Disc-shaped triphenylenes in a smectic organisation
Paul H. J.
Kouwer
,
Jahan
Pourzand
and
Georg H.
Mehl
*
University of Hull, Department of Chemistry, Cottingham Road, Hull, UK HU6 7RX. E-mail: g.h.mehl@hull.ac.uk; Fax: +44 1482 466411
First published on 21st November 2003
Abstract
A layered phase (SmA) was observed in a novel linked disc-rod mesogen, containing a triphenylene mesogen and attached to three cyanobiphenyl moieties.
From the introduction of discotic liquid crystals in the late 1970s, the development of disc-shaped and rod-shaped mesogens has run mostly in parallel. Some attempts to bridge the gap between these differently shaped liquid crystals have resulted in monotropic nematic or soft-crystal phases.1 Recently, we have shown that by attaching discs and rods, materials with stable nematic phases can be obtained. Moreover, these materials were miscible with common disc-shaped nematogens as well as with the rod-shaped precursor.2 When calamitic smectogens were coupled to a discotic nematogen, the nematic phase was only found at high temperatures. At lower temperatuters, various smectic phases were observed.3
The extension of this work to the archetype of discotic mesogens, the triphenylene core4 is not only logical, but also of interest in the light of the resurgence of interest in discotic materials in nanotechnological applications.5 However, triphenylenes, show a strong preference to form columnar phases. In fact, only substitution by (bulky) benzoic acid derivatives can force these discotics into nematic phases.6 Smectic phases with triphenylenes have not been observed so far.
Here, we present the synthesis of a novel covalently linked disc-rod mesogen, containing a triphenylene based disc-shaped moiety and three cyanobiphenyl-based rod shaped moiety, see Fig. 1. The mesogen is designed in a way that cross sections of the discotic part and the calamitic part of the mesogen are matching, favouring the formation of layered phases.7
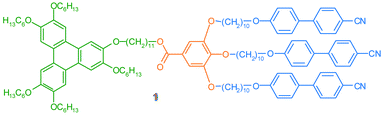 | ||
| Fig. 1 Disc-rod mesogen 1. | ||
The synthesis of 1 is outlined in Scheme 1. The triphenylene core was prepared by a modified literature procedure.8 A C11 spacer was attached to the mono-substituted catechol 2 (easily obtained as the by-product from disubstitution). By converting the alcohol group to an acetyl group, the hydroxyl is protected from the oxidising conditions during the triphenylene coupling reaction. Deprotection yielded the hydroxyl functionalised triphenylene 7 quantitatively. The synthesis of the linked cyanobiphenyl trimer 8 has been described elsewhere.9 Deprotection of the ester group yielded the free carboxylic acid, which was subsequently coupled to 7 by a DCC mediated esterification to give 1.10
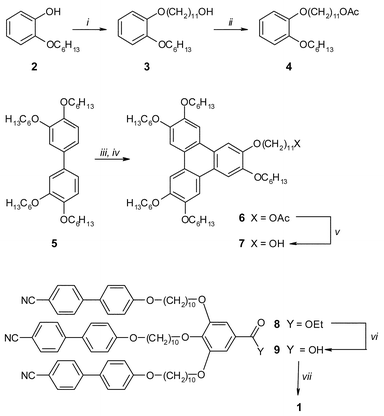 | ||
| Scheme 1 Synthesis of the target compound 1. Key: (i) Br(CH2)11OH, K2CO3, KI, butanone, reflux for16 h; (ii) AcCl, C5H5N, CH2Cl2, room temperature for 20 h; (iii) 4, FeCl3, CH2Cl2, room temperature for 20 h; (iv) precipitation and stirring in cold MeOH for 2 h; (v) pTSA, EtOH, reflux for 16 h; (vi) aq. KOH, EtOH/THF, reflux for 2 h; (vii) 7, DCC, DMAP, pTSA, CH2Cl2, room temperature for 5 d. | ||
The liquid crystalline properties of 1, 7 and 8 have been investigated with optical polarising microscopy (OPM), differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The results are summarised in Table 1.
| Material | Phase behavioura,b |
ΔHclc
/kJ mol−1 |
|||
|---|---|---|---|---|---|
| a Transition temperatures in °C and (latent heat in J g−1). b GSmA = glassy, smectic A phase frozen in, SmA = smectic A, Colh = hexagonal columnar, I = isotropic, Cr = crystalline. c Latent heat at the clearing temperature. d Monotropic phase transition. | |||||
| 1 | GSmA | 3 | SmA | 39 (4.1) I | 8.5 |
| 7 | Cr | [23 (37) | Colh 51 (3.7)]d | 55 (49) I | 3.4 |
| 8 | Cr | 96 (77) | N | 105 (3.6) I | 4.3 |
Optical polarising microscopy experiments show a clear columnar texture for 7 (Fig. 2a) and nematic texture for mesogen 8 (Fig. 2b). On formation from the isotropic phase, the texture of 1 is grainy and inconclusive upon formation from the isotropic phase, but after shearing and annealing domains grow and focal conics can be observed, see Fig. 2c. The texture and presence of large homeotropic domains in the sample is indicative for the formation of a SmA phase. X-Ray experiments support the evidence for a layered phase. As reflections were observed over a wide angular range, separate small and wide angle diffractograms were recorded (Fig. 3).
 | ||
| Fig. 2 Optical polarising microscopy photographs of (a) 7 at 51 °C (Colh); (b) 8 at 110 °C (N) and (c) 1 at 30 °C. All pictures taken on cooling from the isotropic phase. Crossed polarisers. The magnification is indicated by the scale bar. | ||
In the small angle range a sharp reflection is observed at q = 0.70 nm−1, corresponding to a spacing of 90 Å, attributed to the SmA layer spacing. A small second order reflection is visible at 1.36 nm−1 (46 Å), but is more clear in the wide angle diffractogram of Fig. 3a. Furthermore, two diffuse reflections are observed at q = 3.9 nm−1 (marked A) and 14.4 nm−1 (marked B) The latter, corresponding to a spacing of 4.4 Å is found in nearly all liquid crystals and is attributed to the average lateral periodicity of two (rod) shaped mesogens. It coincides with the reflections from the alkyl-groups and the face-on arrangement of two disordered discotic mesogens. The small angle diffuse reflection (A), corresponds to a spacing of 15.9 Å, which is attributed to the average lateral periodicity of two discotic mesogens.
 | ||
| Fig. 3 Integrated wide angle (a) and small angle (b) X-ray diffraction patterns of 1 at T = 30 °C. In the small angle area a clear fundamental reflection is observed as well as a very small (002) reflection (more clear in the wide angle diffractogram). Two diffuse reflections are marked A and B. Circles give experimental data, lines the multi-function fits — Gaussian functions for layer reflections, Lorentzian for A and B. | ||
The X-ray experiments clearly indicate a bilayered phase (SmA2), see Fig. 4. As the layer spacing is slightly smaller than twice the molecular length (2 × 48 Å), we can assume some degree of interdigitation of either the cyano-groups or of the alkyl tails of the triphenylenes; alternatively, this might be due to the disordering of the mesogens in the mesomorphic state.11
 | ||
| Fig. 4 Cartoon of the order of 1 in the SmA2 phase. | ||
The diffuse small angle reflection and the absence of any sharp reflections at 3.5 Å (18 nm−1, face-on spacing between two ordered discotic mesogens) suggest that the layer of triphenylene mesogens is highly disordered. In fact, the discs behave like common calamitic mesogens in the layers. Moreover, any positional order of the discs in this conformation will lead to positional order in three dimensions and hence, the formation of a crystalline phase.
In summary, a novel linked disc-rod mesogen was prepared, based on one triphenylene mesogen and three cyanobiphenyl moieties. Due to the molecular topology, the system orders in a SmA2 phase, where the disc-shaped mesogens, although standing up in the layers, behave indifferently from the calamitic mesogens. To the best of our knowledge, this is the first report on a thermodynamically stable layered phase for triphenylene mesogens.12
We acknowledge Prof. S. J. Picken for allowing us to conduct SAXS studies in his laboratories, the Ramsay Memorial Fund and the EU (HPRN-CT2000-00016) for financial support.
Notes and references
- (a) W. Kreuder, H. Ringsdorf, O. Herrmann-Schönherr and J. H. Wendorff, Angew. Chem., Int. Ed. Engl., 1987, 26, 1249 CrossRef; (b) I. D. Fletcher and G. R. Luckhurst, Liq. Cryst., 1995, 18, 175 CrossRef CAS; (c) J. J. Hunt, R. W. Date, B. A. Timimi, G. R. Luckhurst and D. W. Bruce, J. Am. Chem. Soc., 2001, 123, 10115 CrossRef CAS.
- P. H. J. Kouwer and G. H. Mehl, J. Am. Chem. Soc., 2003, 125, 11172 CrossRef CAS.
- P. H. J. Kouwer and G. H. Mehl, Angew. Chem., in press Search PubMed.
- (a) J. Billard, J. C. Dubois, H. H. Tinh and A. Zann, Nouv. J. Chim., 1978, 2, 535 Search PubMed; (b) C. Destrade, M. C. Mondon and J. Malthête, J. Phys. (Paris), 1979, 40, C3 Search PubMed.
- (a) N. Boden and B. Movaghar, in Handbook of Liquid Crystals, D. Demus, J. W. Goodby, G. W. Gray, H. W. Spiess and V. Vill, eds., Wiley VCH, New York, 1998, vol. 2B, p. 781 Search PubMed; (b) R. J. Bushby and O. W. Lozman, Curr. Op. Coll. Interf. Sci., 2002, 7, 343 Search PubMed and refs. therein.
- For a review on the phase behaviour of a wide variety of substituted triphenylenes: A. N. Cammidge and R. J. Bushby, in Handbook of Liquid Crystals, D. Demus, J. W. Goodby, G. W. Gray, H. W. Spiess and V. Vill, eds., Wiley VCH, New York, 1998, vol. 2B, p. 693 Search PubMed.
- The cross section is the area of the mesogens perpendicular to the phase normal. For the triphenylene discs (in a non-columnar organisation), the cross section is the ØD × dD (diameter × width) = 16–20 × 4 = 64–80 Å2, dependent on the orientation of the tails. Rods have characteristic cross sections of ∼20–25 Å2, and thus 3 rods in the molecule will have a cross section of ∼60–75 Å2.
- (a) In this route, the spacer (with protected functional group) was introduced prior to oxidative synthesis of the triphenylene core. Useful references for the preparation of functionalised triphenylenes; (b) N. Boden, R. J. Bushby, A. N. Cammidge, A. El-Mansoury, P. S. Martin and Z. Lu, J. Mater. Chem., 1999, 9, 1391 RSC.
- P. H. J. Kouwer and G. H. Mehl, Mol. Cryst. Liq. Cryst., 2003, 397, 301 Search PubMed.
- Target compound 1: A mixture of 7 (91 mg, 0.1 mmol), 9 (94 mg, 0.08 mmol), DCC (103 mg, 0.5 mmol), DMAP (6.1 mg, 0.05 mmol) and pTSA (9.5 mg, 0.05 mmol) dissolved in CH2Cl2 (5 mL) and dry THF (5 mL) was stirred for 5 days at room temperature. The solvents were evaporated under reduced pressure, 2 mL of CH2Cl2 was added and the reaction mixture was transferred to a column for chromatography (SiO2, eluent CH2Cl2 to CH2Cl2/EtOAc (20 : 1)) yielding pure 1 (52 mg, 0.025 mmol, 31%) as a sticky white solid. 1H NMR (CDCl3, 400 MHz): δ = 7.77 (s, 6H, CH triphenylene); 7.68–7.64, 7.62–7.58, 7.49–7.45, 6.95–6.91 (4 × m, 4 × 6H, CH cyanobiphenyl); 7.24 (s, 2H, CH linking group); 4.23 (t, 3J(H,H) = 6.8 Hz, 2H, CH2OCO); 4.18–4.15 (m, 12H, CH2O triphenylene); 3.96, 3.92 (2 × t, 3J(H,H) = 6.5 Hz, 2 × 12H, CH2O cyanobiphenyl spacer); 1.95–1.20 (m, 88H, CH2); 0.88 (t, 3J(H,H) = 6.2 Hz, 15H, CH3). 13C NMR (CDCl3, 400 MHz): δ = 166.45, 152.72, 142.19, 125.09, 107.93 (linking group); 159.71, 145.15, 132.49, 131.16, 128.23, 126.96, 119.06, 114.98, 109.96 (cyanobiphenyl); 148.9, 123.54, 107.27 (triphenylene); 73.40, 69.66, 69.09, 68.06, 65.15 (CH2O); 31.6–22.6 (CH2 tails and spacers); 14.02 (CH3).
- B. I. Ostrovskii, Liq. Cryst., 1993, 14, 131.
- Y. Shimizu, A. Kurobe, H. Monobe, N. Terasawa, K. Kiyohara and K. Uchida, Chem. Commun., 2003, 1667 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |
