT, with maxima as high as 73%.45,46 The presence of persistent contrails demonstrates that the UT is ice-supersaturated but will not form clouds unless initiated by the aircraft exhaust.47 Therefore, insoluble BC particles represented by kerosene flame soot in our study may amplify heterogeneous nucleation in the UT.
We should emphasize that the ice supersaturations estimated above give the lower limit for actual nucleation conditions because they were calculated assuming ideal spherical soot particles which are just in contact with each other. Original soot is frequently observed as fused roughly spherical particles (see ref. 36) that may reduce the obtained Si values.
In situ investigations of cirrus clouds have indicated that ice nucleation may occur in air which is saturated with respect to ice but still subsaturated with respect to liquid water.6 Laboratory studies of original lamp soot have also demonstrated ice nucleation at water subsaturated conditions.10 This situation is different for contrail formation where supersaturation with respect to the liquid phase is required.22,47 This requirement is probably correlated with the observation of the relatively high amount of sulfur in ice contrail BC residual particles.4 A high WSF ≈10 wt% was obtained in exhaust particles of an aircraft engine.48 Certainly, particles having such a large amount of soluble material are highly hydrophilic and may be covered by water at water supersaturation conditions and then may freeze rapidly in the cooled plume.
It is important to estimate the saturation with respect to water, Sw, for such hydrophilic partially soluble soots using the Koehler theory16 and compare it with the value of the Sw for insoluble BC particles which may exhibit the inverse Kelvin effect (IKE). Let us assume for estimation purposes T = 235 K as the typical temperature for water saturation in the plume.21,22 For a primary particle of radius R = 20 nm and WSF equal to 10 wt% the Sw was estimated to be 1.014. The corresponding values of Sw from the IKE are shown in Table 1. The effect of the existence of WSF ∼10% is much more considerable and cannot be compared with the IKE on low wettable surfaces. The effect of WSF = 2.2% is much lower, the value of Sw calculated corresponds to that given by the IKE on the insoluble surface characterized by the contact angle of 70°. This clearly emphasises the impact of the IKE in ice nucleation.
Finally, to demonstrate the minor impact of the Fletcher theory for concave partially wettable substrates we compare the critical supersaturation sw calculated in ref. 16 for R = 20 nm assuming the Fletcher theory, with the sw given by the IKE for two contact primary particles of 20 nm. At T = 296 K and θ = 70° the Fletcher theory gives sw = 40% while the IKE yields a result one order of magnitude less, near 4%.
In conclusion, the mechanism of soot formation in the aircraft combustor is very complex and not well characterized. The nonhomogeneous flow and temperature field as well as the numerical changes in combustion conditions during the flight result in heterogeneous distribution of the soluble compounds over the surface of exhaust soot particles. Then, two mechanisms of water nucleation may be competitive; due to both the high hygroscopicity of soluble particles and morphological features of insoluble particles. Particles containing the highest WSF will be covered by water and nucleate first at the water supersaturation in the young plume in correspondence with the Koehler theory. They are the most active as contrail condensation nuclei and will rapidly leave the atmosphere in the precipitation process. The part of the soot exhaust which does not contain many soluble compounds but is relatively wettable (characterized by 60–70° water contact angle) may also impact on the contrail formation and act as cloud condensation nuclei if the water supersaturation in the plume reaches ≈4%. The last part of the most hydrophobic soot is probably not activated in the plume. Such an assumption about the partly activated exhaust is in good agreement with the findings of Petzold and Schroder that one third of emitted BC must be incorporated into the contrail ice particles.49 Also Karcher et al.21 concluded that only about 1% of emitted soot particles in the plume are necessary for a contrail to have an optical depth above the visibility threshold. Therefore, hydrophobic soot particles will have a long lifetime in the atmosphere and will act as cirrus nuclei in the ice supersaturated UT regions, characterized by si > 18%.
Finally, we should mention that we neglected the effects of aging and chemical transformations of the soot surface under long exposure to reactive atmospheric gases50,51 because this important aspect is poorly understood now and further studies will be undertaken in future.
5. Conclusions
Aircraft combustor soot produced by burning sulfur-free gaseous fuel and kerosene flame soots are believed to represent the insoluble part of aircraft-generated soot in the upper atmosphere. Measurements of the water/ice contact angle on surfaces of atmospheric soot surrogates showed a range from 60° to 80°. Aircraft combustor soot appeared to be relatively wettable, θ ≅ 63°, in spite of the absence of any soluble sulfur containing compounds. The outgassing and heating of soots confirmed that volatile organics and adsorbed water covering the surface and filling the pores are factors influencing the soot wettability. This is why the different soot pre-treatments which are frequently used for soot laboratory studies should be used carefully in atmospheric applications because any pretreatment can have a dramatic influence on soot surface chemistry and wetting.Soot was believed to consist largely of hydrophobic material, and the fact that soot aerosols may be incorporated within cloud droplets has been explained in the past by the presence of internally mixed water soluble components in soot particles.14,52 We have proven that the formation of a water coating on the insoluble surface is facilitated by the specific mechanism of water adsorption on surface active sites. Water condenses on partially wettable particles even below saturation conditions because capillary condensation occurs in soot mesopores represented by interparticle cavities with negative curvature. This finding answers the question of how a water coating may form on the surface of insoluble exhaust soot particles.
Obviously, the water soluble mass fraction may not be the only criterion that governs nucleation properties. Following the inverse Kelvin effect the agglomerated soot structure is the major factor in ice nuclei formation on insoluble carbonaceous particles in the UT. It may dominate cirrus formation on insoluble BC particles in the UT as well as contrail formation if the water soluble fraction of the exhaust is relatively small, probably when the low sulfur content in the fuel is low.
Appendix
The simplest system which may simulate the soot agglomerates consists of two identical spherical particles of radius R which are in contact. Fig. 4 shows the pendular ring of condensed water at the contact point. The mean curvature of the water meniscus surface is defined as the sum | (1) |
| u = −sin ε, | (2) |
 | (3) |
In an axisymmetric geometry where the plane separating the two spheres can be regarded as a plane of symmetry eqn. (3) may be resolved only for positive values of y. Then the boundary conditions are expressed
at the sphere: ys = 1 − cos Ψ, us = −sin(θ + Ψ);
at the sphere: yp = 0, up = −1.
 | (4) |
| c = 4HR sin Ψ (HR sin Ψ − sin (θ + Ψ)). | (5) |
 | (6) |
In the range of negative HR the solution HR2 should be chosen, and when HR becomes positive and meridional curvature changes the sign from negative to positive, the other solution HR1 is correct.43 A set of calculations was performed for the mean curvatures with the contact angles measured for the different soots in our study.
The Kelvin equation relates the mean curvature to the equilibrium vapor pressure over the water or ice interface with air. For the saturation ratio in respect to water or ice, Sw or Si respectively, it gives
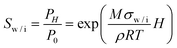 | (7) |
Acknowledgements
This research was funded by CRDF project RC1-2327-MO-02 and by the grant of President of Russian Federation, SSNo.11713.200.2. We are grateful to Dr Talukdar R. (NOAA Aeronomy Laboratory, Boulder, USA) for corrections and improvements as well as for production of lamp soot and suggesting it for the study.References
- M. Kuhn, A. Petzold, D. Baumgardner and F. P. Schroder, Geophys. Res. Lett., 1998, 25, 2679 CrossRef CAS.
- P. J. Sheridan, C. A. Brock and J. C. Wilson, Geophys. Res. Lett., 1994, 21, 2587 CrossRef CAS.
- D. F. Blake and K. Kato, J. Geophys. Res., [Atmos.], 1995, 100, 7195 CrossRef CAS.
- A. Petzold, J. Strom, S. Ohlsson and F. P. Schroder, Atmos. Res., 1998, 49, 21 CrossRef CAS.
- A. Petzold, A. Dopelheuer, C. A. Brock and F. Schroder, J. Geophys. Res., [Atmos.], 1999, 104, 22171 CrossRef CAS.
- IPCC Special report, Aviation and the global atmosphere, ed. J. E. Penner, D. H. Lister, D. J. Griggs, D. J. Dokken and M. McFarland, Cambridge University Press, Cambridge, UK, 1999 Search PubMed.
- E. J. Jensen and O. B. Toon, Geophys. Res. Lett., 1997, 24, 249 CrossRef CAS.
- Y. Chen, S. M. Kreidenweis, L. M. McInnes, D. C. Rogers and P. J. DeMott, Geophys. Res. Lett., 1998, 25, 1391 CrossRef CAS.
- D. Rogers, P. DeMott, S. M. Kreidenweis, Y. Chen, C. Twohy, D. Baumgarder, A. Heymsfield and K. R. Chan, Geophys. Res. Lett., 1998, 25, 1383 CrossRef CAS.
- P. J. DeMott, Y. Chen, S. M. Kreidenweis, D. Rogers and D. E. Sherman, Geophys. Res. Lett., 1999, 26, 2429 CrossRef CAS.
- K. Diehl and S. K. Mitra, Atmos. Environ., 1998, 32, 3145 CrossRef CAS.
- D. E. Hagen, M. B. Trueblood and D. R. White, Aerosol Sci. Technol., 1989, 10, 65.
- J. Hallett, J. G. Hudson and C. F. Rogers, Aerosol Sci. Technol., 1989, 10, 70 CAS.
- S. Lamel and P. Novakov, Atmos. Environ., 1995, 29, 813 CrossRef CAS.
- R. Niessner, B. Damner and D. Klokow, Aerosol Sci. Technol., 1990, 12, 953.
- H. K. Pruppacher and J. D. Klett, Microphysics of Clouds and Precipitation, D. Reidel, Dodrecht, 1978, pp. 714 Search PubMed.
- C.-C. Chen, L.-C. Hung and H.-K. Hsu, J. Colloid Interface Sci., 1993, 157, 465 CrossRef CAS.
- M. Schrader, J. Phys. Chem., 1975, 79, 2508 CrossRef CAS.
- M. L. Studebaker and C. W. Snow, J. Phys. Chem., 1955, 59, 973 CrossRef CAS.
- R. C. Brown, R. C. Miake-Lye, M. K. Anderson and C. E. Kolb, J. Geophys. Res., [Atmos.], 1996, 101, 22939 CrossRef CAS.
- B. Karcher, Th. Peter, U. M. Biermann and U. Schumann, J. Atmos. Sci., 1996, 53, 3066 CrossRef.
- B. Karcher, R. Busen, A. Petzold, F. P. Schroder, U. Schumann and E. J. Jensen, J. Geophys. Res., [Atmos.], 1998, 103, 17129 CrossRef CAS.
- M. M. Dubinin, Carbon, 1980, 18, 355 CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, New York, 2nd edn., 1982 Search PubMed.
- R. Sh. Vartapetian and A. M. Voloshuchuk, Russ. Chem. Rev., 1995, 64, 985 Search PubMed.
- O. B. Popovicheva, N. M. Persiantseva, M. E. Trukhin, G. B. Rulev, N. K. Shonija, Yu. Ya. Buriko, A. M. Starik, B. Demitdjian, D. Ferry and J. Suzanne, Phys. Chem. Chem. Phys., 2000, 2, 4421 RSC.
- O. B. Popovicheva, M. E. Trukhin and N. K. Shonija, Atmos. Environ., 2001, 35, 1673 CrossRef CAS.
- D. Ferry, J. Suzanne, S. Nitsche, O. B. Popovitcheva and N. K Shonija, J. Geophys. Res., [Atmos.], 2002, 107, 4734 CrossRef.
- J. Suzanne, D. Ferry, O. Popovitcheva and N. Shonija, Can. J. Phys., 2003, 81, 423 CrossRef CAS.
- C. H. Twohy and B. W. Gandrud, Geophys. Res. Lett., 1998, 25, 1359 CrossRef CAS.
- A. R. Chughtai, G. R. Williams, M. M. Atteya, N. J. Miller and D. M. Smith, Atmos. Environ., 1999, 33, 2679 CrossRef CAS.
- C. A. Longfellow, A. R. Ravishankara and D. R. Hanson, J. Geophys. Res., [Atmos.], 1999, 104, 13833 CrossRef CAS.
- I. L. Spain, in Chemistry and Physics of Carbon, ed. P. L. Walker and P. A. Thrower, Marcel Dekker, New York, 1981, vol. 16, p. 119 Search PubMed.
- S. J. Gregg and M. I. Pope, Fuel, 1960, 39, 301 CAS.
- S. Ohta and T. Okita, Atmos. Environ., 1984, 18, 2423.
- O. B. Popovicheva, N. M. Persiantseva, B. V. Kuznetsov, T. A. Rakhmanova, N. K. Shonija, J. Suzanne and D. Ferry, J. Phys. Chem. A, 2003, 107, 10046 CrossRef CAS.
- K. Kinoshita, Carbon: Electrochemical and Physicochemical Properties, Wiley-Interscience, New York, 1988 Search PubMed.
- P. Carrott, Carbon, 1992, 50, 201 CrossRef.
- M. L. Gubkina and N. S. Polyakov, Bull. Acad. Sci. USSR, Div. Chem. Sci., 2001, 4, 570 Search PubMed.
- M. Gysel, S. Nyeki, E. Weingartner, U. Baltensperger, H. Giebl and R. Hitzenberger, Geophys. Res. Lett., 2003, 30, 1566 Search PubMed , doi: 10.1029/2003GL016896.
- N. H. Fletcher, J. Chem. Phys., 1958, 29, 572 CAS.
- Y. Crouzet and W. Marlow, Aerosol Sci. Technol., 1995, 22, 43 CAS.
- F. M. Orr, L. E. Scriven and A. P. Rivas, J. Fluid Mech., 1975, 67, 723.
- P. J. DeMott, D. C. Rogers and S. M. Kreidenweis, J. Geophys. Res., [Atmos.], 1997, 102, 19575 CrossRef CAS.
- E. J. Jensen, S. Vay, G. Sachse, J. Podolske, D. Baumgardner and M. Avery, Aviation, aerosol, contrails and cirrus clouds, air pollution research report 74, European Community, Luxembourg, 2001, p. 173 Search PubMed.
- S. A. Vay, B. E. Anderson, E. J. Jensen, G. W. Sachse, J. Ovarlez, G. L. Gregory, S. R. Nolf, J. R. Podolske, T. A. Slate and C. E. Sorenson, J. Geophys. Res., [Atmos.], 2000, 105(D3), 3745 CrossRef CAS.
- E. J. Jensen, O. B. Toon, S. Kinne, G. W. Sachse, B. E. Anderson, K. R. Chan, C. H. Twohy, B. Gandrud, A. Heymsfield and R. C. Miake-Lye, J. Geophys. Res., [Atmos.], 1998, 103, 3929 CrossRef.
- D. E. Hagen, M. B. Trueblood and P. D. Whitefield, Particulate Sci. Technol., 1992, 10, 53 Search PubMed.
- A. Petzold and F. P. Schroder, Aerosol Sci. Technol., 1998, 28, 62 CAS.
- A. R. Chughtai, M. E. Brooks and D. M. Smith, J. Geophys. Res., [Atmos.], 1996, 101, 19505 CrossRef CAS.
- R. Kotzick and R. Niessner, Atmos. Environ., 1999, 33, 2669 CrossRef CAS.
- E. Weingartner, H. Burtscher and U. Baltensperger, Atmos. Environ., 1997, 31, 2311 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |

